 Found 94 hits with Last Name = 'tep' and Initial = 's'
Found 94 hits with Last Name = 'tep' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM50041234

(6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...)Show InChI InChI=1S/C12H8O4/c1-14-12-10-8(4-5-15-10)6-7-2-3-9(13)16-11(7)12/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50041234

(6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...)Show InChI InChI=1S/C12H8O4/c1-14-12-10-8(4-5-15-10)6-7-2-3-9(13)16-11(7)12/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM50008072
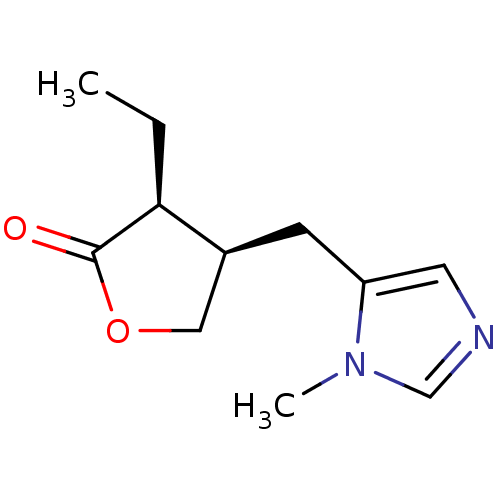
((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...)Show InChI InChI=1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50024210
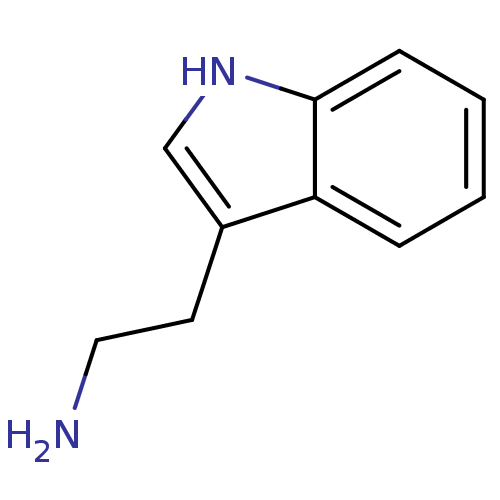
(1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...)Show InChI InChI=1S/C10H12N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50240850
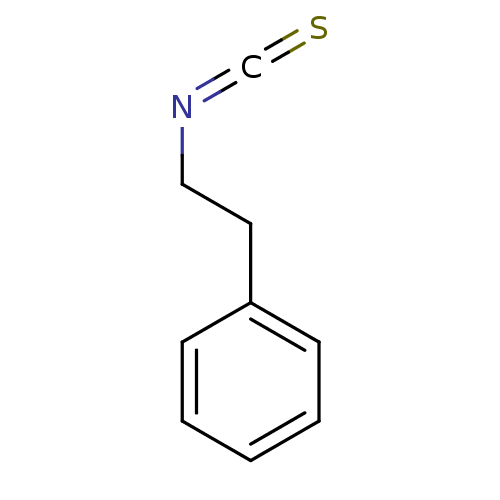
((2-Isothiocyanato-ethyl)-benzene | CHEMBL151649 | ...)Show InChI InChI=1S/C9H9NS/c11-8-10-7-6-9-4-2-1-3-5-9/h1-5H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50101991
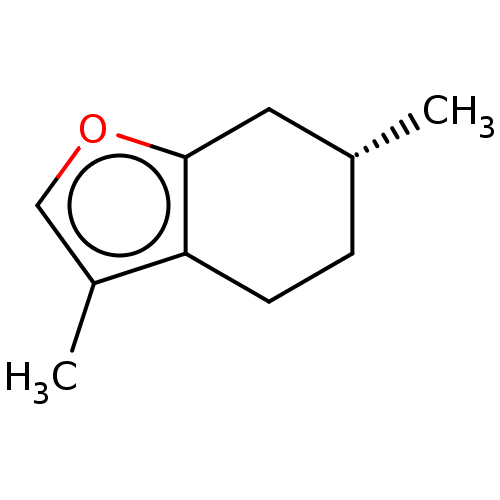
(CHEBI:6750 | CHEMBL3526658)Show InChI InChI=1S/C10H14O/c1-7-3-4-9-8(2)6-11-10(9)5-7/h6-7H,3-5H2,1-2H3/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50008072

((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...)Show InChI InChI=1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50101990
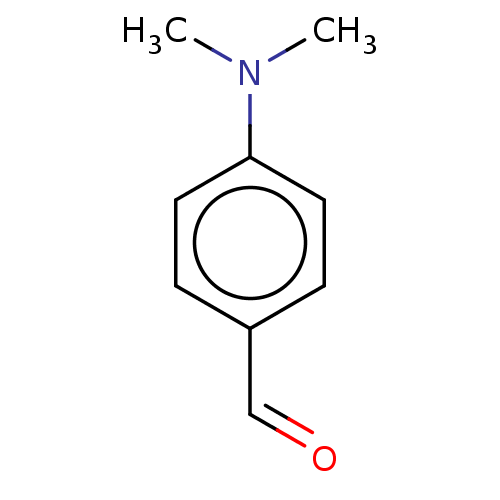
(CHEMBL3188333)Show InChI InChI=1S/C9H11NO/c1-10(2)9-5-3-8(7-11)4-6-9/h3-7H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM50240850

((2-Isothiocyanato-ethyl)-benzene | CHEMBL151649 | ...)Show InChI InChI=1S/C9H9NS/c11-8-10-7-6-9-4-2-1-3-5-9/h1-5H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM109750
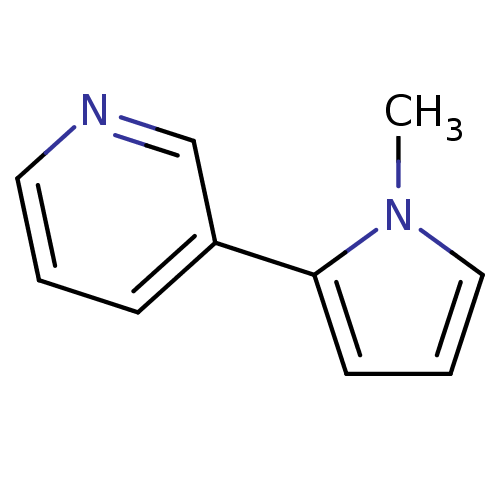
(US8609708, 19 β-Nicotyrine | US8609708, 19 be...)Show InChI InChI=1S/C10H10N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM109750

(US8609708, 19 β-Nicotyrine | US8609708, 19 be...)Show InChI InChI=1S/C10H10N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM50024210

(1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...)Show InChI InChI=1S/C10H12N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM50101990

(CHEMBL3188333)Show InChI InChI=1S/C9H11NO/c1-10(2)9-5-3-8(7-11)4-6-9/h3-7H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM50101991

(CHEBI:6750 | CHEMBL3526658)Show InChI InChI=1S/C10H14O/c1-7-3-4-9-8(2)6-11-10(9)5-7/h6-7H,3-5H2,1-2H3/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Mixed inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A13
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2A13 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2A6 (unknown origin) |
Drug Metab Dispos 40: 1797-802 (2012)
Article DOI: 10.1124/dmd.112.045161
BindingDB Entry DOI: 10.7270/Q2BK1F3P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552588
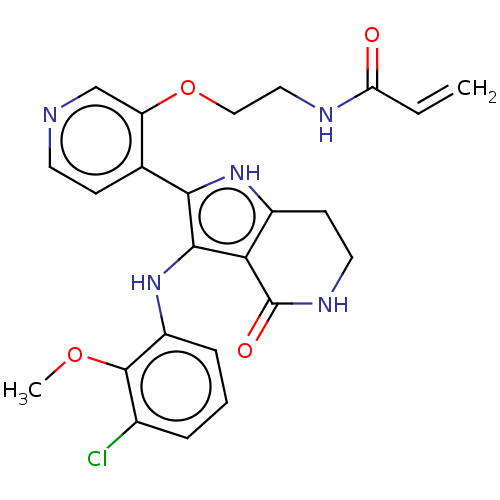
(N-[2-({4-[3-(3-chloro-2-methoxyanilino)-4-oxo-4,5,...)Show SMILES COc1c(Cl)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OCCNC(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552593
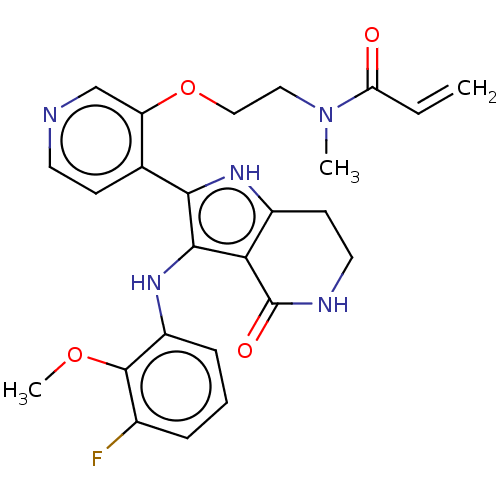
(N-[2-({4-[3-(3-fluoro-2-methoxyanilino)-4-oxo-4,5,...)Show SMILES COc1c(F)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OCCN(C)C(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552600
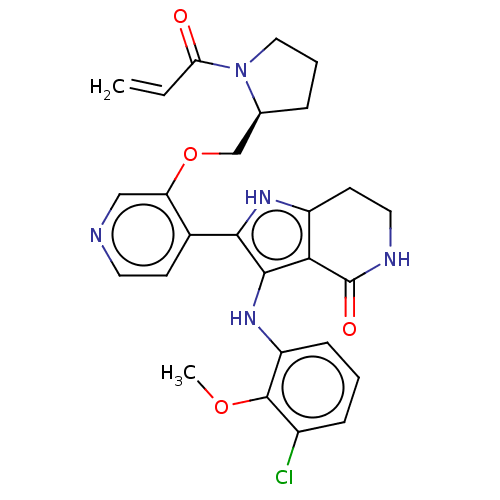
(3-(3-chloro-2-methoxyanilino)-2-(3-{[(2S)-1-(prop-...)Show SMILES COc1c(Cl)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OC[C@@H]1CCCN1C(=O)C=C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552592
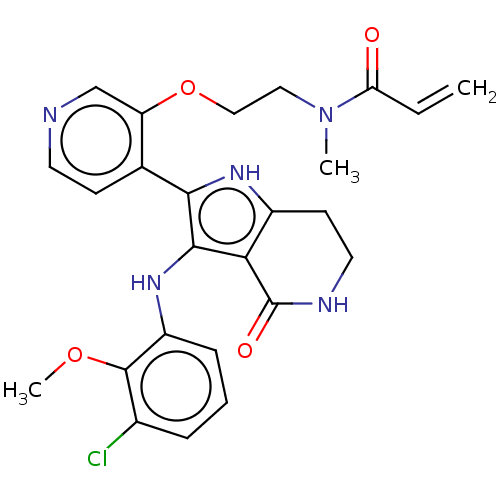
(N-[2-({4-[3-(3-chloro-2-methoxyanilino)-4-oxo-4,5,...)Show SMILES COc1c(Cl)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OCCN(C)C(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552590
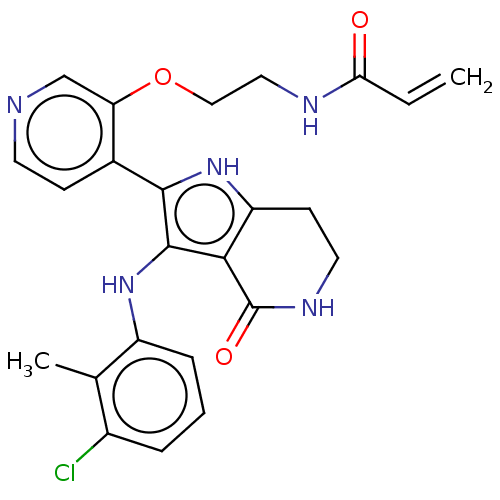
(N-[2-({4-[3-(3-chloro-2-methylanilino)-4-oxo-4,5,6...)Show SMILES Cc1c(Cl)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OCCNC(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552591
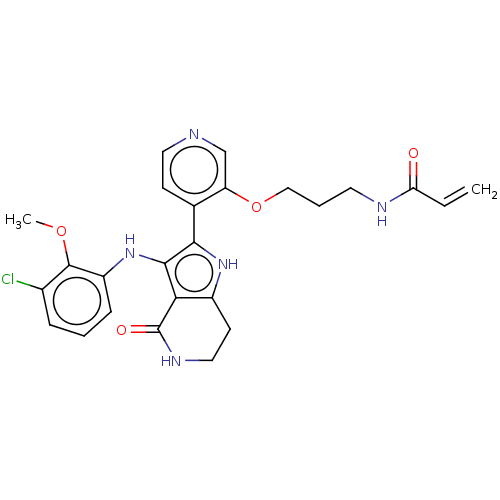
(N-[3-({4-[3-(3-chloro-2-methoxyanilino)-4-oxo-4,5,...)Show SMILES COc1c(Cl)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OCCCNC(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552589
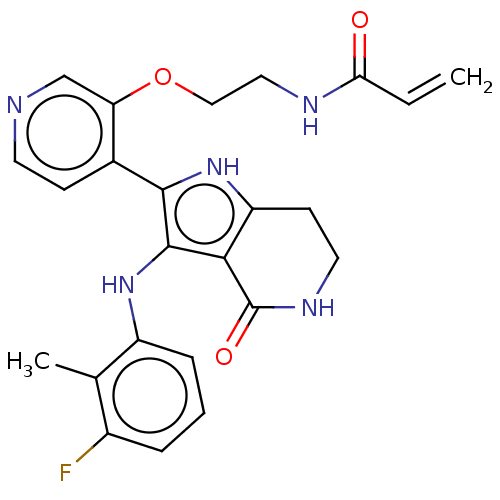
(N-[2-({4-[3-(3-fluoro-2-methylanilino)-4-oxo-4,5,6...)Show SMILES Cc1c(F)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OCCNC(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552598
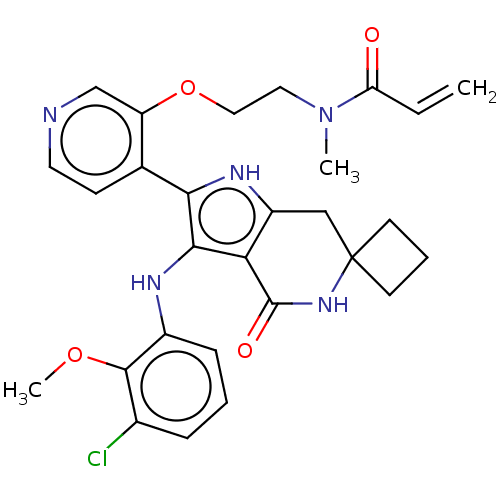
(N-[2-({4-[3'-(3-chloro-2-methoxyanilino)-4'-oxo-1'...)Show SMILES COc1c(Cl)cccc1Nc1c([nH]c2CC3(CCC3)NC(=O)c12)-c1ccncc1OCCN(C)C(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552595
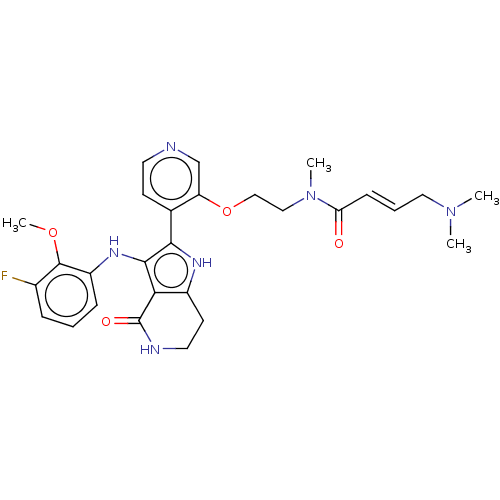
((2E)-4-(dimethylamino)-N-[2-({4-[3-(3-fluoro-2-met...)Show SMILES COc1c(F)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OCCN(C)C(=O)\C=C\CN(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552601
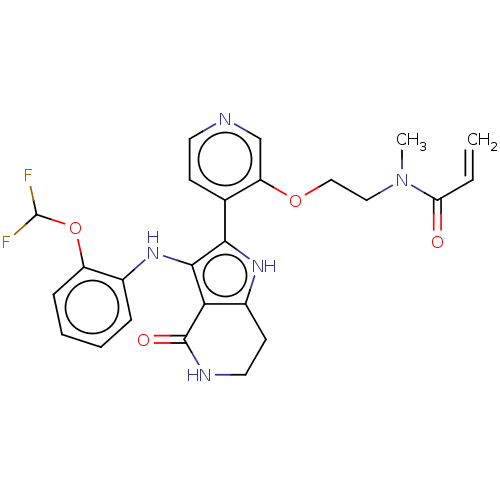
(N-(2-{[4-(3-{[2-(difluoromethoxy)phenyl]amino}-4-o...)Show SMILES CN(CCOc1cnccc1-c1[nH]c2CCNC(=O)c2c1Nc1ccccc1OC(F)F)C(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552599
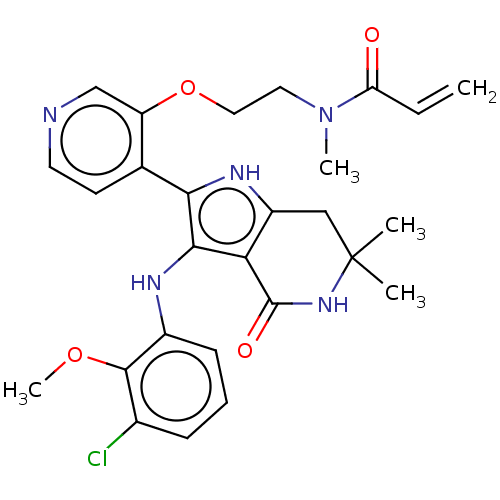
(N-[2-({4-[3-(3-chloro-2-methoxyanilino)-6,6-dimeth...)Show SMILES COc1c(Cl)cccc1Nc1c([nH]c2CC(C)(C)NC(=O)c12)-c1ccncc1OCCN(C)C(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552594
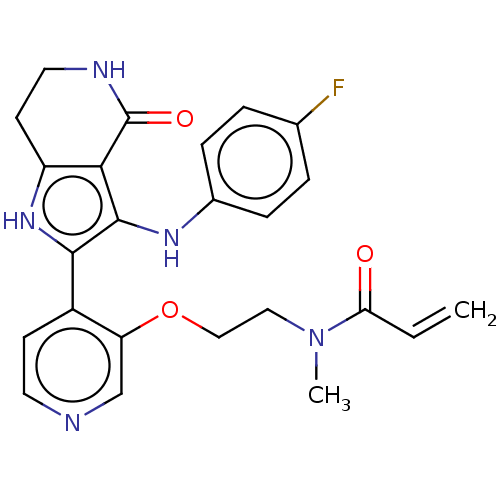
(N-[2-({4-[3-(4-fluoroanilino)-4-oxo-4,5,6,7-tetrah...)Show SMILES CN(CCOc1cnccc1-c1[nH]c2CCNC(=O)c2c1Nc1ccc(F)cc1)C(=O)C=C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552596
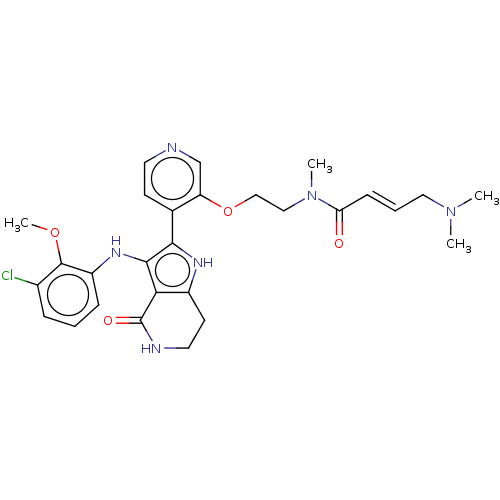
((2E)-N-[2-({4-[3-(3-chloro-2-methoxyanilino)-4-oxo...)Show SMILES COc1c(Cl)cccc1Nc1c([nH]c2CCNC(=O)c12)-c1ccncc1OCCN(C)C(=O)\C=C\CN(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [669-770,'SVD',771-1210]
(Homo sapiens (Human)) | BDBM552597
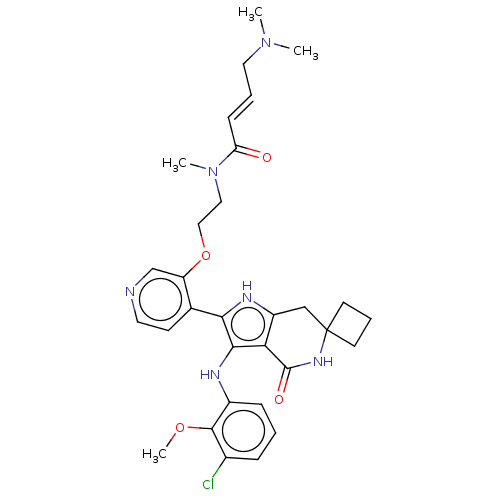
((2E)-N-[2-({4-[3'-(3-chloro-2-methoxyanilino)-4'-o...)Show SMILES COc1c(Cl)cccc1Nc1c([nH]c2CC3(CCC3)NC(=O)c12)-c1ccncc1OCCN(C)C(=O)\C=C\CN(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| WIPO WO2022101184
| n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542RSQ |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426433
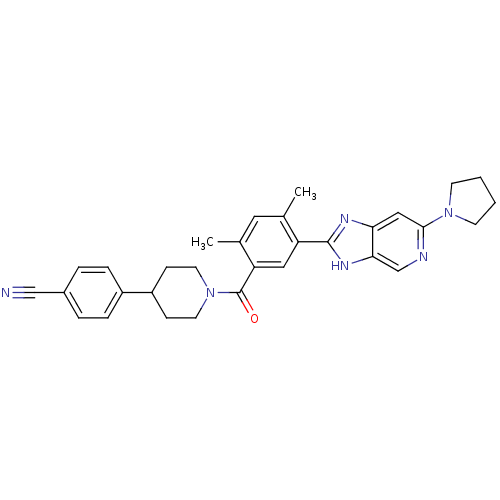
(CHEMBL2322360)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2cc(ncc2[nH]1)N1CCCC1 Show InChI InChI=1S/C31H32N6O/c1-20-15-21(2)26(31(38)37-13-9-24(10-14-37)23-7-5-22(18-32)6-8-23)16-25(20)30-34-27-17-29(33-19-28(27)35-30)36-11-3-4-12-36/h5-8,15-17,19,24H,3-4,9-14H2,1-2H3,(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FASN in human HeLa cells assessed as reduction of de novo synthesis of palmitate |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426433

(CHEMBL2322360)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2cc(ncc2[nH]1)N1CCCC1 Show InChI InChI=1S/C31H32N6O/c1-20-15-21(2)26(31(38)37-13-9-24(10-14-37)23-7-5-22(18-32)6-8-23)16-25(20)30-34-27-17-29(33-19-28(27)35-30)36-11-3-4-12-36/h5-8,15-17,19,24H,3-4,9-14H2,1-2H3,(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426447
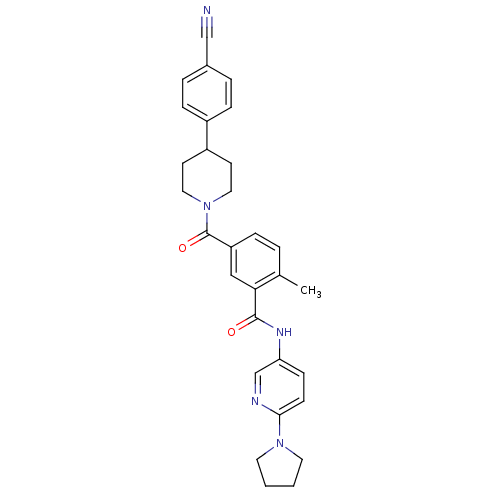
(CHEMBL2322358)Show SMILES Cc1ccc(cc1C(=O)Nc1ccc(nc1)N1CCCC1)C(=O)N1CCC(CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C30H31N5O2/c1-21-4-7-25(30(37)35-16-12-24(13-17-35)23-8-5-22(19-31)6-9-23)18-27(21)29(36)33-26-10-11-28(32-20-26)34-14-2-3-15-34/h4-11,18,20,24H,2-3,12-17H2,1H3,(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426435

(CHEMBL2322370)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2cc(ncc2[nH]1)N1CCOCC1 Show InChI InChI=1S/C31H32N6O2/c1-20-15-21(2)26(31(38)37-9-7-24(8-10-37)23-5-3-22(18-32)4-6-23)16-25(20)30-34-27-17-29(33-19-28(27)35-30)36-11-13-39-14-12-36/h3-6,15-17,19,24H,7-14H2,1-2H3,(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426432
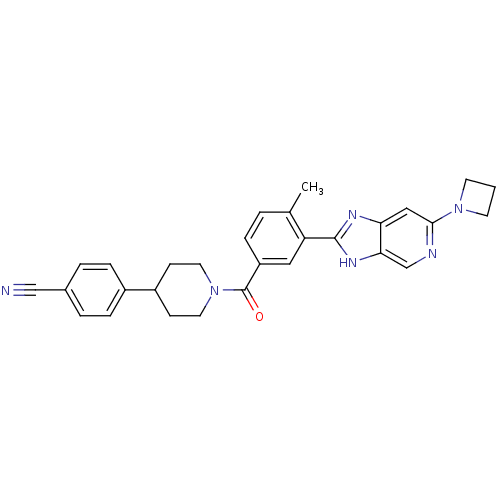
(CHEMBL2322361)Show SMILES Cc1ccc(cc1-c1nc2cc(ncc2[nH]1)N1CCC1)C(=O)N1CCC(CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C29H28N6O/c1-19-3-6-23(29(36)35-13-9-22(10-14-35)21-7-4-20(17-30)5-8-21)15-24(19)28-32-25-16-27(34-11-2-12-34)31-18-26(25)33-28/h3-8,15-16,18,22H,2,9-14H2,1H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426427
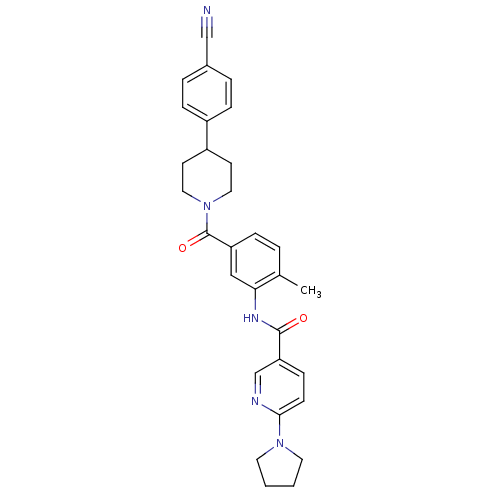
(CHEMBL2322357)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(nc1)N1CCCC1)C(=O)N1CCC(CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C30H31N5O2/c1-21-4-7-25(30(37)35-16-12-24(13-17-35)23-8-5-22(19-31)6-9-23)18-27(21)33-29(36)26-10-11-28(32-20-26)34-14-2-3-15-34/h4-11,18,20,24H,2-3,12-17H2,1H3,(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426434
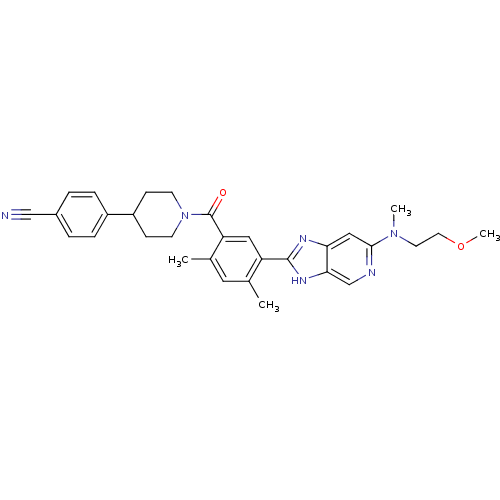
(CHEMBL2322359)Show SMILES COCCN(C)c1cc2nc([nH]c2cn1)-c1cc(C(=O)N2CCC(CC2)c2ccc(cc2)C#N)c(C)cc1C Show InChI InChI=1S/C31H34N6O2/c1-20-15-21(2)26(31(38)37-11-9-24(10-12-37)23-7-5-22(18-32)6-8-23)16-25(20)30-34-27-17-29(33-19-28(27)35-30)36(3)13-14-39-4/h5-8,15-17,19,24H,9-14H2,1-4H3,(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426443
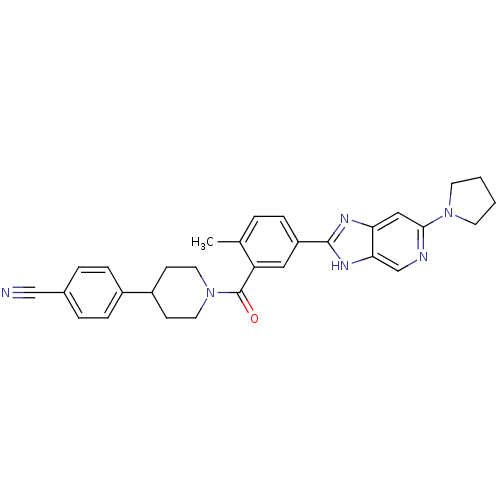
(CHEMBL2322374)Show SMILES Cc1ccc(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2cc(ncc2[nH]1)N1CCCC1 Show InChI InChI=1S/C30H30N6O/c1-20-4-7-24(29-33-26-17-28(32-19-27(26)34-29)35-12-2-3-13-35)16-25(20)30(37)36-14-10-23(11-15-36)22-8-5-21(18-31)6-9-22/h4-9,16-17,19,23H,2-3,10-15H2,1H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Rattus norvegicus) | BDBM50426433

(CHEMBL2322360)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2cc(ncc2[nH]1)N1CCCC1 Show InChI InChI=1S/C31H32N6O/c1-20-15-21(2)26(31(38)37-13-9-24(10-14-37)23-7-5-22(18-32)6-8-23)16-25(20)30-34-27-17-29(33-19-28(27)35-30)36-11-3-4-12-36/h5-8,15-17,19,24H,3-4,9-14H2,1-2H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FASN in rat NMU cells assessed as reduction of de novo synthesis of palmitate |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426430
(CHEMBL2322363)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2ncc(cc2[nH]1)S(C)(=O)=O Show InChI InChI=1S/C28H27N5O3S/c1-17-12-18(2)24(14-23(17)26-31-25-13-22(37(3,35)36)16-30-27(25)32-26)28(34)33-10-8-21(9-11-33)20-6-4-19(15-29)5-7-20/h4-7,12-14,16,21H,8-11H2,1-3H3,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
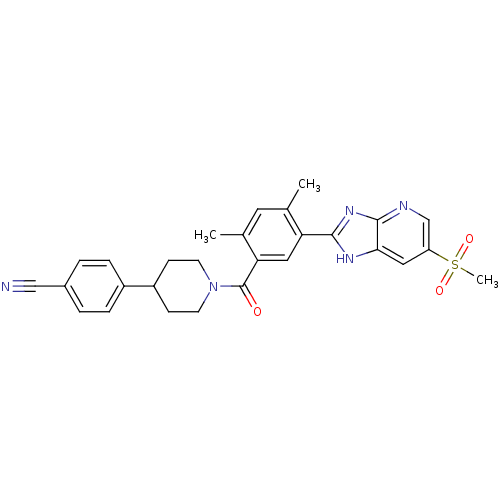
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426429

(CHEMBL2322364)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2nc(ccc2[nH]1)N1CCC1 Show InChI InChI=1S/C30H30N6O/c1-19-16-20(2)25(30(37)36-14-10-23(11-15-36)22-6-4-21(18-31)5-7-22)17-24(19)28-32-26-8-9-27(33-29(26)34-28)35-12-3-13-35/h4-9,16-17,23H,3,10-15H2,1-2H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426431

(CHEMBL2322362)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2cc(ncc2[nH]1)S(C)(=O)=O Show InChI InChI=1S/C28H27N5O3S/c1-17-12-18(2)23(13-22(17)27-31-24-14-26(37(3,35)36)30-16-25(24)32-27)28(34)33-10-8-21(9-11-33)20-6-4-19(15-29)5-7-20/h4-7,12-14,16,21H,8-11H2,1-3H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426446

(CHEMBL2322371)Show SMILES Cc1ccc(cc1-c1nc2cc(ncc2[nH]1)N1CCCC1)C(=O)N1CCC(CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C30H30N6O/c1-20-4-7-24(30(37)36-14-10-23(11-15-36)22-8-5-21(18-31)6-9-22)16-25(20)29-33-26-17-28(32-19-27(26)34-29)35-12-2-3-13-35/h4-9,16-17,19,23H,2-3,10-15H2,1H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426445
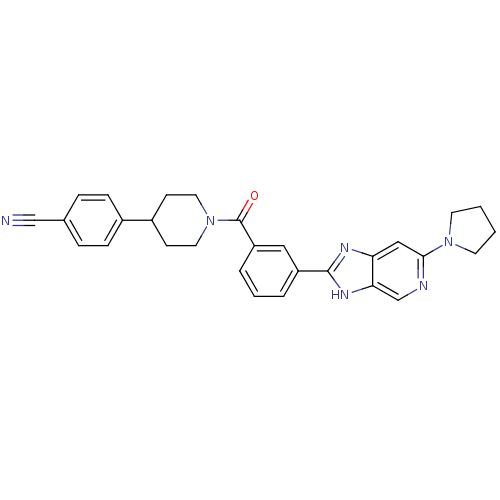
(CHEMBL2322372)Show SMILES O=C(N1CCC(CC1)c1ccc(cc1)C#N)c1cccc(c1)-c1nc2cc(ncc2[nH]1)N1CCCC1 Show InChI InChI=1S/C29H28N6O/c30-18-20-6-8-21(9-7-20)22-10-14-35(15-11-22)29(36)24-5-3-4-23(16-24)28-32-25-17-27(31-19-26(25)33-28)34-12-1-2-13-34/h3-9,16-17,19,22H,1-2,10-15H2,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Rattus norvegicus) | BDBM50426433

(CHEMBL2322360)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2cc(ncc2[nH]1)N1CCCC1 Show InChI InChI=1S/C31H32N6O/c1-20-15-21(2)26(31(38)37-13-9-24(10-14-37)23-7-5-22(18-32)6-8-23)16-25(20)30-34-27-17-29(33-19-28(27)35-30)36-11-3-4-12-36/h5-8,15-17,19,24H,3-4,9-14H2,1-2H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50426439

(CHEMBL2322366)Show SMILES Cc1ccc(cc1-c1nc2cc(ncc2[nH]1)N1CC(O)C1)C(=O)N1CCC(CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C29H28N6O2/c1-18-2-5-22(29(37)34-10-8-21(9-11-34)20-6-3-19(14-30)4-7-20)12-24(18)28-32-25-13-27(31-15-26(25)33-28)35-16-23(36)17-35/h2-7,12-13,15,21,23,36H,8-11,16-17H2,1H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Rattus norvegicus) | BDBM50426435

(CHEMBL2322370)Show SMILES Cc1cc(C)c(cc1C(=O)N1CCC(CC1)c1ccc(cc1)C#N)-c1nc2cc(ncc2[nH]1)N1CCOCC1 Show InChI InChI=1S/C31H32N6O2/c1-20-15-21(2)26(31(38)37-9-7-24(8-10-37)23-5-3-22(18-32)4-6-23)16-25(20)30-34-27-17-29(33-19-28(27)35-30)36-11-13-39-14-12-36/h3-6,15-17,19,24H,7-14H2,1-2H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Rattus norvegicus) | BDBM50426434

(CHEMBL2322359)Show SMILES COCCN(C)c1cc2nc([nH]c2cn1)-c1cc(C(=O)N2CCC(CC2)c2ccc(cc2)C#N)c(C)cc1C Show InChI InChI=1S/C31H34N6O2/c1-20-15-21(2)26(31(38)37-11-9-24(10-12-37)23-7-5-22(18-32)6-8-23)16-25(20)30-34-27-17-29(33-19-28(27)35-30)36(3)13-14-39-4/h5-8,15-17,19,24H,9-14H2,1-4H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
3-V Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat FASN assessed as release of co-enzyme A |
ACS Med Chem Lett 4: 113-7 (2013)
Article DOI: 10.1021/ml300335r
BindingDB Entry DOI: 10.7270/Q2P84D76 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data