 Found 46 hits with Last Name = 'yamaguchi' and Initial = 's'
Found 46 hits with Last Name = 'yamaguchi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM9948
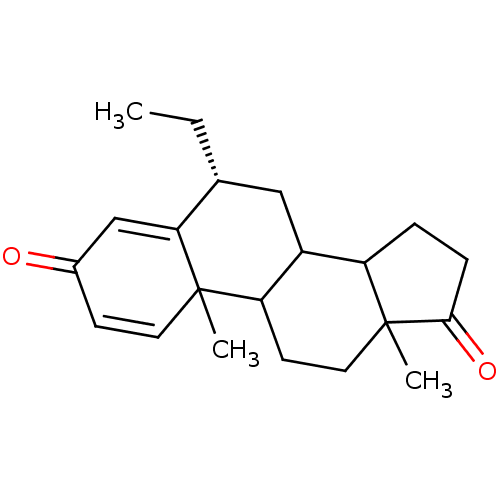
((8R)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...)Show SMILES CC[C@@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:19,t:23| Show InChI InChI=1S/C21H28O2/c1-4-13-11-15-16-5-6-19(23)21(16,3)10-8-17(15)20(2)9-7-14(22)12-18(13)20/h7,9,12-13,15-17H,4-6,8,10-11H2,1-3H3/t13-,15?,16?,17?,20?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | -49.4 | 54 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9951
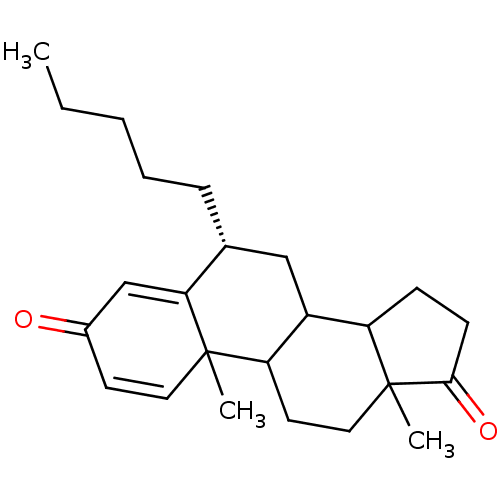
((8R)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...)Show SMILES CCCCC[C@@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:22,t:26| Show InChI InChI=1S/C24H34O2/c1-4-5-6-7-16-14-18-19-8-9-22(26)24(19,3)13-11-20(18)23(2)12-10-17(25)15-21(16)23/h10,12,15-16,18-20H,4-9,11,13-14H2,1-3H3/t16-,18?,19?,20?,23?,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | -49.3 | 54 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9949

((8R)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...)Show SMILES CCC[C@@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:20,t:24| Show InChI InChI=1S/C22H30O2/c1-4-5-14-12-16-17-6-7-20(24)22(17,3)11-9-18(16)21(2)10-8-15(23)13-19(14)21/h8,10,13-14,16-18H,4-7,9,11-12H2,1-3H3/t14-,16?,17?,18?,21?,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -48.4 | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9953

((8R)-8-heptyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7...)Show SMILES CCCCCCC[C@@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:24,t:28| Show InChI InChI=1S/C26H38O2/c1-4-5-6-7-8-9-18-16-20-21-10-11-24(28)26(21,3)15-13-22(20)25(2)14-12-19(27)17-23(18)25/h12,14,17-18,20-22H,4-11,13,15-16H2,1-3H3/t18-,20?,21?,22?,25?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | -48.1 | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9952
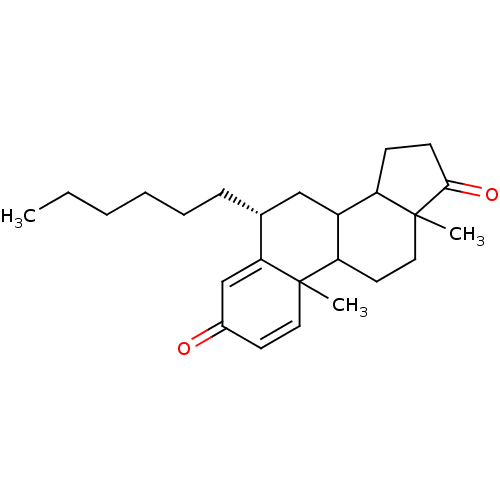
((8R)-8-hexyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...)Show SMILES CCCCCC[C@@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:23,t:27| Show InChI InChI=1S/C25H36O2/c1-4-5-6-7-8-17-15-19-20-9-10-23(27)25(20,3)14-12-21(19)24(2)13-11-18(26)16-22(17)24/h11,13,16-17,19-21H,4-10,12,14-15H2,1-3H3/t17-,19?,20?,21?,24?,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -48.1 | 76 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9944
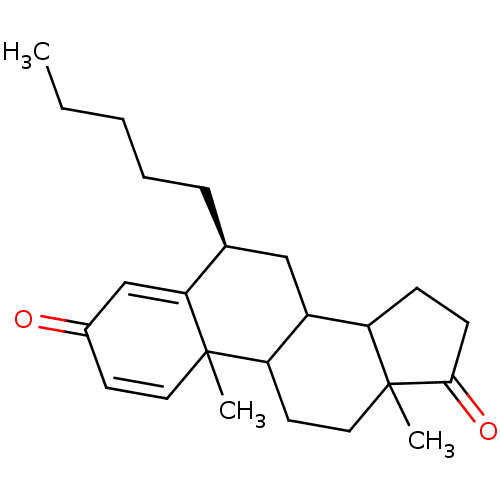
((8S)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...)Show SMILES CCCCC[C@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:22,t:26| Show InChI InChI=1S/C24H34O2/c1-4-5-6-7-16-14-18-19-8-9-22(26)24(19,3)13-11-20(18)23(2)12-10-17(25)15-21(16)23/h10,12,15-16,18-20H,4-9,11,13-14H2,1-3H3/t16-,18?,19?,20?,23?,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | -47.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9950

((8R)-8-butyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...)Show SMILES CCCC[C@@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:21,t:25| Show InChI InChI=1S/C23H32O2/c1-4-5-6-15-13-17-18-7-8-21(25)23(18,3)12-10-19(17)22(2)11-9-16(24)14-20(15)22/h9,11,14-15,17-19H,4-8,10,12-13H2,1-3H3/t15-,17?,18?,19?,22?,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | -46.6 | 180 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9943
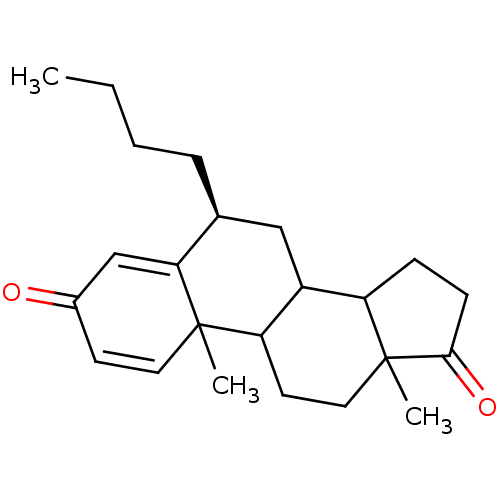
((8S)-8-butyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...)Show SMILES CCCC[C@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:21,t:25| Show InChI InChI=1S/C23H32O2/c1-4-5-6-15-13-17-18-7-8-21(25)23(18,3)12-10-19(17)22(2)11-9-16(24)14-20(15)22/h9,11,14-15,17-19H,4-8,10,12-13H2,1-3H3/t15-,17?,18?,19?,22?,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | -46.3 | 200 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9941
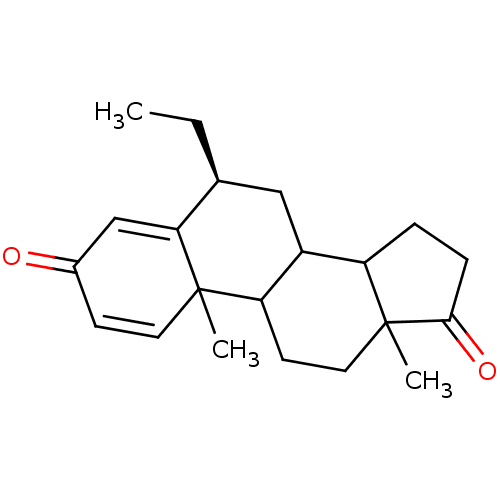
((8S)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...)Show SMILES CC[C@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:19,t:23| Show InChI InChI=1S/C21H28O2/c1-4-13-11-15-16-5-6-19(23)21(16,3)10-8-17(15)20(2)9-7-14(22)12-18(13)20/h7,9,12-13,15-17H,4-6,8,10-11H2,1-3H3/t13-,15?,16?,17?,20?,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | -45.8 | 250 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9946
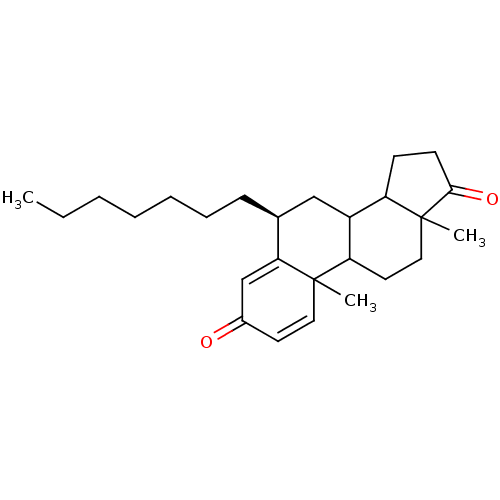
((8S)-8-heptyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7...)Show SMILES CCCCCCC[C@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:24,t:28| Show InChI InChI=1S/C26H38O2/c1-4-5-6-7-8-9-18-16-20-21-10-11-24(28)26(21,3)15-13-22(20)25(2)14-12-19(27)17-23(18)25/h12,14,17-18,20-22H,4-11,13,15-16H2,1-3H3/t18-,20?,21?,22?,25?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | -45.7 | 250 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8592
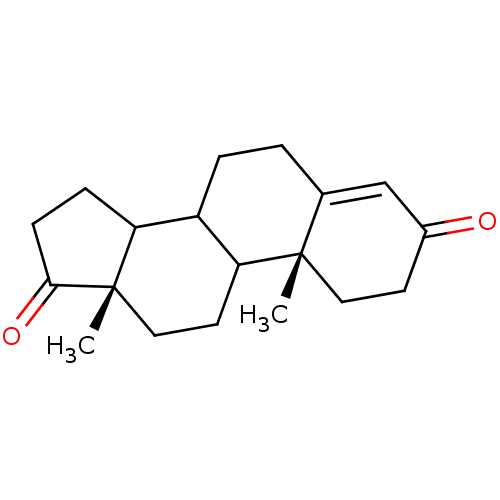
((2R,15S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CCC2=O |r,t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14?,15?,16?,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | -45.7 | 300 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM9942
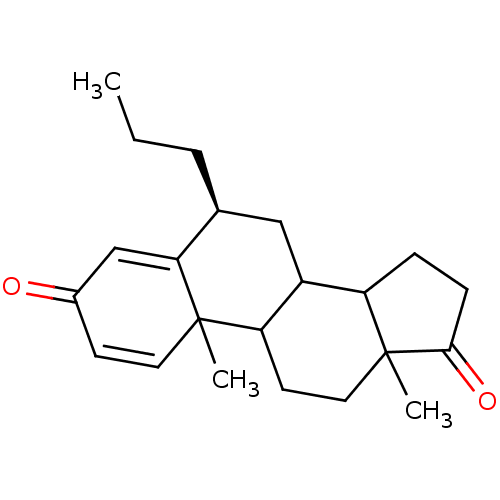
((8S)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...)Show SMILES CCC[C@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:20,t:24| Show InChI InChI=1S/C22H30O2/c1-4-5-14-12-16-17-6-7-20(24)22(17,3)11-9-18(16)21(2)10-8-15(23)13-19(14)21/h8,10,13-14,16-18H,4-7,9,11-12H2,1-3H3/t14-,16?,17?,18?,21?,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | -45.5 | 270 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9945
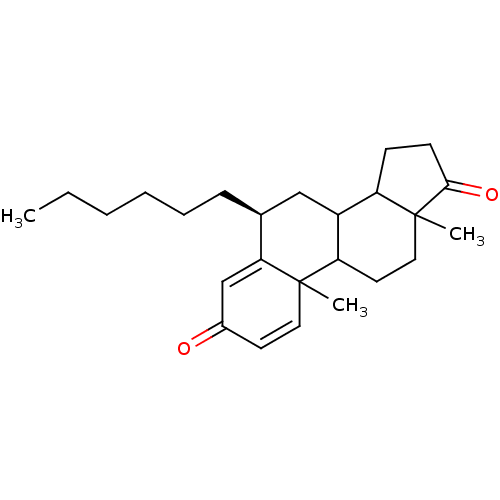
((8S)-8-hexyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...)Show SMILES CCCCCC[C@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:23,t:27| Show InChI InChI=1S/C25H36O2/c1-4-5-6-7-8-17-15-19-20-9-10-23(27)25(20,3)14-12-21(19)24(2)13-11-18(26)16-22(17)24/h11,13,16-17,19-21H,4-10,12,14-15H2,1-3H3/t17-,19?,20?,21?,24?,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | -45.1 | 280 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9947
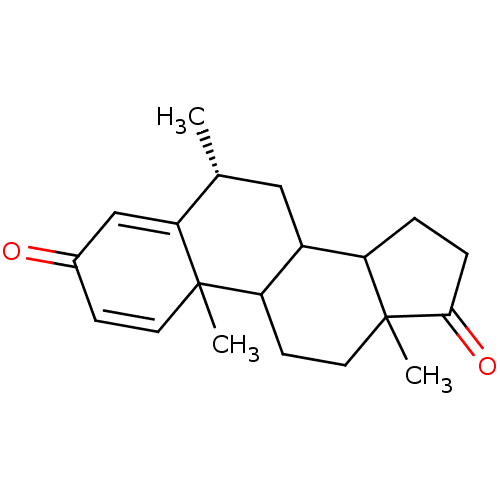
((8R)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...)Show SMILES C[C@@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:18,t:22| Show InChI InChI=1S/C20H26O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11-12,14-16H,4-5,7,9-10H2,1-3H3/t12-,14?,15?,16?,19?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | -43.7 | 520 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9954

(1,4-Androsten-3,17-dione | 2,15-dimethyltetracyclo...)Show SMILES CC12CCC3C(CCC4=CC(=O)C=CC34C)C1CCC2=O |c:12,t:8| Show InChI InChI=1S/C19H24O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h7,9,11,14-16H,3-6,8,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 43 | -43.7 | 530 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9940

((8S)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...)Show SMILES C[C@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)C=CC(=O)C=C12 |r,c:18,t:22| Show InChI InChI=1S/C20H26O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11-12,14-16H,4-5,7,9-10H2,1-3H3/t12-,14?,15?,16?,19?,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | -43.2 | 620 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 1033-8 (1996)
Article DOI: 10.1021/jm950720u
BindingDB Entry DOI: 10.7270/Q21V5C6P |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM32020
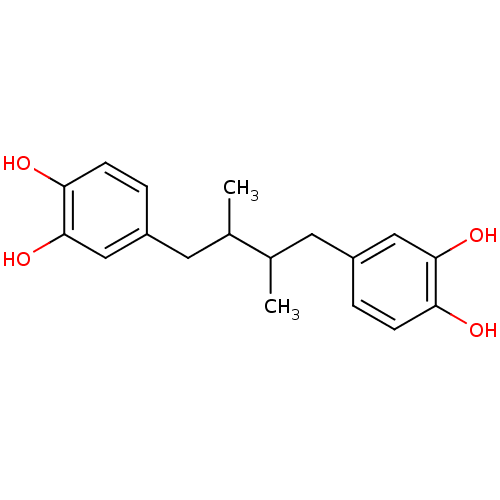
(4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...)Show InChI InChI=1S/C18H22O4/c1-11(7-13-3-5-15(19)17(21)9-13)12(2)8-14-4-6-16(20)18(22)10-14/h3-6,9-12,19-22H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50275172

(CHEMBL456234 | furan S)Show InChI InChI=1S/C13H14O3/c1-3-10-5-7-12(16-10)9-4-6-11(14)13(8-9)15-2/h4-8,14H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50275173

(CHEMBL456235 | furan L)Show InChI InChI=1S/C17H22O3/c1-3-4-5-6-7-14-9-11-16(20-14)13-8-10-15(18)17(12-13)19-2/h8-12,18H,3-7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50240419
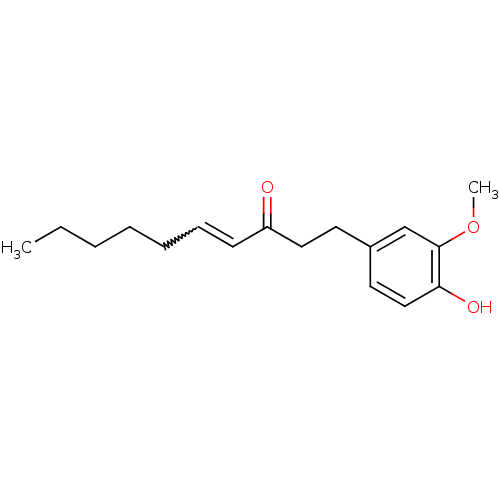
((E)-1-(4-Hydroxy-3-methoxy-phenyl)-dec-4-en-3-one ...)Show InChI InChI=1S/C17H24O3/c1-3-4-5-6-7-8-15(18)11-9-14-10-12-16(19)17(13-14)20-2/h7-8,10,12-13,19H,3-6,9,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50275173

(CHEMBL456235 | furan L)Show InChI InChI=1S/C17H22O3/c1-3-4-5-6-7-14-9-11-16(20-14)13-8-10-15(18)17(12-13)19-2/h8-12,18H,3-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50275172

(CHEMBL456234 | furan S)Show InChI InChI=1S/C13H14O3/c1-3-10-5-7-12(16-10)9-4-6-11(14)13(8-9)15-2/h4-8,14H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50275172

(CHEMBL456234 | furan S)Show InChI InChI=1S/C13H14O3/c1-3-10-5-7-12(16-10)9-4-6-11(14)13(8-9)15-2/h4-8,14H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50275173

(CHEMBL456235 | furan L)Show InChI InChI=1S/C17H22O3/c1-3-4-5-6-7-14-9-11-16(20-14)13-8-10-15(18)17(12-13)19-2/h8-12,18H,3-7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50275147

(6-Gingerol | BDBM50317427 | CHEMBL446043 | Gingero...)Show InChI InChI=1S/C17H26O4/c1-3-4-5-6-14(18)12-15(19)9-7-13-8-10-17(21-2)16(20)11-13/h8,10-11,14,18,20H,3-7,9,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50275147

(6-Gingerol | BDBM50317427 | CHEMBL446043 | Gingero...)Show InChI InChI=1S/C17H26O4/c1-3-4-5-6-14(18)12-15(19)9-7-13-8-10-17(21-2)16(20)11-13/h8,10-11,14,18,20H,3-7,9,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem 16: 10332-7 (2008)
Article DOI: 10.1016/j.bmc.2008.10.038
BindingDB Entry DOI: 10.7270/Q24M94C2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271379
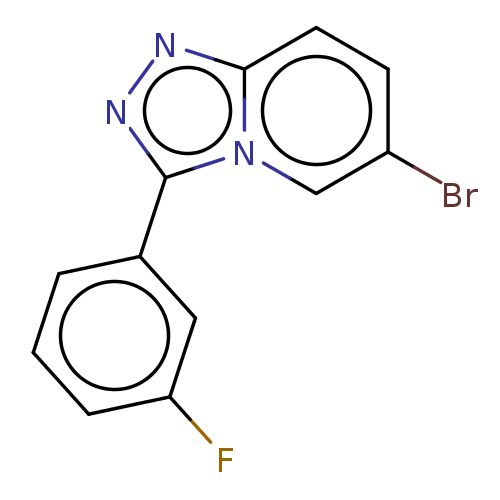
(CHEMBL4128394)Show InChI InChI=1S/C12H7BrFN3/c13-9-4-5-11-15-16-12(17(11)7-9)8-2-1-3-10(14)6-8/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271381

(CHEMBL4129762)Show InChI InChI=1S/C13H15FN2O/c14-10-3-4-12-9(6-10)8-16(13(12)17)11-2-1-5-15-7-11/h3-4,6,11,15H,1-2,5,7-8H2/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271382
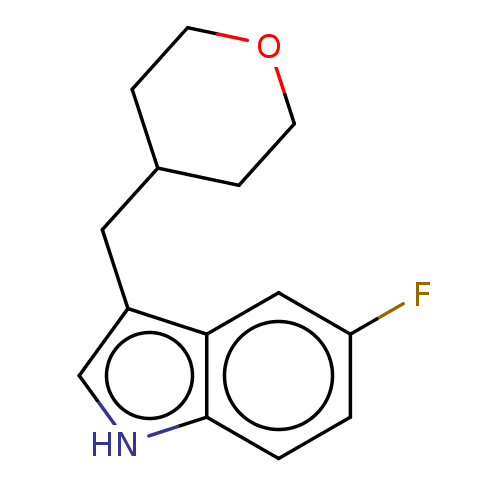
(CHEMBL4127943)Show InChI InChI=1S/C14H16FNO/c15-12-1-2-14-13(8-12)11(9-16-14)7-10-3-5-17-6-4-10/h1-2,8-10,16H,3-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.29E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271382

(CHEMBL4127943)Show InChI InChI=1S/C14H16FNO/c15-12-1-2-14-13(8-12)11(9-16-14)7-10-3-5-17-6-4-10/h1-2,8-10,16H,3-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.10E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by isothermal titration calorimetry |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271383
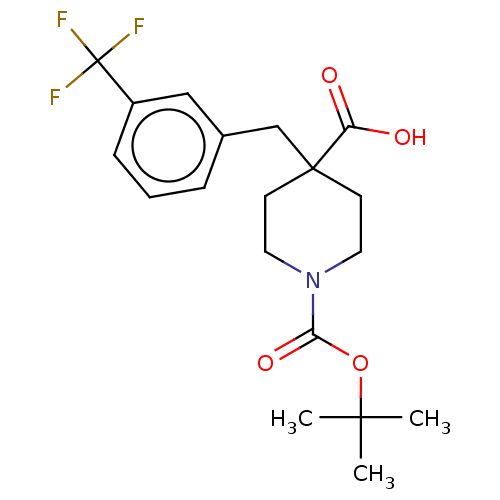
(CHEMBL4126554)Show SMILES CC(C)(C)OC(=O)N1CCC(Cc2cccc(c2)C(F)(F)F)(CC1)C(O)=O Show InChI InChI=1S/C19H24F3NO4/c1-17(2,3)27-16(26)23-9-7-18(8-10-23,15(24)25)12-13-5-4-6-14(11-13)19(20,21)22/h4-6,11H,7-10,12H2,1-3H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271384
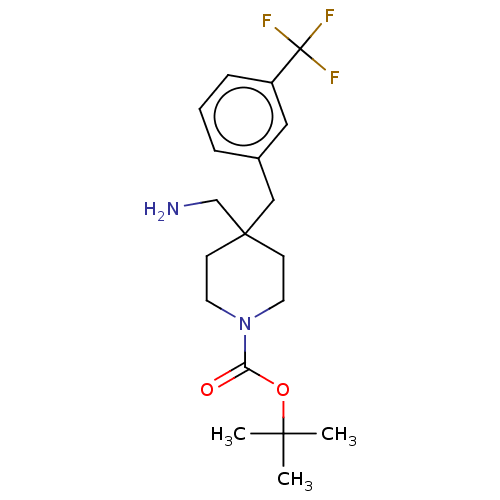
(CHEMBL4126972)Show SMILES CC(C)(C)OC(=O)N1CCC(CN)(Cc2cccc(c2)C(F)(F)F)CC1 Show InChI InChI=1S/C19H27F3N2O2/c1-17(2,3)26-16(25)24-9-7-18(13-23,8-10-24)12-14-5-4-6-15(11-14)19(20,21)22/h4-6,11H,7-10,12-13,23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271385
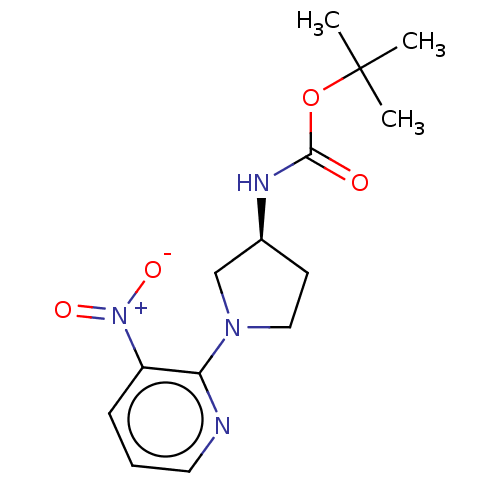
(CHEMBL4130243)Show SMILES CC(C)(C)OC(=O)N[C@H]1CCN(C1)c1ncccc1[N+]([O-])=O |r| Show InChI InChI=1S/C14H20N4O4/c1-14(2,3)22-13(19)16-10-6-8-17(9-10)12-11(18(20)21)5-4-7-15-12/h4-5,7,10H,6,8-9H2,1-3H3,(H,16,19)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271386
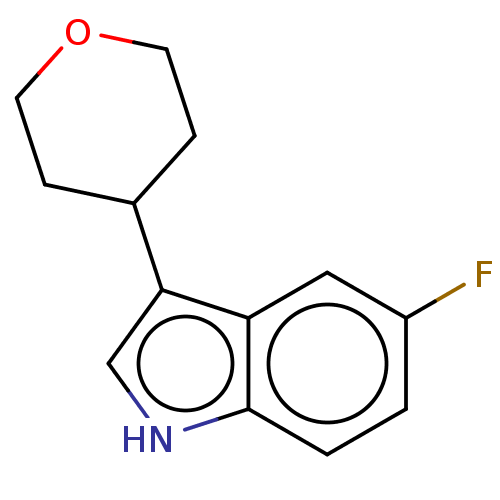
(CHEMBL4125742)Show InChI InChI=1S/C13H14FNO/c14-10-1-2-13-11(7-10)12(8-15-13)9-3-5-16-6-4-9/h1-2,7-9,15H,3-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271387

(CHEMBL4129032)Show InChI InChI=1S/C13H15FN2O/c14-10-1-2-12-9(7-10)8-16(13(12)17)11-3-5-15-6-4-11/h1-2,7,11,15H,3-6,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271388
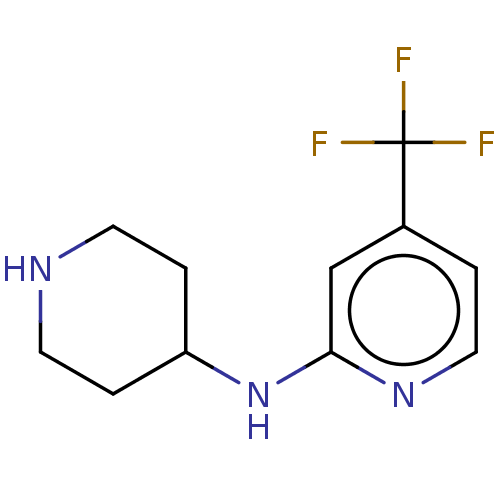
(CHEMBL4130139)Show InChI InChI=1S/C11H14F3N3/c12-11(13,14)8-1-6-16-10(7-8)17-9-2-4-15-5-3-9/h1,6-7,9,15H,2-5H2,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.89E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271389
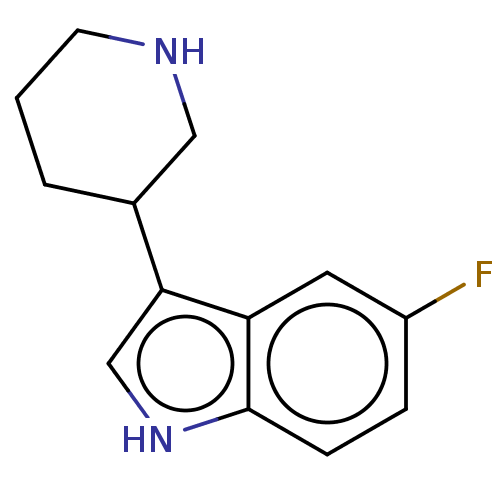
(CHEMBL4127417)Show InChI InChI=1S/C13H15FN2/c14-10-3-4-13-11(6-10)12(8-16-13)9-2-1-5-15-7-9/h3-4,6,8-9,15-16H,1-2,5,7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271394
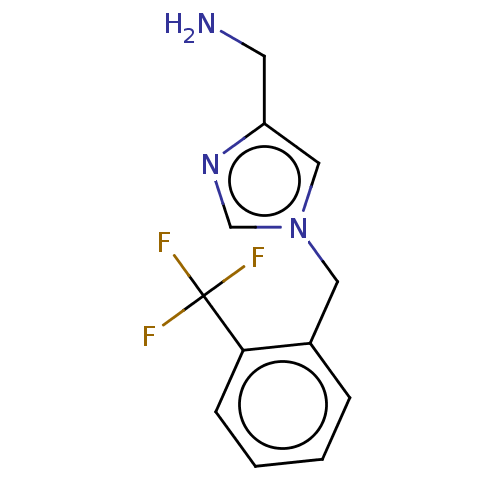
(CHEMBL4125992)Show InChI InChI=1S/C12H12F3N3/c13-12(14,15)11-4-2-1-3-9(11)6-18-7-10(5-16)17-8-18/h1-4,7-8H,5-6,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271377
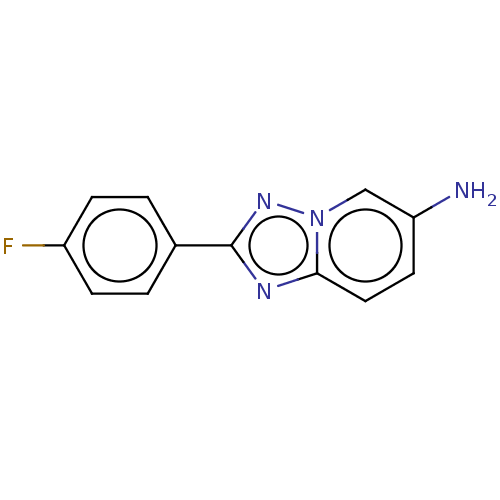
(CHEMBL4129626)Show InChI InChI=1S/C12H9FN4/c13-9-3-1-8(2-4-9)12-15-11-6-5-10(14)7-17(11)16-12/h1-7H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271376

(CHEMBL4127157)Show SMILES N[C@H]1CC[C@@H](CC1)N1Cc2c(cccc2F)C1=O |r,wU:4.7,wD:1.0,(75.47,-9.36,;73.93,-9.37,;73.15,-8.03,;71.61,-8.04,;70.86,-9.38,;71.63,-10.71,;73.16,-10.7,;69.32,-9.39,;68.4,-8.14,;66.94,-8.63,;66.95,-10.17,;65.61,-10.95,;64.27,-10.18,;64.28,-8.64,;65.6,-7.87,;65.6,-6.33,;68.42,-10.64,;68.9,-12.1,)| Show InChI InChI=1S/C14H17FN2O/c15-13-3-1-2-11-12(13)8-17(14(11)18)10-6-4-9(16)5-7-10/h1-3,9-10H,4-8,16H2/t9-,10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.95E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271375

(CHEMBL4129368)Show InChI InChI=1S/C11H9F3N4/c12-11(13,14)7-1-3-9(4-2-7)18-10-16-5-8(15)6-17-10/h1-6H,15H2,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271374

(CHEMBL4127139)Show InChI InChI=1S/C11H14FN3O2/c12-9-2-1-3-10(11(9)15(16)17)14-6-4-8(13)5-7-14/h1-3,8H,4-7,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271373

(CHEMBL4129350)Show InChI InChI=1S/C14H16FNO/c15-12-1-2-13-11(9-16-14(13)8-12)7-10-3-5-17-6-4-10/h1-2,8-10,16H,3-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271372
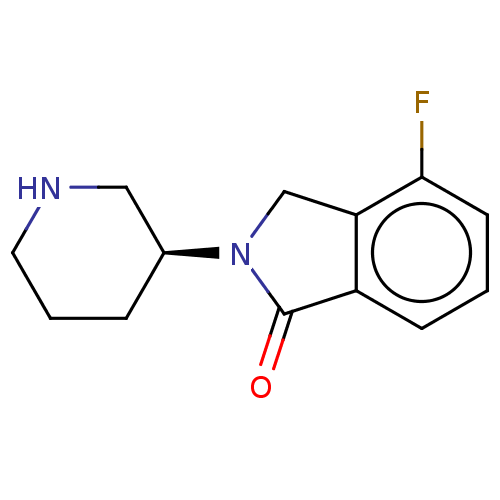
(CHEMBL4128128)Show InChI InChI=1S/C13H15FN2O/c14-12-5-1-4-10-11(12)8-16(13(10)17)9-3-2-6-15-7-9/h1,4-5,9,15H,2-3,6-8H2/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271395
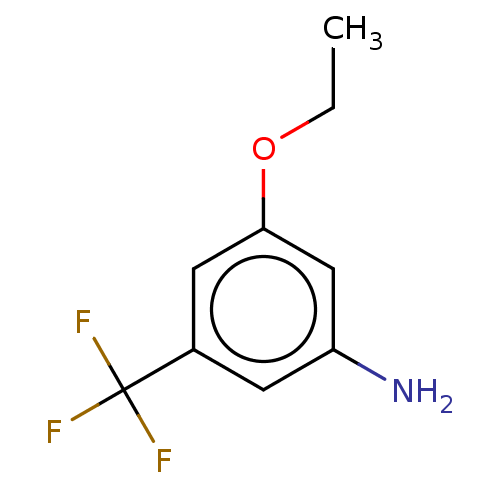
(CHEMBL4128535)Show InChI InChI=1S/C9H10F3NO/c1-2-14-8-4-6(9(10,11)12)3-7(13)5-8/h3-5H,2,13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50271378
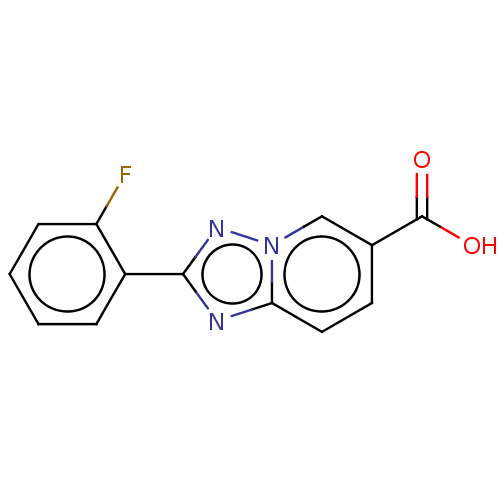
(CHEMBL4126333)Show InChI InChI=1S/C13H8FN3O2/c14-10-4-2-1-3-9(10)12-15-11-6-5-8(13(18)19)7-17(11)16-12/h1-7H,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ATP-binding site of ERK2 (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem 26: 1929-1938 (2018)
Article DOI: 10.1016/j.bmc.2018.02.041
BindingDB Entry DOI: 10.7270/Q28W3GTR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data