 Found 172 hits with Last Name = 'aster' and Initial = 'sd'
Found 172 hits with Last Name = 'aster' and Initial = 'sd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 5
(Mus musculus) | BDBM50468129
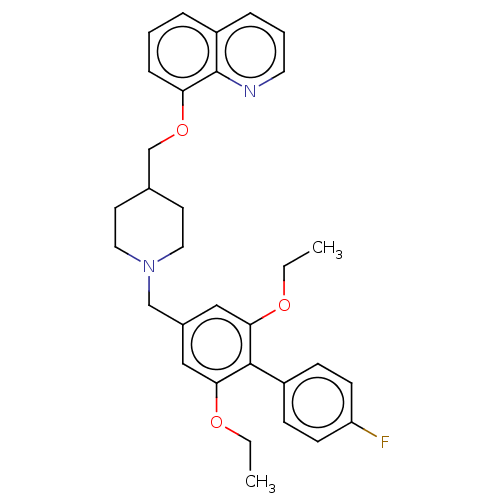
(CHEMBL4288848)Show SMILES CCOc1cc(CN2CCC(COc3cccc4cccnc34)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O3/c1-3-36-29-19-24(20-30(37-4-2)31(29)25-10-12-27(33)13-11-25)21-35-17-14-23(15-18-35)22-38-28-9-5-7-26-8-6-16-34-32(26)28/h5-13,16,19-20,23H,3-4,14-15,17-18,21-22H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468133
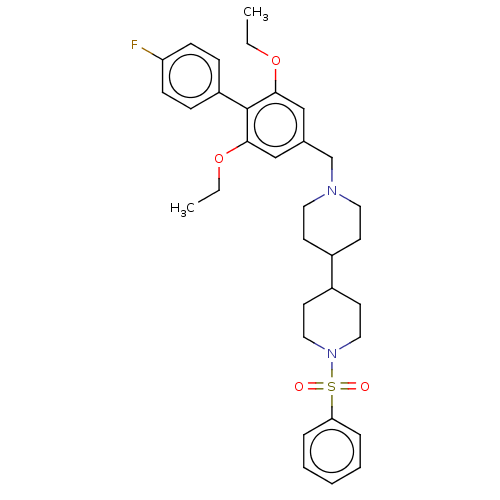
(CHEMBL4278161)Show SMILES CCOc1cc(CN2CCC(CC2)C2CCN(CC2)S(=O)(=O)c2ccccc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C33H41FN2O4S/c1-3-39-31-22-25(23-32(40-4-2)33(31)28-10-12-29(34)13-11-28)24-35-18-14-26(15-19-35)27-16-20-36(21-17-27)41(37,38)30-8-6-5-7-9-30/h5-13,22-23,26-27H,3-4,14-21,24H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468133

(CHEMBL4278161)Show SMILES CCOc1cc(CN2CCC(CC2)C2CCN(CC2)S(=O)(=O)c2ccccc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C33H41FN2O4S/c1-3-39-31-22-25(23-32(40-4-2)33(31)28-10-12-29(34)13-11-28)24-35-18-14-26(15-19-35)27-16-20-36(21-17-27)41(37,38)30-8-6-5-7-9-30/h5-13,22-23,26-27H,3-4,14-21,24H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377736

(CHEMBL403898)Show InChI InChI=1S/C18H15ClN4/c1-22-11-10-12-13(7-5-9-16(12)22)17-20-21-18(23(17)2)14-6-3-4-8-15(14)19/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377747

(CHEMBL258388)Show SMILES Cn1c(nnc1-c1ccccc1C(F)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C17H11F6N3/c1-26-14(10-6-2-4-8-12(10)16(18,19)20)24-25-15(26)11-7-3-5-9-13(11)17(21,22)23/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377735

(CHEMBL257779)Show SMILES Cn1c(nnc1-c1cccc2n(C)ccc12)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C19H15F3N4/c1-25-11-10-12-13(7-5-9-16(12)25)17-23-24-18(26(17)2)14-6-3-4-8-15(14)19(20,21)22/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377756

(CHEMBL256298)Show InChI InChI=1S/C16H11BrF3N3/c1-23-14(10-6-2-4-8-12(10)16(18,19)20)21-22-15(23)11-7-3-5-9-13(11)17/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50322981
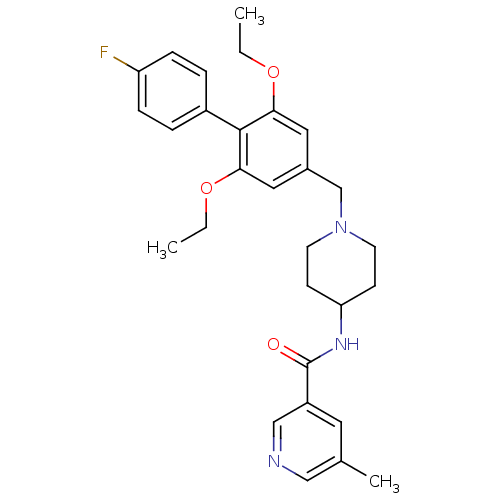
(CHEMBL1210207 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H34FN3O3/c1-4-35-26-15-21(16-27(36-5-2)28(26)22-6-8-24(30)9-7-22)19-33-12-10-25(11-13-33)32-29(34)23-14-20(3)17-31-18-23/h6-9,14-18,25H,4-5,10-13,19H2,1-3H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Angiotensin I converting enzyme |
J Med Chem 29: 251-60 (1986)
BindingDB Entry DOI: 10.7270/Q2J67HH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50377736

(CHEMBL403898)Show InChI InChI=1S/C18H15ClN4/c1-22-11-10-12-13(7-5-9-16(12)22)17-20-21-18(23(17)2)14-6-3-4-8-15(14)19/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50377735

(CHEMBL257779)Show SMILES Cn1c(nnc1-c1cccc2n(C)ccc12)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C19H15F3N4/c1-25-11-10-12-13(7-5-9-16(12)25)17-23-24-18(26(17)2)14-6-3-4-8-15(14)19(20,21)22/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377712

(CHEMBL401909)Show SMILES Cn1c(nnc1-c1ccccc1C(F)(F)F)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C17H11F6N3O/c1-26-14(10-6-2-4-8-12(10)16(18,19)20)24-25-15(26)11-7-3-5-9-13(11)27-17(21,22)23/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377746
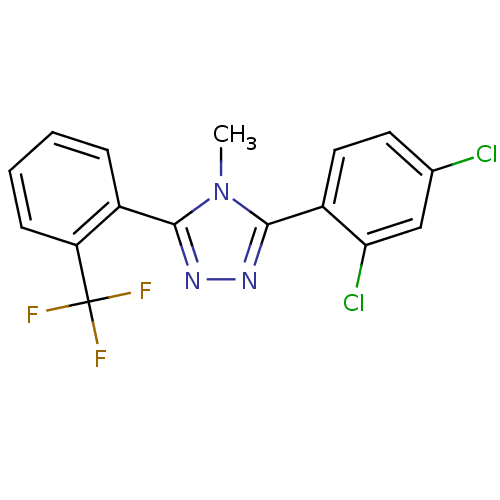
(CHEMBL403811)Show InChI InChI=1S/C16H10Cl2F3N3/c1-24-14(10-4-2-3-5-12(10)16(19,20)21)22-23-15(24)11-7-6-9(17)8-13(11)18/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322981

(CHEMBL1210207 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H34FN3O3/c1-4-35-26-15-21(16-27(36-5-2)28(26)22-6-8-24(30)9-7-22)19-33-12-10-25(11-13-33)32-29(34)23-14-20(3)17-31-18-23/h6-9,14-18,25H,4-5,10-13,19H2,1-3H3,(H,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377730
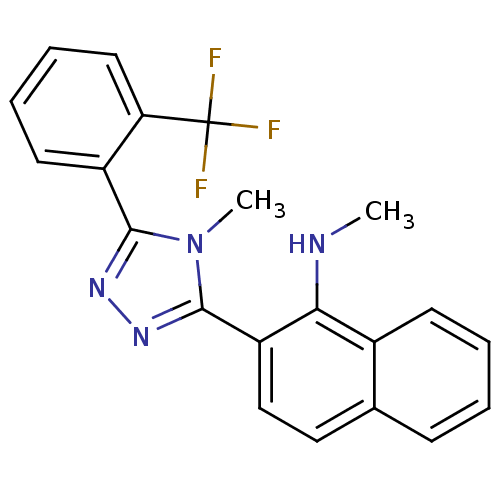
(CHEMBL257601)Show SMILES CNc1c(ccc2ccccc12)-c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H17F3N4/c1-25-18-14-8-4-3-7-13(14)11-12-16(18)20-27-26-19(28(20)2)15-9-5-6-10-17(15)21(22,23)24/h3-12,25H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468130

(CHEMBL4292255)Show SMILES CCOc1cc(CN2CCC3(CC(Cc4ccc(F)cc4)C(=O)O3)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35F2NO4/c1-3-37-28-18-23(19-29(38-4-2)30(28)24-7-11-27(34)12-8-24)21-35-15-13-32(14-16-35)20-25(31(36)39-32)17-22-5-9-26(33)10-6-22/h5-12,18-19,25H,3-4,13-17,20-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377724
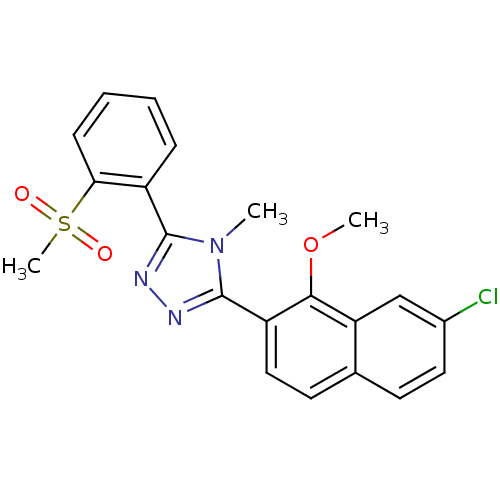
(CHEMBL401883)Show SMILES COc1c(ccc2ccc(Cl)cc12)-c1nnc(-c2ccccc2S(C)(=O)=O)n1C Show InChI InChI=1S/C21H18ClN3O3S/c1-25-20(15-6-4-5-7-18(15)29(3,26)27)23-24-21(25)16-11-9-13-8-10-14(22)12-17(13)19(16)28-2/h4-12H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50377724

(CHEMBL401883)Show SMILES COc1c(ccc2ccc(Cl)cc12)-c1nnc(-c2ccccc2S(C)(=O)=O)n1C Show InChI InChI=1S/C21H18ClN3O3S/c1-25-20(15-6-4-5-7-18(15)29(3,26)27)23-24-21(25)16-11-9-13-8-10-14(22)12-17(13)19(16)28-2/h4-12H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377750
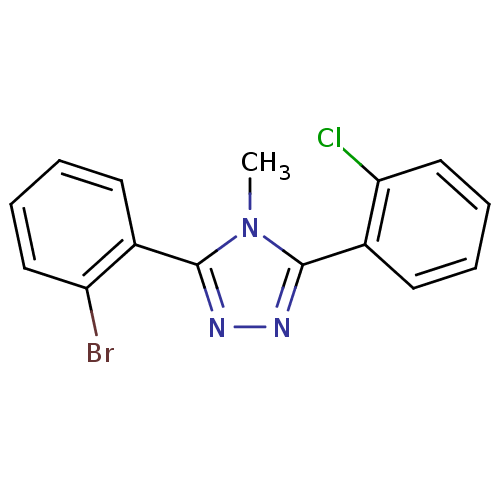
(CHEMBL401908)Show InChI InChI=1S/C15H11BrClN3/c1-20-14(10-6-2-4-8-12(10)16)18-19-15(20)11-7-3-5-9-13(11)17/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50452268

(CHEMBL2114219)Show SMILES OC(=O)CN1CCCCC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O |r| Show InChI InChI=1S/C19H26N2O5/c22-17(23)13-21-12-6-2-5-9-15(18(21)24)20-16(19(25)26)11-10-14-7-3-1-4-8-14/h1,3-4,7-8,15-16,20H,2,5-6,9-13H2,(H,22,23)(H,25,26)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration (isomer B) against Angiotensin I converting enzyme |
J Med Chem 29: 251-60 (1986)
BindingDB Entry DOI: 10.7270/Q2J67HH7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50377730

(CHEMBL257601)Show SMILES CNc1c(ccc2ccccc12)-c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H17F3N4/c1-25-18-14-8-4-3-7-13(14)11-12-16(18)20-27-26-19(28(20)2)15-9-5-6-10-17(15)21(22,23)24/h3-12,25H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50377725

(CHEMBL401884)Show SMILES COc1c(cc(Cl)c2ccccc12)-c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H15ClF3N3O/c1-28-19(14-9-5-6-10-16(14)21(23,24)25)26-27-20(28)15-11-17(22)12-7-3-4-8-13(12)18(15)29-2/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377725

(CHEMBL401884)Show SMILES COc1c(cc(Cl)c2ccccc12)-c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H15ClF3N3O/c1-28-19(14-9-5-6-10-16(14)21(23,24)25)26-27-20(28)15-11-17(22)12-7-3-4-8-13(12)18(15)29-2/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468130

(CHEMBL4292255)Show SMILES CCOc1cc(CN2CCC3(CC(Cc4ccc(F)cc4)C(=O)O3)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35F2NO4/c1-3-37-28-18-23(19-29(38-4-2)30(28)24-7-11-27(34)12-8-24)21-35-15-13-32(14-16-35)20-25(31(36)39-32)17-22-5-9-26(33)10-6-22/h5-12,18-19,25H,3-4,13-17,20-21H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468131
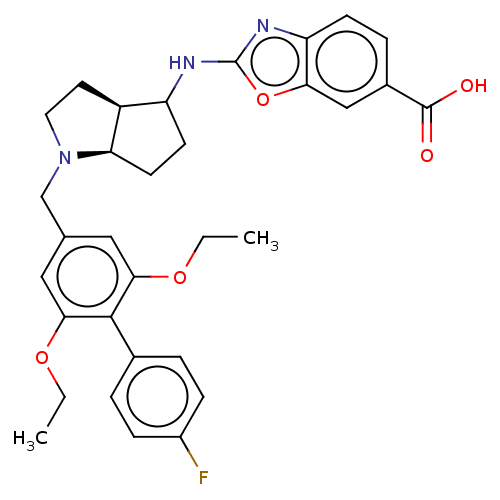
(CHEMBL4277106)Show SMILES [H][C@@]12CCN(Cc3cc(OCC)c(c(OCC)c3)-c3ccc(F)cc3)[C@]1([H])CCC2Nc1nc2ccc(cc2o1)C(O)=O |r| Show InChI InChI=1S/C32H34FN3O5/c1-3-39-28-15-19(16-29(40-4-2)30(28)20-5-8-22(33)9-6-20)18-36-14-13-23-24(11-12-26(23)36)34-32-35-25-10-7-21(31(37)38)17-27(25)41-32/h5-10,15-17,23-24,26H,3-4,11-14,18H2,1-2H3,(H,34,35)(H,37,38)/t23-,24?,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377757

(CHEMBL256986)Show InChI InChI=1S/C16H10ClF4N3/c1-24-14(10-4-2-3-5-12(10)16(19,20)21)22-23-15(24)11-7-6-9(18)8-13(11)17/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50322981

(CHEMBL1210207 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H34FN3O3/c1-4-35-26-15-21(16-27(36-5-2)28(26)22-6-8-24(30)9-7-22)19-33-12-10-25(11-13-33)32-29(34)23-14-20(3)17-31-18-23/h6-9,14-18,25H,4-5,10-13,19H2,1-3H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468127
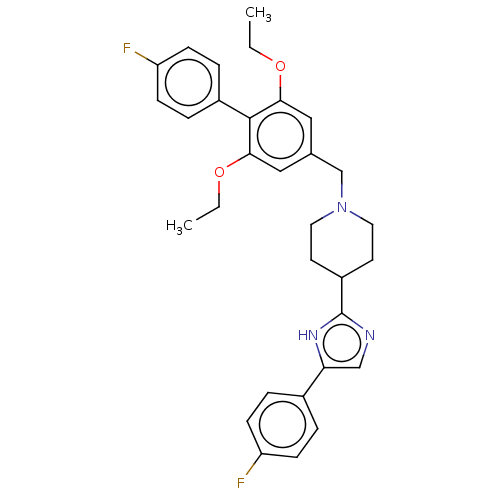
(CHEMBL4288417)Show SMILES CCOc1cc(CN2CCC(CC2)c2ncc([nH]2)-c2ccc(F)cc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C31H33F2N3O2/c1-3-37-28-17-21(18-29(38-4-2)30(28)23-7-11-26(33)12-8-23)20-36-15-13-24(14-16-36)31-34-19-27(35-31)22-5-9-25(32)10-6-22/h5-12,17-19,24H,3-4,13-16,20H2,1-2H3,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377728

(CHEMBL401975)Show SMILES COc1c(cc2ccccc2c1Cl)-c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H15ClF3N3O/c1-28-19(14-9-5-6-10-16(14)21(23,24)25)26-27-20(28)15-11-12-7-3-4-8-13(12)17(22)18(15)29-2/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50377732

(CHEMBL257752)Show SMILES Cn1c(nnc1-c1ccccc1C(F)(F)F)-c1ccc2ccccc2c1F Show InChI InChI=1S/C20H13F4N3/c1-27-18(14-8-4-5-9-16(14)20(22,23)24)25-26-19(27)15-11-10-12-6-2-3-7-13(12)17(15)21/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377749
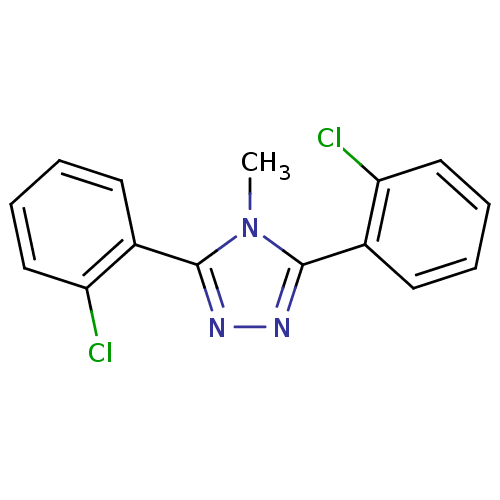
(CHEMBL258389)Show InChI InChI=1S/C15H11Cl2N3/c1-20-14(10-6-2-4-8-12(10)16)18-19-15(20)11-7-3-5-9-13(11)17/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377726

(CHEMBL257129)Show SMILES Cn1c(nnc1-c1cc2ccccc2cc1Cl)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C20H13ClF3N3/c1-27-18(14-8-4-5-9-16(14)20(22,23)24)25-26-19(27)15-10-12-6-2-3-7-13(12)11-17(15)21/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468129

(CHEMBL4288848)Show SMILES CCOc1cc(CN2CCC(COc3cccc4cccnc34)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O3/c1-3-36-29-19-24(20-30(37-4-2)31(29)25-10-12-27(33)13-11-25)21-35-17-14-23(15-18-35)22-38-28-9-5-7-26-8-6-16-34-32(26)28/h5-13,16,19-20,23H,3-4,14-15,17-18,21-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50377722

(CHEMBL401988)Show SMILES CCCCCc1ccc(cc1)-c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H22F3N3/c1-3-4-5-8-15-11-13-16(14-12-15)19-25-26-20(27(19)2)17-9-6-7-10-18(17)21(22,23)24/h6-7,9-14H,3-5,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322981

(CHEMBL1210207 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H34FN3O3/c1-4-35-26-15-21(16-27(36-5-2)28(26)22-6-8-24(30)9-7-22)19-33-12-10-25(11-13-33)32-29(34)23-14-20(3)17-31-18-23/h6-9,14-18,25H,4-5,10-13,19H2,1-3H3,(H,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377751

(CHEMBL256158)Show InChI InChI=1S/C16H11ClF3N3/c1-23-14(10-6-2-4-8-12(10)16(18,19)20)21-22-15(23)11-7-3-5-9-13(11)17/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377745

(CHEMBL402737)Show SMILES Cn1c(nnc1-c1cccc(c1C(F)(F)F)C(F)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C18H10F9N3/c1-30-14(9-5-2-3-7-11(9)16(19,20)21)28-29-15(30)10-6-4-8-12(17(22,23)24)13(10)18(25,26)27/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377737

(CHEMBL257963)Show InChI InChI=1S/C18H16N4O/c1-21-11-10-14-15(4-3-5-16(14)21)18-20-19-17(22(18)2)12-6-8-13(23)9-7-12/h3-11,23H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377732

(CHEMBL257752)Show SMILES Cn1c(nnc1-c1ccccc1C(F)(F)F)-c1ccc2ccccc2c1F Show InChI InChI=1S/C20H13F4N3/c1-27-18(14-8-4-5-9-16(14)20(22,23)24)25-26-19(27)15-11-10-12-6-2-3-7-13(12)17(15)21/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468131

(CHEMBL4277106)Show SMILES [H][C@@]12CCN(Cc3cc(OCC)c(c(OCC)c3)-c3ccc(F)cc3)[C@]1([H])CCC2Nc1nc2ccc(cc2o1)C(O)=O |r| Show InChI InChI=1S/C32H34FN3O5/c1-3-39-28-15-19(16-29(40-4-2)30(28)20-5-8-22(33)9-6-20)18-36-14-13-23-24(11-12-26(23)36)34-32-35-25-10-7-21(31(37)38)17-27(25)41-32/h5-10,15-17,23-24,26H,3-4,11-14,18H2,1-2H3,(H,34,35)(H,37,38)/t23-,24?,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50377712

(CHEMBL401909)Show SMILES Cn1c(nnc1-c1ccccc1C(F)(F)F)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C17H11F6N3O/c1-26-14(10-6-2-4-8-12(10)16(18,19)20)24-25-15(26)11-7-3-5-9-13(11)27-17(21,22)23/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377723
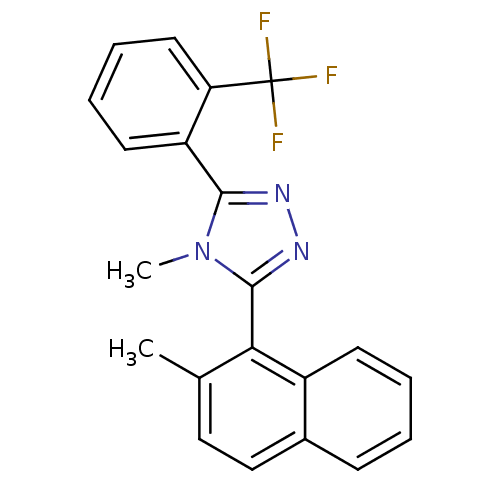
(CHEMBL401830)Show SMILES Cc1ccc2ccccc2c1-c1nnc(-c2ccccc2C(F)(F)F)n1C |(-7.03,-10.86,;-8.23,-9.89,;-9.66,-10.44,;-10.86,-9.47,;-10.61,-7.95,;-11.8,-6.99,;-11.57,-5.48,;-10.13,-4.92,;-8.94,-5.89,;-9.18,-7.4,;-7.98,-8.37,;-6.55,-7.83,;-6.14,-6.35,;-4.61,-6.27,;-4.06,-7.71,;-2.57,-8.11,;-2.18,-9.61,;-.7,-10.01,;.4,-8.92,;0,-7.43,;-1.48,-7.03,;-1.88,-5.54,;-2.29,-4.04,;-.39,-5.14,;-3.37,-5.95,;-5.26,-8.68,;-5.19,-10.22,)| Show InChI InChI=1S/C21H16F3N3/c1-13-11-12-14-7-3-4-8-15(14)18(13)20-26-25-19(27(20)2)16-9-5-6-10-17(16)21(22,23)24/h3-12H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021267
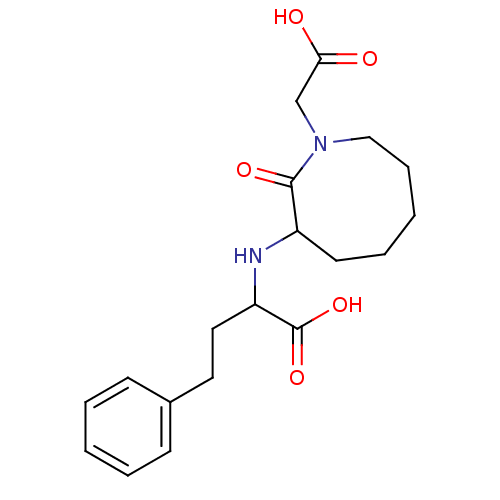
(2-(1-Carboxymethyl-2-oxo-azocan-3-ylamino)-4-pheny...)Show InChI InChI=1S/C19H26N2O5/c22-17(23)13-21-12-6-2-5-9-15(18(21)24)20-16(19(25)26)11-10-14-7-3-1-4-8-14/h1,3-4,7-8,15-16,20H,2,5-6,9-13H2,(H,22,23)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration (isomer B) against Angiotensin I converting enzyme |
J Med Chem 29: 251-60 (1986)
BindingDB Entry DOI: 10.7270/Q2J67HH7 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377722

(CHEMBL401988)Show SMILES CCCCCc1ccc(cc1)-c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H22F3N3/c1-3-4-5-8-15-11-13-16(14-12-15)19-25-26-20(27(19)2)17-9-6-7-10-18(17)21(22,23)24/h6-7,9-14H,3-5,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377755
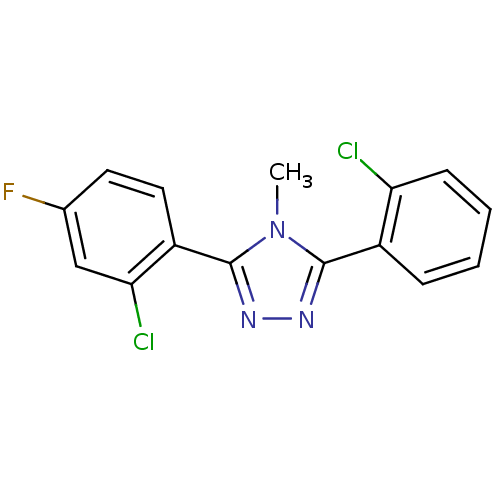
(CHEMBL404638)Show InChI InChI=1S/C15H10Cl2FN3/c1-21-14(10-4-2-3-5-12(10)16)19-20-15(21)11-7-6-9(18)8-13(11)17/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 2799-804 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J9678F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468131

(CHEMBL4277106)Show SMILES [H][C@@]12CCN(Cc3cc(OCC)c(c(OCC)c3)-c3ccc(F)cc3)[C@]1([H])CCC2Nc1nc2ccc(cc2o1)C(O)=O |r| Show InChI InChI=1S/C32H34FN3O5/c1-3-39-28-15-19(16-29(40-4-2)30(28)20-5-8-22(33)9-6-20)18-36-14-13-23-24(11-12-26(23)36)34-32-35-25-10-7-21(31(37)38)17-27(25)41-32/h5-10,15-17,23-24,26H,3-4,11-14,18H2,1-2H3,(H,34,35)(H,37,38)/t23-,24?,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data