 Found 415 hits with Last Name = 'yoo' and Initial = 'se'
Found 415 hits with Last Name = 'yoo' and Initial = 'se' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50201454
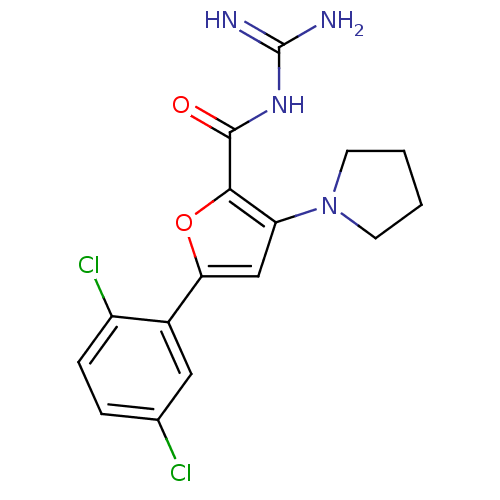
(CHEMBL392093 | N-(diaminomethylene)-5-(2,5-dichlor...)Show SMILES NC(=N)NC(=O)c1oc(cc1N1CCCC1)-c1cc(Cl)ccc1Cl Show InChI InChI=1S/C16H16Cl2N4O2/c17-9-3-4-11(18)10(7-9)13-8-12(22-5-1-2-6-22)14(24-13)15(23)21-16(19)20/h3-4,7-8H,1-2,5-6H2,(H4,19,20,21,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human NHE1 expressed in PS120 cells |
Bioorg Med Chem Lett 17: 1291-5 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.012
BindingDB Entry DOI: 10.7270/Q2MW2GS1 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340346
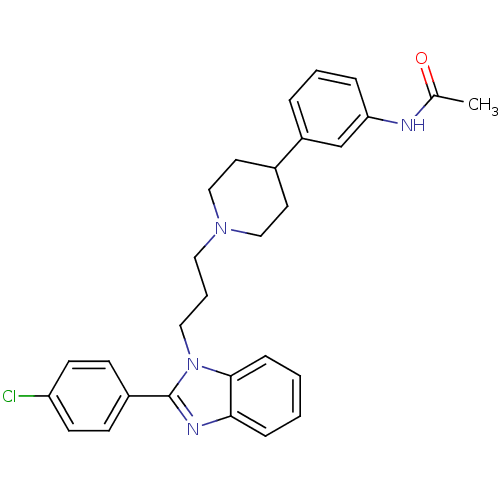
(2-(4-chlorophenyl)-1-{3-[4-(3-acetylaminophenyl)pi...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C29H31ClN4O/c1-21(35)31-26-7-4-6-24(20-26)22-14-18-33(19-15-22)16-5-17-34-28-9-3-2-8-27(28)32-29(34)23-10-12-25(30)13-11-23/h2-4,6-13,20,22H,5,14-19H2,1H3,(H,31,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340362

(2-(4-chloro-2-fluorophenyl)-1-{3-[4-(3-acetylamino...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(Cl)cc2F)CC1 Show InChI InChI=1S/C29H30ClFN4O/c1-20(36)32-24-7-4-6-22(18-24)21-12-16-34(17-13-21)14-5-15-35-28-9-3-2-8-27(28)33-29(35)25-11-10-23(30)19-26(25)31/h2-4,6-11,18-19,21H,5,12-17H2,1H3,(H,32,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337787
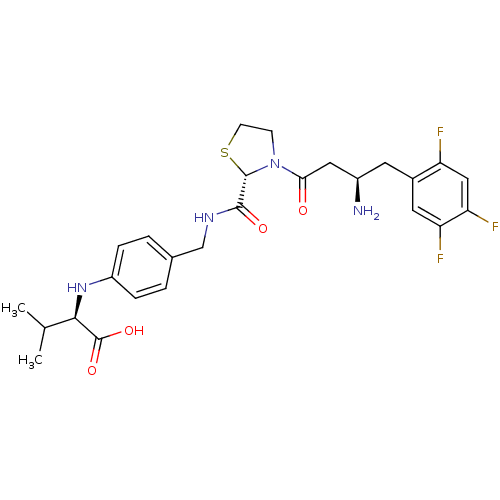
((R)-2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifluoroph...)Show SMILES CC(C)[C@@H](Nc1ccc(CNC(=O)[C@@H]2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H31F3N4O4S/c1-14(2)23(26(36)37)32-18-5-3-15(4-6-18)13-31-24(35)25-33(7-8-38-25)22(34)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25,32H,7-9,11,13,30H2,1-2H3,(H,31,35)(H,36,37)/t17-,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200844

(CHEMBL219480 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cc(F)cc(F)c1)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C26H26ClF2NO2/c1-17(30-25(31)26(2,3)32-23-15-21(28)14-22(29)16-23)24(19-7-5-4-6-8-19)13-18-9-11-20(27)12-10-18/h4-12,14-17,24H,13H2,1-3H3,(H,30,31)/t17-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337789
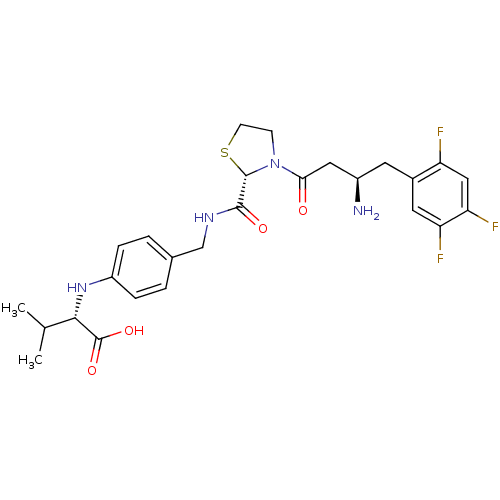
((S)-2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifluoroph...)Show SMILES CC(C)[C@H](Nc1ccc(CNC(=O)[C@@H]2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H31F3N4O4S/c1-14(2)23(26(36)37)32-18-5-3-15(4-6-18)13-31-24(35)25-33(7-8-38-25)22(34)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25,32H,7-9,11,13,30H2,1-2H3,(H,31,35)(H,36,37)/t17-,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200836
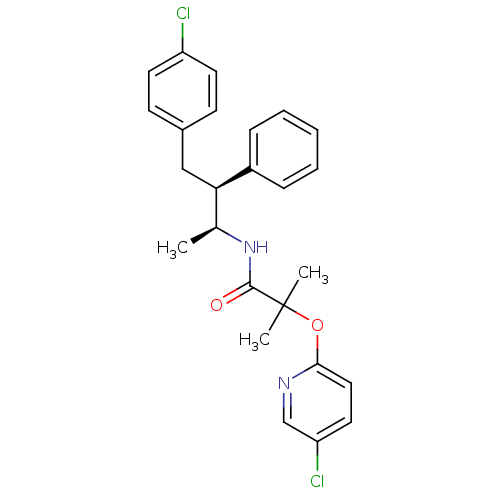
(CHEMBL387027 | N-[(1S,2S)-3-(4-chlorophenyl)-1-met...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(Cl)cn1)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C25H26Cl2N2O2/c1-17(29-24(30)25(2,3)31-23-14-13-21(27)16-28-23)22(19-7-5-4-6-8-19)15-18-9-11-20(26)12-10-18/h4-14,16-17,22H,15H2,1-3H3,(H,29,30)/t17-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200830

(CHEMBL385033 | N-[(1S,2S)-3-(4-chlorophenyl)-1-met...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cccc(Cl)c1)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C26H27Cl2NO2/c1-18(29-25(30)26(2,3)31-23-11-7-10-22(28)17-23)24(20-8-5-4-6-9-20)16-19-12-14-21(27)15-13-19/h4-15,17-18,24H,16H2,1-3H3,(H,29,30)/t18-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174295
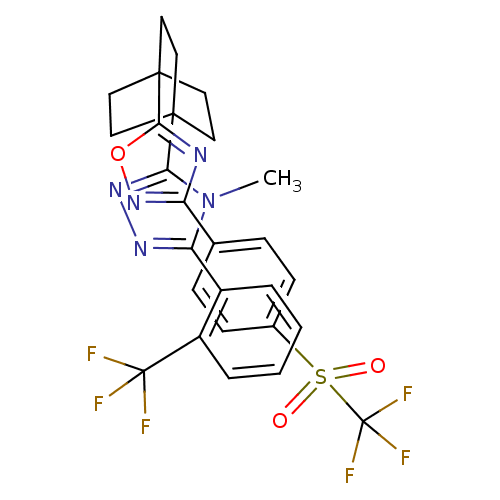
(4-methyl-3-(2-(trifluoromethyl)phenyl)-5-(4-(3-(4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)S(=O)(=O)C(F)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H23F6N5O3S/c1-38-21(18-4-2-3-5-19(18)26(28,29)30)35-36-22(38)24-10-13-25(14-11-24,15-12-24)23-34-20(37-41-23)16-6-8-17(9-7-16)42(39,40)27(31,32)33/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29094
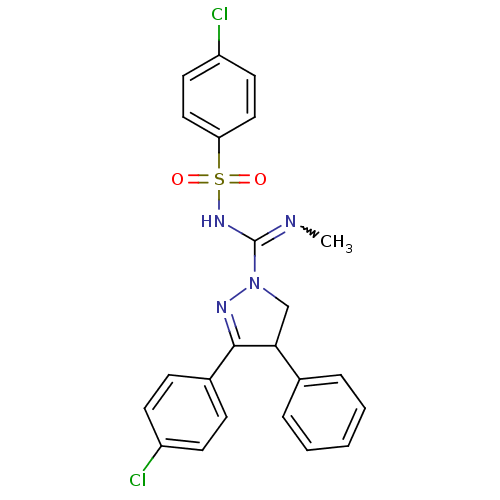
((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...)Show SMILES CN=C(NS(=O)(=O)c1ccc(Cl)cc1)N1CC(C(=N1)c1ccc(Cl)cc1)c1ccccc1 |w:1.0,c:18| Show InChI InChI=1S/C23H20Cl2N4O2S/c1-26-23(28-32(30,31)20-13-11-19(25)12-14-20)29-15-21(16-5-3-2-4-6-16)22(27-29)17-7-9-18(24)10-8-17/h2-14,21H,15H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174281
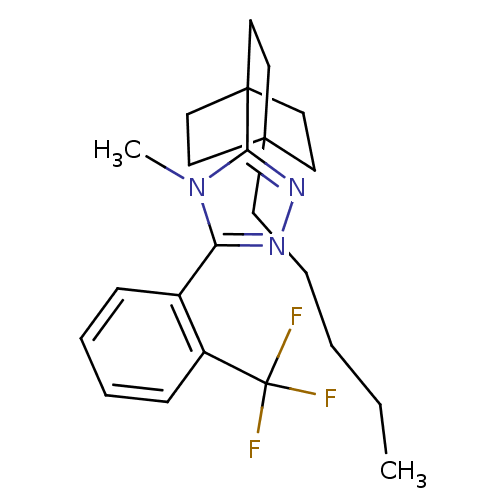
(4-methyl-3-(4-pentylbicyclo[2.2.2]octan-1-yl)-5-(2...)Show SMILES CCCCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C23H30F3N3/c1-3-4-7-10-21-11-14-22(15-12-21,16-13-21)20-28-27-19(29(20)2)17-8-5-6-9-18(17)23(24,25)26/h5-6,8-9H,3-4,7,10-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174298
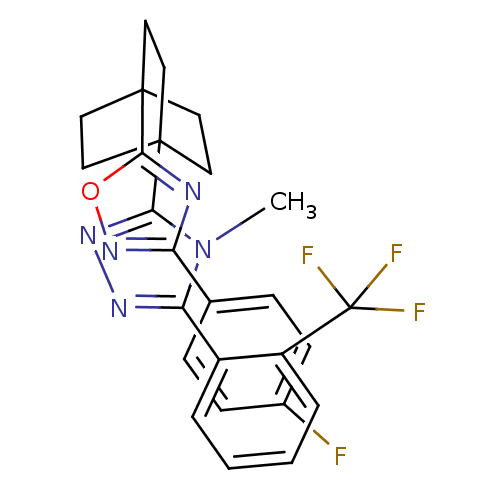
(3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340341
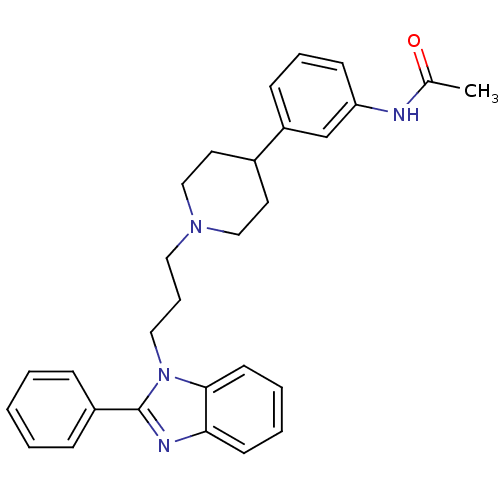
(2-phenyl-1-{3-[4-(3-acetylaminophenyl)piperidin-1-...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccccc2)CC1 Show InChI InChI=1S/C29H32N4O/c1-22(34)30-26-12-7-11-25(21-26)23-15-19-32(20-16-23)17-8-18-33-28-14-6-5-13-27(28)31-29(33)24-9-3-2-4-10-24/h2-7,9-14,21,23H,8,15-20H2,1H3,(H,30,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174297
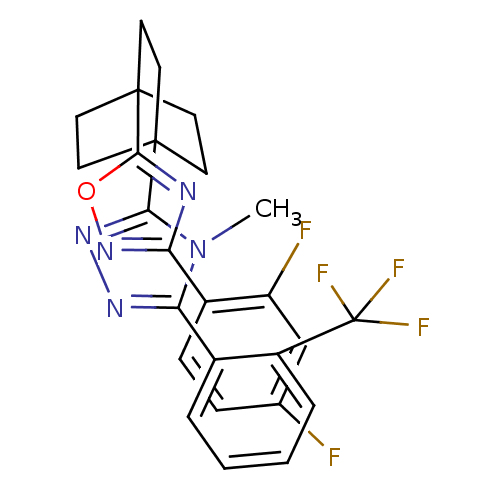
(3-(4-(3-(2,4-difluorophenyl)-1,2,4-oxadiazol-5-yl)...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H22F5N5O/c1-36-21(16-4-2-3-5-18(16)26(29,30)31)33-34-22(36)24-8-11-25(12-9-24,13-10-24)23-32-20(35-37-23)17-7-6-15(27)14-19(17)28/h2-7,14H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340346

(2-(4-chlorophenyl)-1-{3-[4-(3-acetylaminophenyl)pi...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C29H31ClN4O/c1-21(35)31-26-7-4-6-24(20-26)22-14-18-33(19-15-22)16-5-17-34-28-9-3-2-8-27(28)32-29(34)23-10-12-25(30)13-11-23/h2-4,6-13,20,22H,5,14-19H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition hERG by patch clamp method |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337785

(2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...)Show SMILES CC(C)C(Nc1ccc(CNC(=O)C2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H31F3N4O4S/c1-14(2)23(26(36)37)32-18-5-3-15(4-6-18)13-31-24(35)25-33(7-8-38-25)22(34)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25,32H,7-9,11,13,30H2,1-2H3,(H,31,35)(H,36,37)/t17-,23?,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340360

(2-(2,4-dichlorophenyl)-1-{3-[4-(3-acetylaminopheny...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(Cl)cc2Cl)CC1 Show InChI InChI=1S/C29H30Cl2N4O/c1-20(36)32-24-7-4-6-22(18-24)21-12-16-34(17-13-21)14-5-15-35-28-9-3-2-8-27(28)33-29(35)25-11-10-23(30)19-26(25)31/h2-4,6-11,18-19,21H,5,12-17H2,1H3,(H,32,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340349
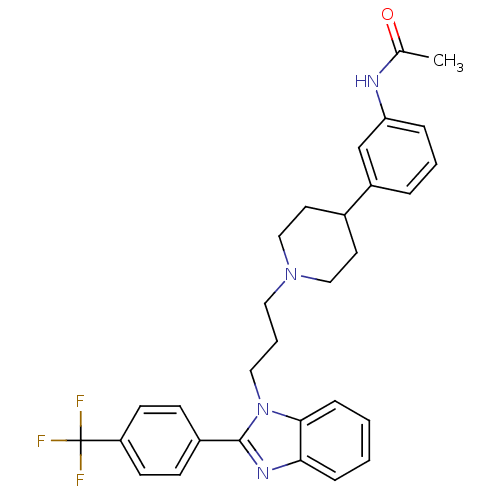
(2-(4-trifluoromethylphenyl)-1-{3-[4-(3-acetylamino...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(cc2)C(F)(F)F)CC1 Show InChI InChI=1S/C30H31F3N4O/c1-21(38)34-26-7-4-6-24(20-26)22-14-18-36(19-15-22)16-5-17-37-28-9-3-2-8-27(28)35-29(37)23-10-12-25(13-11-23)30(31,32)33/h2-4,6-13,20,22H,5,14-19H2,1H3,(H,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174283
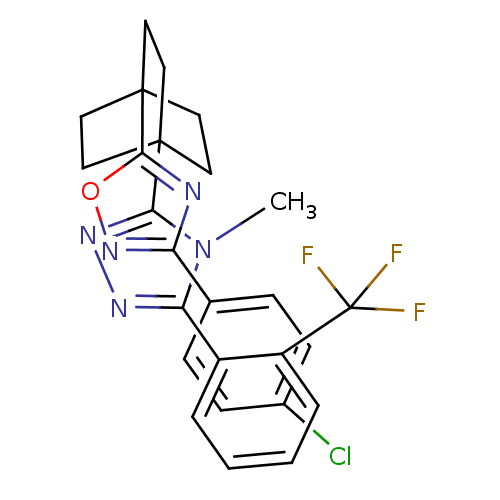
(3-(4-(3-(4-chlorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(Cl)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23ClF3N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340364
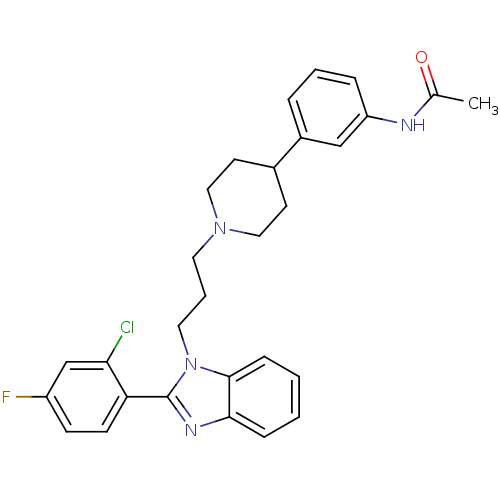
(2-(2-chloro-4-fluorophenyl)-1-{3-[4-(3-acetylamino...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(F)cc2Cl)CC1 Show InChI InChI=1S/C29H30ClFN4O/c1-20(36)32-24-7-4-6-22(18-24)21-12-16-34(17-13-21)14-5-15-35-28-9-3-2-8-27(28)33-29(35)25-11-10-23(31)19-26(25)30/h2-4,6-11,18-19,21H,5,12-17H2,1H3,(H,32,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340363
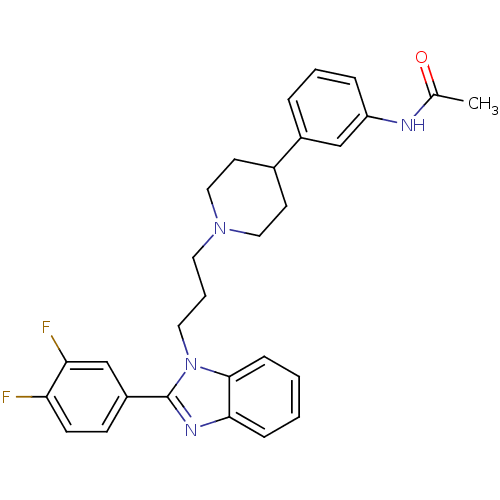
(2-(3,4-difluorophenyl)-1-{3-[4-(3-acetylaminopheny...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(F)c(F)c2)CC1 Show InChI InChI=1S/C29H30F2N4O/c1-20(36)32-24-7-4-6-22(18-24)21-12-16-34(17-13-21)14-5-15-35-28-9-3-2-8-27(28)33-29(35)23-10-11-25(30)26(31)19-23/h2-4,6-11,18-19,21H,5,12-17H2,1H3,(H,32,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174278
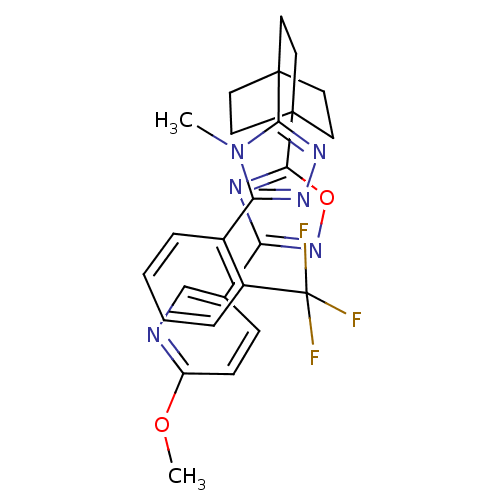
(2-methoxy-5-(5-(4-(4-methyl-5-(2-(trifluoromethyl)...)Show SMILES COc1ccc(cn1)-c1noc(n1)C12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C26H25F3N6O2/c1-35-21(17-5-3-4-6-18(17)26(27,28)29)32-33-22(35)24-9-12-25(13-10-24,14-11-24)23-31-20(34-37-23)16-7-8-19(36-2)30-15-16/h3-8,15H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
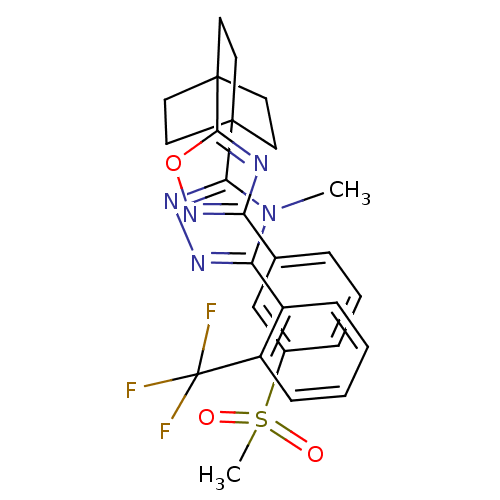
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174301
(4-methyl-3-(4-(3-(3-(methylsulfonyl)phenyl)-1,2,4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1cccc(c1)S(C)(=O)=O)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H26F3N5O3S/c1-35-22(19-8-3-4-9-20(19)27(28,29)30)32-33-23(35)25-10-13-26(14-11-25,15-12-25)24-31-21(34-38-24)17-6-5-7-18(16-17)39(2,36)37/h3-9,16H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50411932

(CHEMBL403283)Show SMILES Cn1c(cnc1C12CCC(CC1)(CC2)c1nnc([nH]1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H25F4N5/c1-36-21(19-4-2-3-5-20(19)27(29,30)31)16-32-24(36)26-13-10-25(11-14-26,12-15-26)23-33-22(34-35-23)17-6-8-18(28)9-7-17/h2-9,16H,10-15H2,1H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
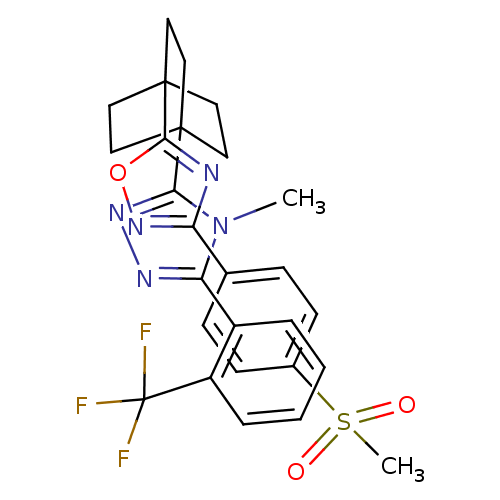
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174284
(4-methyl-3-(4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)S(C)(=O)=O)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H26F3N5O3S/c1-35-22(19-5-3-4-6-20(19)27(28,29)30)32-33-23(35)25-11-14-26(15-12-25,16-13-25)24-31-21(34-38-24)17-7-9-18(10-8-17)39(2,36)37/h3-10H,11-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
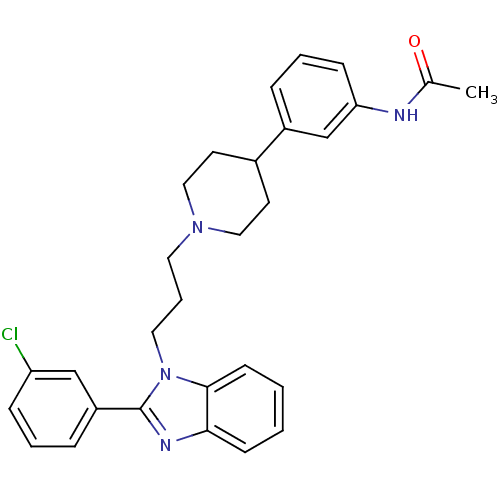
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340345
(2-(3-chlorophenyl)-1-{3-[4-(3-acetylaminophenyl)pi...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2cccc(Cl)c2)CC1 Show InChI InChI=1S/C29H31ClN4O/c1-21(35)31-26-10-5-7-23(20-26)22-13-17-33(18-14-22)15-6-16-34-28-12-3-2-11-27(28)32-29(34)24-8-4-9-25(30)19-24/h2-5,7-12,19-20,22H,6,13-18H2,1H3,(H,31,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
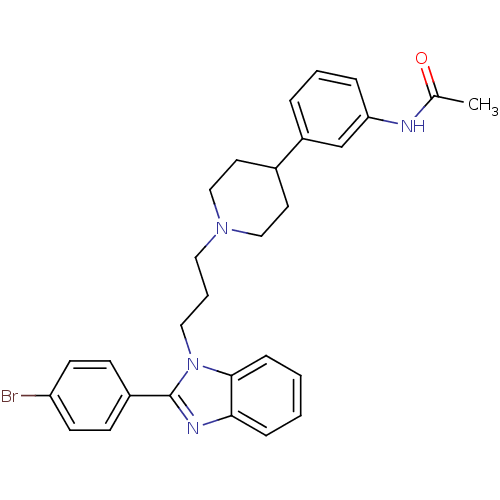
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340354
(2-(4-bromophenyl)-1-{3-[4-(3-acetylaminophenyl)pip...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(Br)cc2)CC1 Show InChI InChI=1S/C29H31BrN4O/c1-21(35)31-26-7-4-6-24(20-26)22-14-18-33(19-15-22)16-5-17-34-28-9-3-2-8-27(28)32-29(34)23-10-12-25(30)13-11-23/h2-4,6-13,20,22H,5,14-19H2,1H3,(H,31,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340367
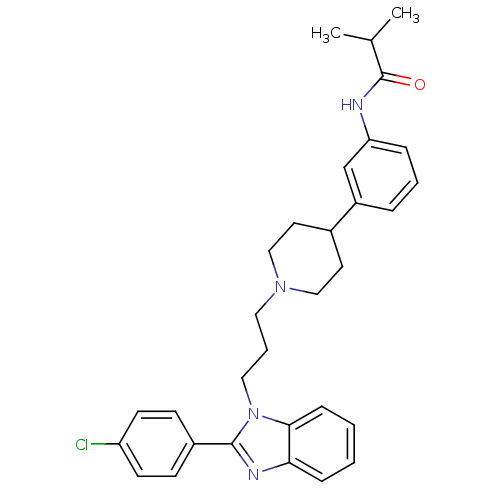
(2-(4-chlorophenyl)-1-{3-[4-(3-isobutyrylaminopheny...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C31H35ClN4O/c1-22(2)31(37)33-27-8-5-7-25(21-27)23-15-19-35(20-16-23)17-6-18-36-29-10-4-3-9-28(29)34-30(36)24-11-13-26(32)14-12-24/h3-5,7-14,21-23H,6,15-20H2,1-2H3,(H,33,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13756
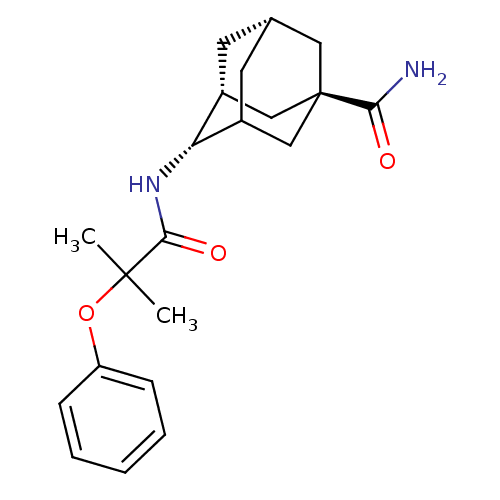
((1R,3R,4S,7S)-4-(2-methyl-2-phenoxypropanamido)ada...)Show SMILES CC(C)(Oc1ccccc1)C(=O)N[C@H]1C2C[C@@H]3C[C@@H]1C[C@](C3)(C2)C(N)=O |r,wU:16.16,18.18,13.13,wD:20.26,TLB:22:20:17:15.14.13,THB:15:16:19:22.14.13,21:16:13:22.19.20,23:20:17:15.14.13,(10.84,-3.12,;11.61,-4.45,;12.7,-3.37,;12.94,-5.22,;14.43,-4.83,;14.43,-3.29,;15.76,-2.52,;17.1,-3.29,;17.1,-4.83,;15.76,-5.6,;10.27,-5.22,;10.27,-6.76,;8.94,-4.45,;7.61,-5.22,;7.61,-6.76,;6.94,-7.93,;4.72,-7.16,;4.72,-5.12,;6.04,-4.32,;4.24,-5.07,;4.24,-6.31,;3.21,-7.32,;6.26,-7.03,;2.91,-5.54,;1.58,-6.31,;2.51,-4.05,)| Show InChI InChI=1S/C21H28N2O3/c1-20(2,26-16-6-4-3-5-7-16)19(25)23-17-14-8-13-9-15(17)12-21(10-13,11-14)18(22)24/h3-7,13-15,17H,8-12H2,1-2H3,(H2,22,24)(H,23,25)/t13-,14+,15?,17+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50411927
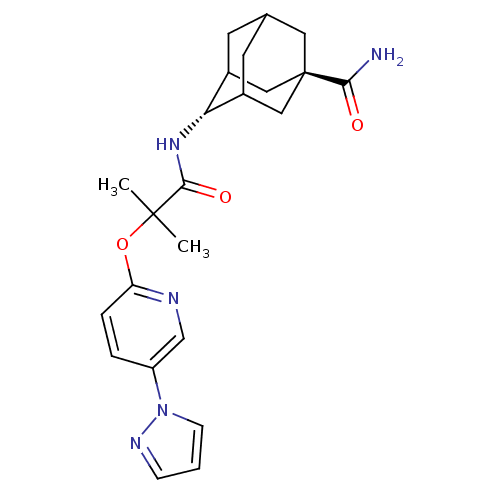
(CHEMBL407945)Show SMILES CC(C)(Oc1ccc(cn1)-n1cccn1)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |wU:18.19,wD:25.32,TLB:17:18:26.21.22:24,18:19:26:22.23.24,THB:20:21:24:27.19.18,20:19:26.21.22:24,18:23:26:27.20.19,(24.88,-39.51,;24.1,-38.2,;23.27,-36.87,;25.41,-37.39,;26.77,-38.13,;26.81,-39.68,;28.16,-40.41,;29.48,-39.61,;29.44,-38.06,;28.08,-37.32,;30.83,-40.34,;31.04,-41.86,;32.55,-42.14,;33.28,-40.79,;32.22,-39.67,;22.79,-39.01,;22.85,-40.56,;21.42,-38.3,;20.12,-39.12,;20.15,-40.64,;19.18,-41.95,;17.76,-41.42,;17.71,-39.84,;18.71,-38.58,;17.38,-39.1,;17.43,-40.58,;16.27,-41.88,;18.77,-41.03,;15.94,-40.17,;14.85,-41.26,;15.54,-38.69,)| Show InChI InChI=1S/C23H29N5O3/c1-22(2,31-18-5-4-17(13-25-18)28-7-3-6-26-28)21(30)27-19-15-8-14-9-16(19)12-23(10-14,11-15)20(24)29/h3-7,13-16,19H,8-12H2,1-2H3,(H2,24,29)(H,27,30)/t14?,15?,16?,19-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50192679

((3r,9r)-9-(2-(4-chlorophenoxy)-2-methylpropanamido...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2CCCC1C[C@H](C2)C(N)=O |wU:21.25,wD:14.14,TLB:13:14:18.17.16:22.21.20,23:21:18.17.16:14,(18.27,-20.26,;16.94,-19.49,;15.59,-20.24,;18.27,-18.72,;18.28,-17.18,;19.61,-16.41,;19.61,-14.87,;18.28,-14.1,;18.28,-12.56,;16.94,-14.88,;16.94,-16.41,;15.61,-18.72,;15.61,-17.18,;14.27,-19.48,;12.95,-18.72,;11.93,-19.52,;12.05,-21.27,;11.23,-22.21,;12.68,-22.15,;12.7,-20.2,;10.93,-20.21,;9.82,-20.99,;10.23,-19.52,;8.49,-20.21,;8.48,-18.67,;7.14,-20.99,)| Show InChI InChI=1S/C20H27ClN2O3/c1-20(2,26-16-8-6-15(21)7-9-16)19(25)23-17-12-4-3-5-13(17)11-14(10-12)18(22)24/h6-9,12-14,17H,3-5,10-11H2,1-2H3,(H2,22,24)(H,23,25)/t12?,13?,14-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50411936
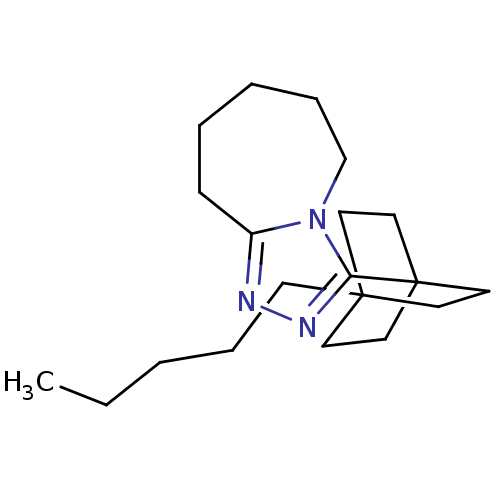
(CHEMBL402046)Show InChI InChI=1S/C20H33N3/c1-2-3-6-9-19-10-13-20(14-11-19,15-12-19)18-22-21-17-8-5-4-7-16-23(17)18/h2-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50411933

(CHEMBL257059)Show SMILES Cn1c(cnc1C12CCC(CC1)(CC2)c1nc(c[nH]1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C28H26F4N4/c1-36-23(20-4-2-3-5-21(20)28(30,31)32)17-34-25(36)27-13-10-26(11-14-27,12-15-27)24-33-16-22(35-24)18-6-8-19(29)9-7-18/h2-9,16-17H,10-15H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50337779

(2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...)Show SMILES CC(C)C(Oc1ccc(CNC(=O)C2SCCN2C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C26H30F3N3O5S/c1-14(2)23(26(35)36)37-18-5-3-15(4-6-18)13-31-24(34)25-32(7-8-38-25)22(33)11-17(30)9-16-10-20(28)21(29)12-19(16)27/h3-6,10,12,14,17,23,25H,7-9,11,13,30H2,1-2H3,(H,31,34)(H,35,36)/t17-,23?,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader |
Bioorg Med Chem Lett 21: 1366-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.041
BindingDB Entry DOI: 10.7270/Q25B02R9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50411930
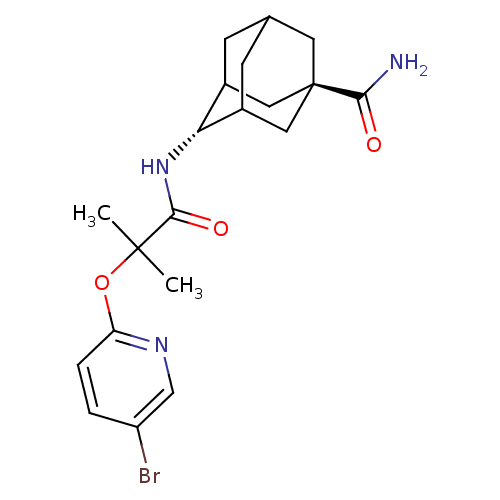
(CHEMBL257011)Show SMILES CC(C)(Oc1ccc(Br)cn1)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |wU:14.14,wD:21.27,TLB:13:14:22.17.18:20,14:15:22:18.19.20,THB:16:17:20:23.15.14,16:15:22.17.18:20,14:19:22:23.16.15,(27.85,-18.03,;27.07,-16.72,;26.25,-15.4,;28.39,-15.91,;29.74,-16.65,;29.78,-18.2,;31.13,-18.93,;32.44,-18.13,;33.8,-18.86,;32.4,-16.58,;31.04,-15.85,;25.76,-17.54,;25.82,-19.08,;24.4,-16.82,;23.1,-17.64,;23.13,-19.16,;22.16,-20.46,;20.74,-19.94,;20.7,-18.36,;21.7,-17.1,;20.37,-17.62,;20.42,-19.1,;19.26,-20.4,;21.75,-19.55,;18.92,-18.69,;17.84,-19.77,;18.53,-17.21,)| Show InChI InChI=1S/C20H26BrN3O3/c1-19(2,27-15-4-3-14(21)10-23-15)18(26)24-16-12-5-11-6-13(16)9-20(7-11,8-12)17(22)25/h3-4,10-13,16H,5-9H2,1-2H3,(H2,22,25)(H,24,26)/t11?,12?,13?,16-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13760

((1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methylpropa...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2C[C@@H]3C[C@@H]1C[C@](C3)(C2)C(N)=O |r,wU:17.17,19.19,14.14,wD:21.27,TLB:23:21:18:16.15.14,THB:16:17:20:23.15.14,22:17:14:23.20.21,24:21:18:16.15.14,(10.84,-3.12,;11.61,-4.45,;12.7,-3.37,;12.94,-5.22,;14.43,-4.83,;14.43,-3.29,;15.76,-2.52,;17.1,-3.29,;18.43,-2.52,;17.1,-4.83,;15.76,-5.6,;10.27,-5.22,;10.27,-6.76,;8.94,-4.45,;7.61,-5.22,;7.61,-6.76,;6.94,-7.93,;4.72,-7.16,;4.72,-5.12,;6.04,-4.32,;4.24,-5.07,;4.24,-6.31,;3.21,-7.32,;6.26,-7.03,;2.91,-5.54,;1.58,-6.31,;2.51,-4.05,)| Show InChI InChI=1S/C21H27ClN2O3/c1-20(2,27-16-5-3-15(22)4-6-16)19(26)24-17-13-7-12-8-14(17)11-21(9-12,10-13)18(23)25/h3-6,12-14,17H,7-11H2,1-2H3,(H2,23,25)(H,24,26)/t12-,13+,14?,17+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13761
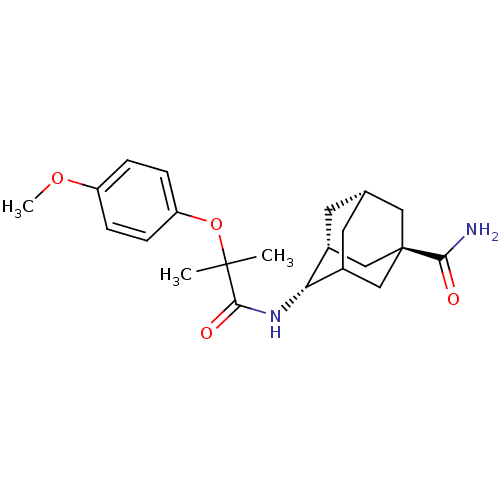
((1R,3R,4S,7S)-4-[2-(4-methoxyphenoxy)-2-methylprop...)Show SMILES COc1ccc(OC(C)(C)C(=O)N[C@H]2C3C[C@@H]4C[C@@H]2C[C@](C4)(C3)C(N)=O)cc1 |r,wU:16.15,18.17,13.12,wD:20.25,TLB:22:20:17:15.14.13,THB:15:16:19:22.14.13,21:16:13:22.19.20,23:20:17:15.14.13,(19.76,-3.29,;18.43,-2.52,;17.1,-3.29,;15.76,-2.52,;14.43,-3.29,;14.43,-4.83,;12.94,-5.22,;11.61,-4.45,;10.84,-3.12,;12.7,-3.37,;10.27,-5.22,;10.27,-6.76,;8.94,-4.45,;7.61,-5.22,;7.61,-6.76,;6.94,-7.93,;4.72,-7.16,;4.72,-5.12,;6.04,-4.32,;4.24,-5.07,;4.24,-6.31,;3.21,-7.32,;6.26,-7.03,;2.91,-5.54,;1.58,-6.31,;2.51,-4.05,;15.76,-5.6,;17.1,-4.83,)| Show InChI InChI=1S/C22H30N2O4/c1-21(2,28-17-6-4-16(27-3)5-7-17)20(26)24-18-14-8-13-9-15(18)12-22(10-13,11-14)19(23)25/h4-7,13-15,18H,8-12H2,1-3H3,(H2,23,25)(H,24,26)/t13-,14+,15?,18+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50413757

(CHEMBL511907)Show SMILES CC(C)(C)c1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H27Cl3N4O/c1-25(2,3)21-22(24(33)30-31-13-5-4-6-14-31)29-32(20-12-11-18(27)15-19(20)28)23(21)16-7-9-17(26)10-8-16/h7-12,15H,4-6,13-14H2,1-3H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160112

(6-(2,4-Dichloro-phenyl)-2-(3,4-difluoro-benzyloxy)...)Show SMILES Cc1ccc(cc1)-c1cc(C#N)c(OCc2ccc(F)c(F)c2)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H16Cl2F2N2O/c1-15-2-5-17(6-3-15)21-11-18(13-31)26(33-14-16-4-9-23(29)24(30)10-16)32-25(21)20-8-7-19(27)12-22(20)28/h2-12H,14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340351
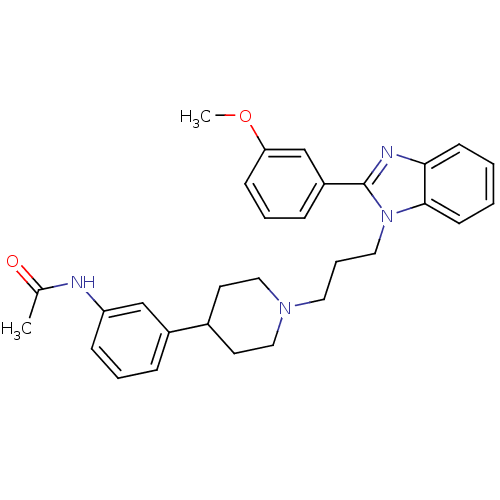
(2-(3-methoxyphenyl)-1-{3-[4-(3-acetylaminophenyl)p...)Show SMILES COc1cccc(c1)-c1nc2ccccc2n1CCCN1CCC(CC1)c1cccc(NC(C)=O)c1 Show InChI InChI=1S/C30H34N4O2/c1-22(35)31-26-10-5-8-24(20-26)23-14-18-33(19-15-23)16-7-17-34-29-13-4-3-12-28(29)32-30(34)25-9-6-11-27(21-25)36-2/h3-6,8-13,20-21,23H,7,14-19H2,1-2H3,(H,31,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174292
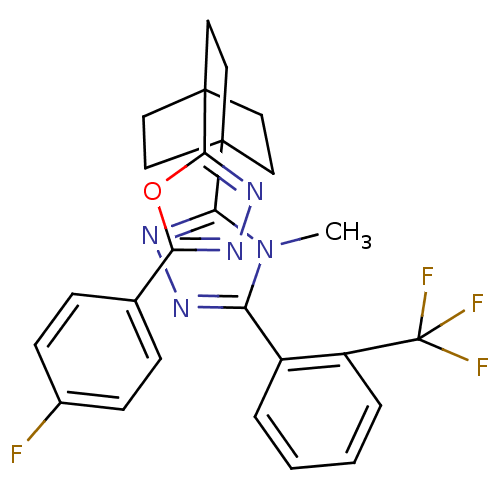
(3-(4-(5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nnc(o1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-20(18-4-2-3-5-19(18)26(28,29)30)31-33-22(35)24-10-13-25(14-11-24,15-12-24)23-34-32-21(36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13757
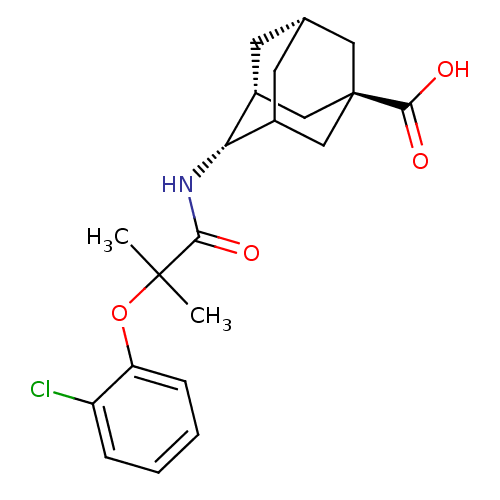
((1R,3R,4S,7S)-4-[2-(2-chlorophenoxy)-2-methylpropa...)Show SMILES CC(C)(Oc1ccccc1Cl)C(=O)N[C@H]1C2C[C@@H]3C[C@@H]1C[C@](C3)(C2)C(O)=O |r,wU:17.17,19.19,14.14,wD:21.27,TLB:23:21:18:16.15.14,THB:16:17:20:23.15.14,22:17:14:23.20.21,24:21:18:16.15.14,(10.84,-3.12,;11.61,-4.45,;12.7,-3.37,;12.94,-5.22,;14.43,-4.83,;14.43,-3.29,;15.76,-2.52,;17.1,-3.29,;17.1,-4.83,;15.76,-5.6,;15.76,-7.14,;10.27,-5.22,;10.27,-6.76,;8.94,-4.45,;7.61,-5.22,;7.61,-6.76,;6.94,-7.93,;4.72,-7.16,;4.72,-5.12,;6.04,-4.32,;4.24,-5.07,;4.24,-6.31,;3.21,-7.32,;6.26,-7.03,;2.91,-5.54,;1.58,-6.31,;2.51,-4.05,)| Show InChI InChI=1S/C21H26ClNO4/c1-20(2,27-16-6-4-3-5-15(16)22)18(24)23-17-13-7-12-8-14(17)11-21(9-12,10-13)19(25)26/h3-6,12-14,17H,7-11H2,1-2H3,(H,23,24)(H,25,26)/t12-,13+,14?,17+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50340357
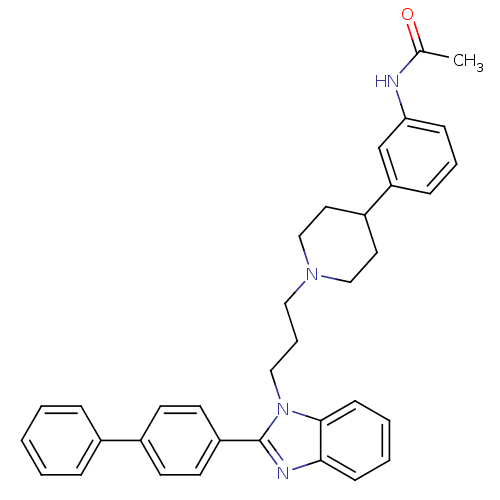
(2-(4-biphenyl)-1-{3-[4-(3-acetylaminophenyl)piperi...)Show SMILES CC(=O)Nc1cccc(c1)C1CCN(CCCn2c(nc3ccccc23)-c2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C35H36N4O/c1-26(40)36-32-12-7-11-31(25-32)29-19-23-38(24-20-29)21-8-22-39-34-14-6-5-13-33(34)37-35(39)30-17-15-28(16-18-30)27-9-3-2-4-10-27/h2-7,9-18,25,29H,8,19-24H2,1H3,(H,36,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50201465
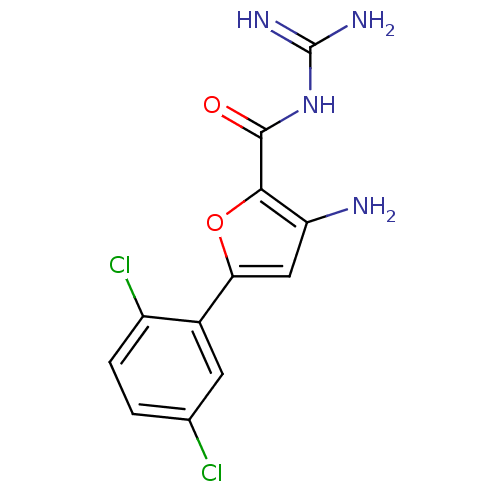
(3-amino-N-(diaminomethylene)-5-(2,5-dichlorophenyl...)Show InChI InChI=1S/C12H10Cl2N4O2/c13-5-1-2-7(14)6(3-5)9-4-8(15)10(20-9)11(19)18-12(16)17/h1-4H,15H2,(H4,16,17,18,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human NHE1 expressed in PS120 cells |
Bioorg Med Chem Lett 17: 1291-5 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.012
BindingDB Entry DOI: 10.7270/Q2MW2GS1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM35873
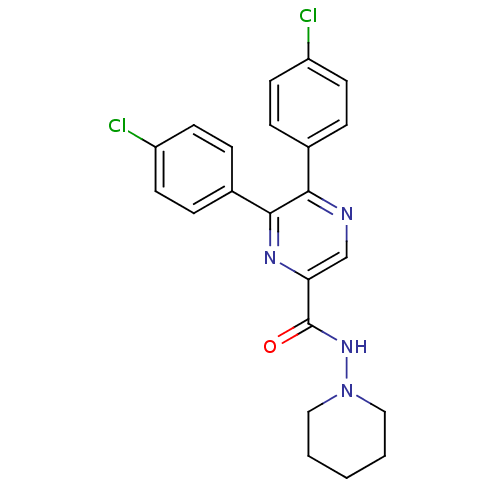
(5,6-bis(4-chlorophenyl)-N-(piperidin-1-yl)pyrazine...)Show SMILES Clc1ccc(cc1)-c1ncc(nc1-c1ccc(Cl)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H20Cl2N4O/c23-17-8-4-15(5-9-17)20-21(16-6-10-18(24)11-7-16)26-19(14-25-20)22(29)27-28-12-2-1-3-13-28/h4-11,14H,1-3,12-13H2,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174290
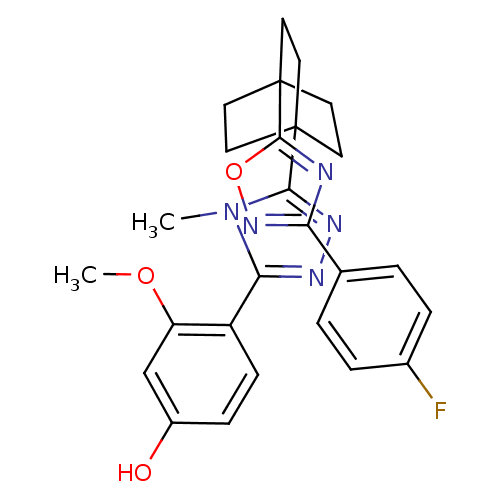
(4-(5-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)b...)Show SMILES COc1cc(O)ccc1-c1nnc(n1C)C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1 Show InChI InChI=1S/C26H26FN5O3/c1-32-22(19-8-7-18(33)15-20(19)34-2)29-30-23(32)25-9-12-26(13-10-25,14-11-25)24-28-21(31-35-24)16-3-5-17(27)6-4-16/h3-8,15,33H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13770

(2-(4-chlorophenoxy)-N-[(1R,2S,5R,7S)-5-(hydroxymet...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2C[C@@H]3C[C@@H]1C[C@](CO)(C3)C2 |r,wU:17.17,19.19,14.14,wD:21.23,TLB:25:21:18:16.15.14,THB:16:17:20:25.15.14,24:17:14:25.20.21,22:21:18:16.15.14,(10.84,-3.12,;11.61,-4.45,;12.7,-3.37,;12.94,-5.22,;14.43,-4.83,;14.43,-3.29,;15.76,-2.52,;17.1,-3.29,;18.43,-2.52,;17.1,-4.83,;15.76,-5.6,;10.27,-5.22,;10.27,-6.76,;8.94,-4.45,;7.61,-5.22,;7.61,-6.76,;6.94,-7.93,;4.72,-7.16,;4.72,-5.12,;6.04,-4.32,;4.24,-5.07,;4.24,-6.31,;2.91,-5.54,;1.58,-6.31,;3.21,-7.32,;6.26,-7.03,)| Show InChI InChI=1S/C21H28ClNO3/c1-20(2,26-17-5-3-16(22)4-6-17)19(25)23-18-14-7-13-8-15(18)11-21(9-13,10-14)12-24/h3-6,13-15,18,24H,7-12H2,1-2H3,(H,23,25)/t13-,14+,15?,18+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 |
Bioorg Med Chem Lett 18: 2479-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.042
BindingDB Entry DOI: 10.7270/Q2QC04Q0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50201443

(CHEMBL395897 | N-(diaminomethylene)-5-(2,5-dichlor...)Show InChI InChI=1S/C14H14Cl2N4O2/c1-20(2)10-6-11(8-5-7(15)3-4-9(8)16)22-12(10)13(21)19-14(17)18/h3-6H,1-2H3,(H4,17,18,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human NHE1 expressed in PS120 cells |
Bioorg Med Chem Lett 17: 1291-5 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.012
BindingDB Entry DOI: 10.7270/Q2MW2GS1 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
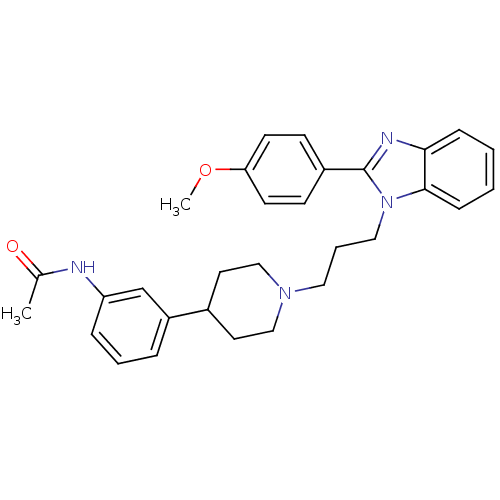
(Homo sapiens (Human)) | BDBM50340352
(2-(4-methoxyphenyl)-1-{3-[4-(3-acetylaminophenyl)p...)Show SMILES COc1ccc(cc1)-c1nc2ccccc2n1CCCN1CCC(CC1)c1cccc(NC(C)=O)c1 Show InChI InChI=1S/C30H34N4O2/c1-22(35)31-26-8-5-7-25(21-26)23-15-19-33(20-16-23)17-6-18-34-29-10-4-3-9-28(29)32-30(34)24-11-13-27(36-2)14-12-24/h3-5,7-14,21,23H,6,15-20H2,1-2H3,(H,31,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of Eu-labeled MCH from human MCH-R1 expressed in CHO by time-resolved fluorometric assay |
Bioorg Med Chem Lett 21: 2309-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.099
BindingDB Entry DOI: 10.7270/Q20002DT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data