 Found 195 hits with Last Name = 'hsieh' and Initial = 'sh'
Found 195 hits with Last Name = 'hsieh' and Initial = 'sh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330230
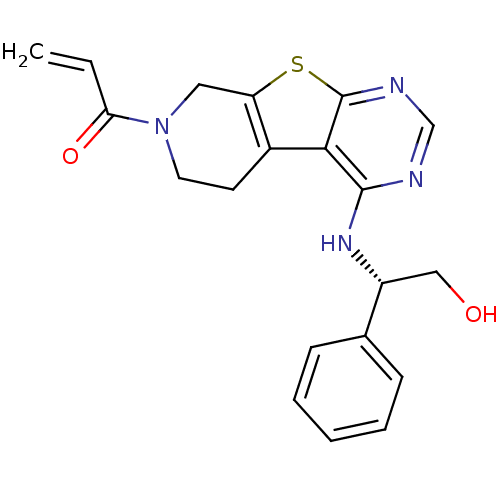
(1-(4-(((1S)-2-Hydroxy-1-phenylethyl)amino)-5,6,7,8...)Show SMILES OC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C20H20N4O2S/c1-2-17(26)24-9-8-14-16(10-24)27-20-18(14)19(21-12-22-20)23-15(11-25)13-6-4-3-5-7-13/h2-7,12,15,25H,1,8-11H2,(H,21,22,23)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330238

((E)-4-(Diethylamino)-1-(4-(((1R)-2-hydroxy-1-pheny...)Show SMILES CCN(CC)C\C=C\C(=O)N1CCc2c(C1)sc1ncnc(N[C@H](CO)c3ccccc3)c21 |r| Show InChI InChI=1S/C25H31N5O2S/c1-3-29(4-2)13-8-11-22(32)30-14-12-19-21(15-30)33-25-23(19)24(26-17-27-25)28-20(16-31)18-9-6-5-7-10-18/h5-11,17,20,31H,3-4,12-16H2,1-2H3,(H,26,27,28)/b11-8+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249053

(3-({2-[(2S),4-2S-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES Cc1ncsc1[C@H]1C[C@@H]1NC(=O)CC(C)(C)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C19H26FN5O2S/c1-11-18(28-10-22-11)14-5-15(14)24-16(26)6-19(2,3)23-8-17(27)25-9-12(20)4-13(25)7-21/h10,12-15,23H,4-6,8-9H2,1-3H3,(H,24,26)/t12-,13-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 in presence of 50% human serum |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330241
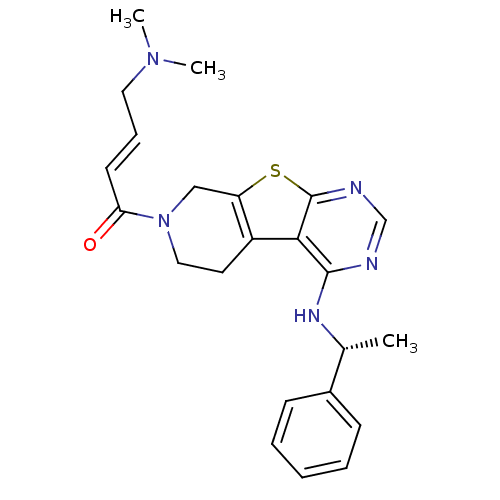
((E)-4-(Dimethylamino)-1-(4-[(1R)-1-phenylethyl]ami...)Show SMILES C[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN(C)C)c1ccccc1 |r| Show InChI InChI=1S/C23H27N5OS/c1-16(17-8-5-4-6-9-17)26-22-21-18-11-13-28(20(29)10-7-12-27(2)3)14-19(18)30-23(21)25-15-24-22/h4-10,15-16H,11-14H2,1-3H3,(H,24,25,26)/b10-7+/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330242
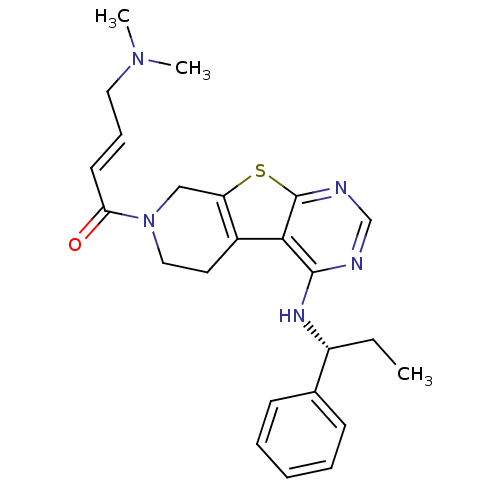
((E)-4-(Dimethylamino)-1-(4-[(1S)-1-phenylpropyl]am...)Show SMILES CC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN(C)C)c1ccccc1 |r| Show InChI InChI=1S/C24H29N5OS/c1-4-19(17-9-6-5-7-10-17)27-23-22-18-12-14-29(21(30)11-8-13-28(2)3)15-20(18)31-24(22)26-16-25-23/h5-11,16,19H,4,12-15H2,1-3H3,(H,25,26,27)/b11-8+/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249053

(3-({2-[(2S),4-2S-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES Cc1ncsc1[C@H]1C[C@@H]1NC(=O)CC(C)(C)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C19H26FN5O2S/c1-11-18(28-10-22-11)14-5-15(14)24-16(26)6-19(2,3)23-8-17(27)25-9-12(20)4-13(25)7-21/h10,12-15,23H,4-6,8-9H2,1-3H3,(H,24,26)/t12-,13-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 in presence of 50% rat serum |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248621

(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(Cl)c1)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C21H26ClFN4O2/c1-21(2,25-11-20(29)27-12-15(23)7-16(27)10-24)9-19(28)26-18-8-17(18)13-4-3-5-14(22)6-13/h3-6,15-18,25H,7-9,11-12H2,1-2H3,(H,26,28)/t15-,16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249053

(3-({2-[(2S),4-2S-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES Cc1ncsc1[C@H]1C[C@@H]1NC(=O)CC(C)(C)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C19H26FN5O2S/c1-11-18(28-10-22-11)14-5-15(14)24-16(26)6-19(2,3)23-8-17(27)25-9-12(20)4-13(25)7-21/h10,12-15,23H,4-6,8-9H2,1-3H3,(H,24,26)/t12-,13-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248693

(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES CCOc1cccc(c1)[C@H]1C[C@@H]1NC(=O)CC(C)(C)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C23H31FN4O3/c1-4-31-18-7-5-6-15(8-18)19-10-20(19)27-21(29)11-23(2,3)26-13-22(30)28-14-16(24)9-17(28)12-25/h5-8,16-17,19-20,26H,4,9-11,13-14H2,1-3H3,(H,27,29)/t16-,17-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248620
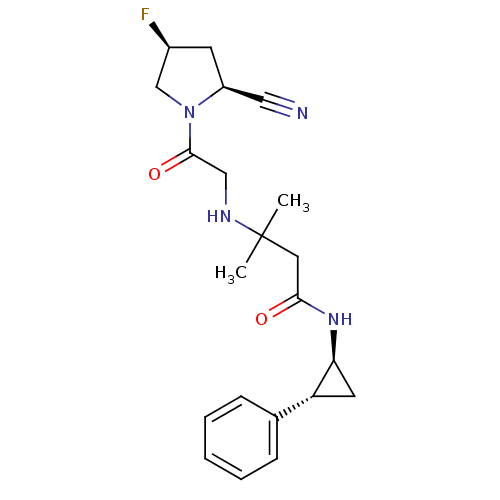
(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1ccccc1)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C21H27FN4O2/c1-21(2,24-12-20(28)26-13-15(22)8-16(26)11-23)10-19(27)25-18-9-17(18)14-6-4-3-5-7-14/h3-7,15-18,24H,8-10,12-13H2,1-2H3,(H,25,27)/t15-,16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330234

(1-(4-[(1R)-1-Phenylethyl]amino-5,6,7,8-tetrahydrop...)Show SMILES C[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C20H20N4OS/c1-3-17(25)24-10-9-15-16(11-24)26-20-18(15)19(21-12-22-20)23-13(2)14-7-5-4-6-8-14/h3-8,12-13H,1,9-11H2,2H3,(H,21,22,23)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248694
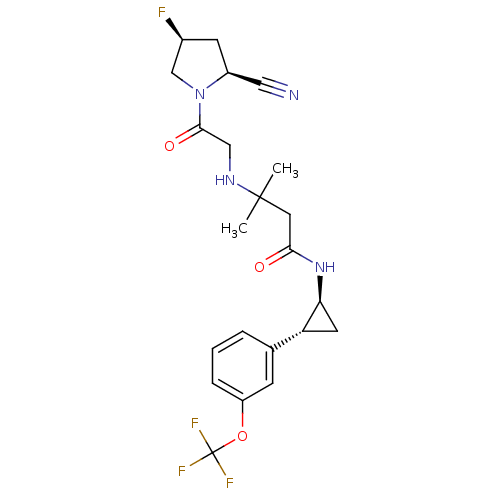
(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(OC(F)(F)F)c1)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C22H26F4N4O3/c1-21(2,28-11-20(32)30-12-14(23)7-15(30)10-27)9-19(31)29-18-8-17(18)13-4-3-5-16(6-13)33-22(24,25)26/h3-6,14-15,17-18,28H,7-9,11-12H2,1-2H3,(H,29,31)/t14-,15-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248622

(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(F)c1)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C21H26F2N4O2/c1-21(2,25-11-20(29)27-12-15(23)7-16(27)10-24)9-19(28)26-18-8-17(18)13-4-3-5-14(22)6-13/h3-6,15-18,25H,7-9,11-12H2,1-2H3,(H,26,28)/t15-,16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330239
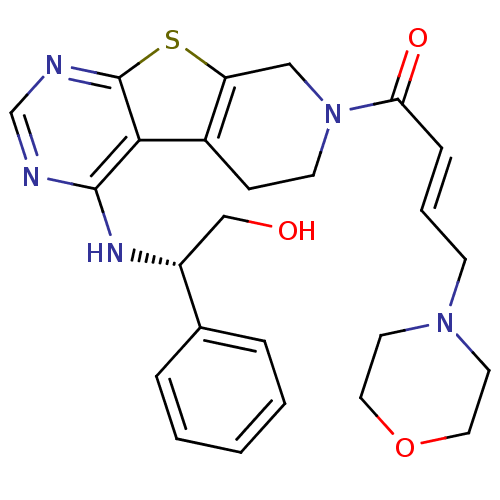
((E)-1-(4-[(1R)-2-Hydroxy-1-phenylethyl]amino-5,6,7...)Show SMILES OC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)\C=C\CN1CCOCC1)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3S/c31-16-20(18-5-2-1-3-6-18)28-24-23-19-8-10-30(15-21(19)34-25(23)27-17-26-24)22(32)7-4-9-29-11-13-33-14-12-29/h1-7,17,20,31H,8-16H2,(H,26,27,28)/b7-4+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248654
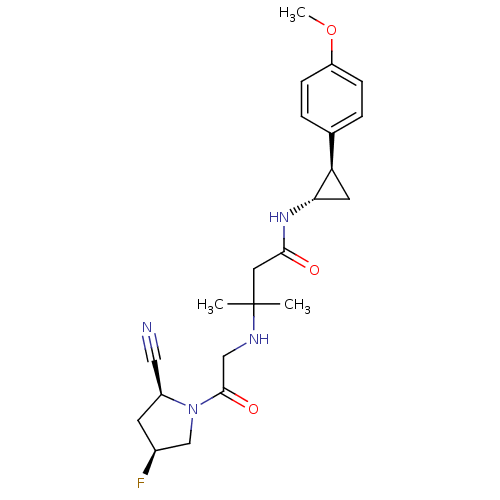
(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES COc1ccc(cc1)[C@H]1C[C@@H]1NC(=O)CC(C)(C)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C22H29FN4O3/c1-22(2,25-12-21(29)27-13-15(23)8-16(27)11-24)10-20(28)26-19-9-18(19)14-4-6-17(30-3)7-5-14/h4-7,15-16,18-19,25H,8-10,12-13H2,1-3H3,(H,26,28)/t15-,16-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249052

(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccnc1)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C20H26FN5O2/c1-20(2,24-11-19(28)26-12-14(21)6-15(26)9-22)8-18(27)25-17-7-16(17)13-4-3-5-23-10-13/h3-5,10,14-17,24H,6-8,11-12H2,1-2H3,(H,25,27)/t14-,15-,16+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248692

(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES COc1cccc(c1)[C@H]1C[C@@H]1NC(=O)CC(C)(C)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C22H29FN4O3/c1-22(2,25-12-21(29)27-13-15(23)8-16(27)11-24)10-20(28)26-19-9-18(19)14-5-4-6-17(7-14)30-3/h4-7,15-16,18-19,25H,8-10,12-13H2,1-3H3,(H,26,28)/t15-,16-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584094
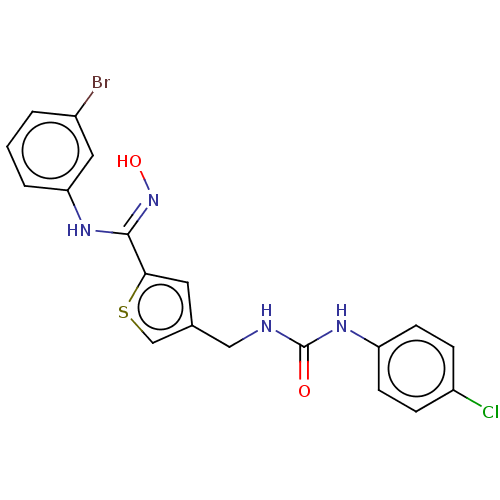
(CHEMBL5077002)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2ccc(Cl)cc2)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248695

(3-({2-[(2S)-2-Cyano-4,4-difluoropyrrolidin-1-yl]-2...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(Cl)c1)NCC(=O)N1CC(F)(F)C[C@H]1C#N |r| Show InChI InChI=1S/C21H25ClF2N4O2/c1-20(2,26-11-19(30)28-12-21(23,24)8-15(28)10-25)9-18(29)27-17-7-16(17)13-4-3-5-14(22)6-13/h3-6,15-17,26H,7-9,11-12H2,1-2H3,(H,27,29)/t15-,16+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248619
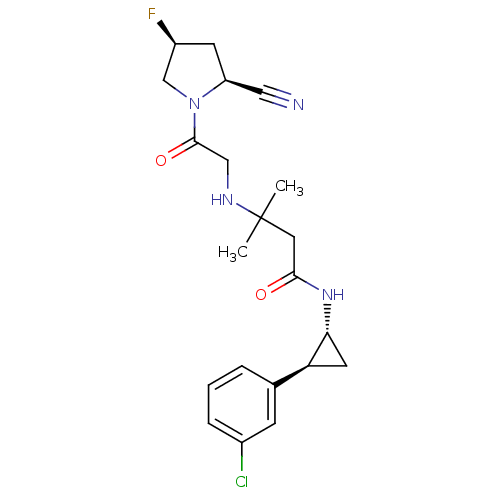
(3-({2-[(2S,4S)-2-Cyano-4-fluoropyrrolidin-1-yl]-2-...)Show SMILES CC(C)(CC(=O)N[C@@H]1C[C@H]1c1cccc(Cl)c1)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C21H26ClFN4O2/c1-21(2,25-11-20(29)27-12-15(23)7-16(27)10-24)9-19(28)26-18-8-17(18)13-4-3-5-14(22)6-13/h3-6,15-18,25H,7-9,11-12H2,1-2H3,(H,26,28)/t15-,16-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584075
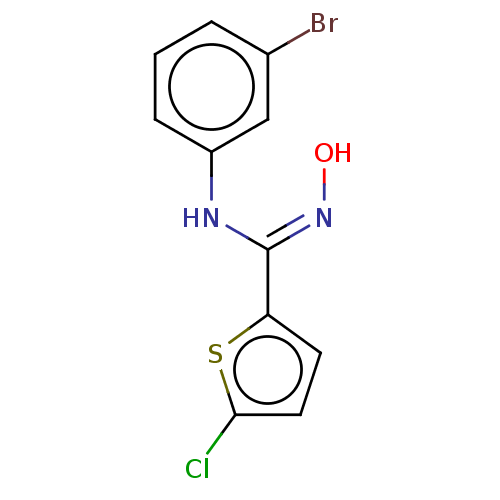
(CHEMBL5076745) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584093

(CHEMBL5087302)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2ccc(F)cc2)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248618
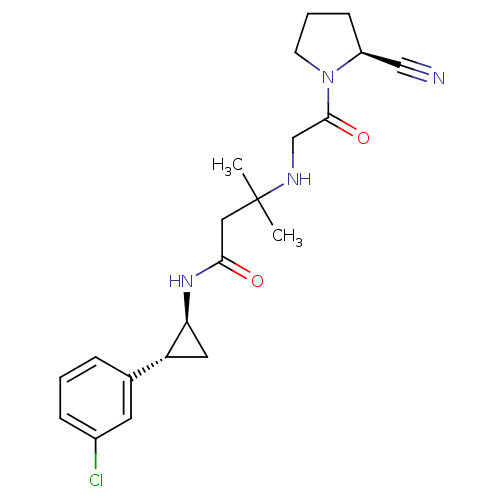
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(Cl)c1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H27ClN4O2/c1-21(2,24-13-20(28)26-8-4-7-16(26)12-23)11-19(27)25-18-10-17(18)14-5-3-6-15(22)9-14/h3,5-6,9,16-18,24H,4,7-8,10-11,13H2,1-2H3,(H,25,27)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248618

(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(Cl)c1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H27ClN4O2/c1-21(2,24-13-20(28)26-8-4-7-16(26)12-23)11-19(27)25-18-10-17(18)14-5-3-6-15(22)9-14/h3,5-6,9,16-18,24H,4,7-8,10-11,13H2,1-2H3,(H,25,27)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330235
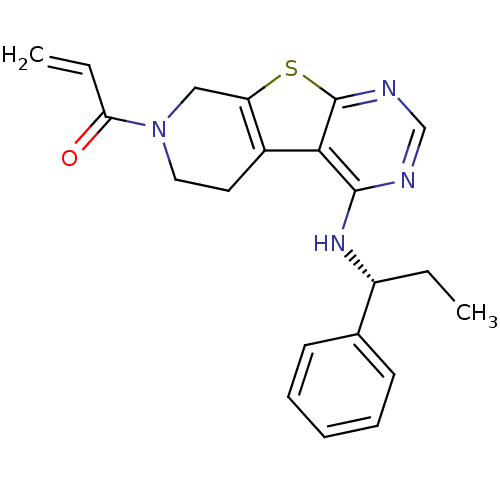
(1-(4-[(1R)-1-Phenylpropyl]amino-5,6,7,8-tetrahydro...)Show SMILES CC[C@@H](Nc1ncnc2sc3CN(CCc3c12)C(=O)C=C)c1ccccc1 |r| Show InChI InChI=1S/C21H22N4OS/c1-3-16(14-8-6-5-7-9-14)24-20-19-15-10-11-25(18(26)4-2)12-17(15)27-21(19)23-13-22-20/h4-9,13,16H,2-3,10-12H2,1H3,(H,22,23,24)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248975
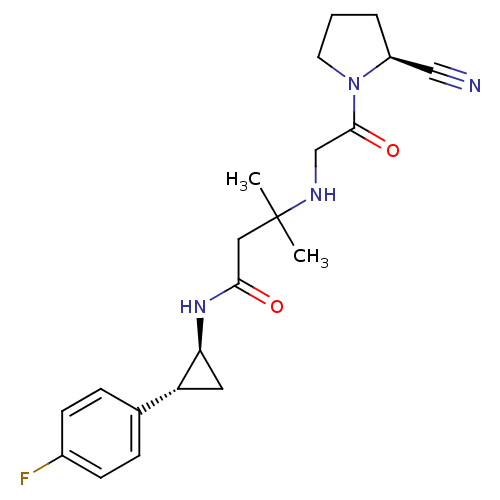
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1ccc(F)cc1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H27FN4O2/c1-21(2,24-13-20(28)26-9-3-4-16(26)12-23)11-19(27)25-18-10-17(18)14-5-7-15(22)8-6-14/h5-8,16-18,24H,3-4,9-11,13H2,1-2H3,(H,25,27)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249054
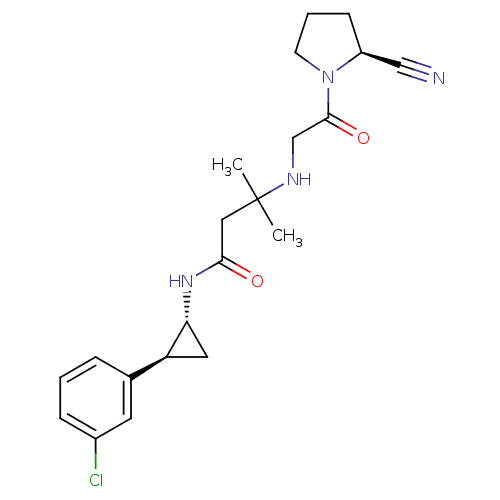
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@@H]1C[C@H]1c1cccc(Cl)c1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H27ClN4O2/c1-21(2,24-13-20(28)26-8-4-7-16(26)12-23)11-19(27)25-18-10-17(18)14-5-3-6-15(22)9-14/h3,5-6,9,16-18,24H,4,7-8,10-11,13H2,1-2H3,(H,25,27)/t16-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249018
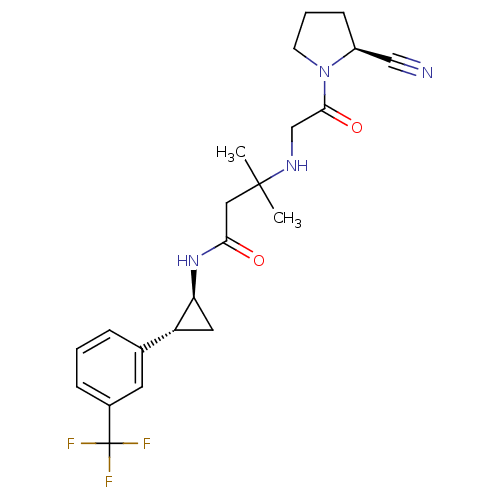
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(c1)C(F)(F)F)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C22H27F3N4O2/c1-21(2,27-13-20(31)29-8-4-7-16(29)12-26)11-19(30)28-18-10-17(18)14-5-3-6-15(9-14)22(23,24)25/h3,5-6,9,16-18,27H,4,7-8,10-11,13H2,1-2H3,(H,28,30)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248996
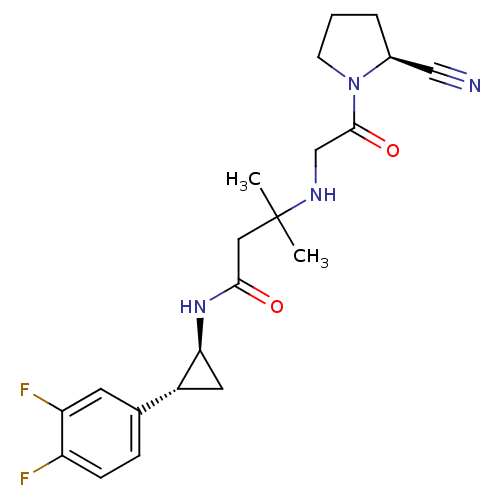
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1ccc(F)c(F)c1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H26F2N4O2/c1-21(2,25-12-20(29)27-7-3-4-14(27)11-24)10-19(28)26-18-9-15(18)13-5-6-16(22)17(23)8-13/h5-6,8,14-15,18,25H,3-4,7,9-10,12H2,1-2H3,(H,26,28)/t14-,15+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584087

(CHEMBL5093619)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2ccc(cc2)C#N)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248993
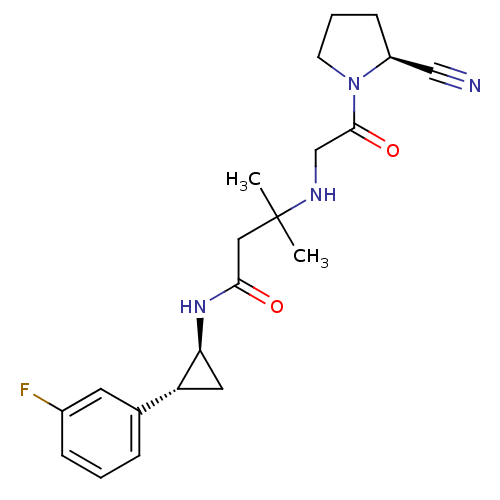
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(F)c1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H27FN4O2/c1-21(2,24-13-20(28)26-8-4-7-16(26)12-23)11-19(27)25-18-10-17(18)14-5-3-6-15(22)9-14/h3,5-6,9,16-18,24H,4,7-8,10-11,13H2,1-2H3,(H,25,27)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584085
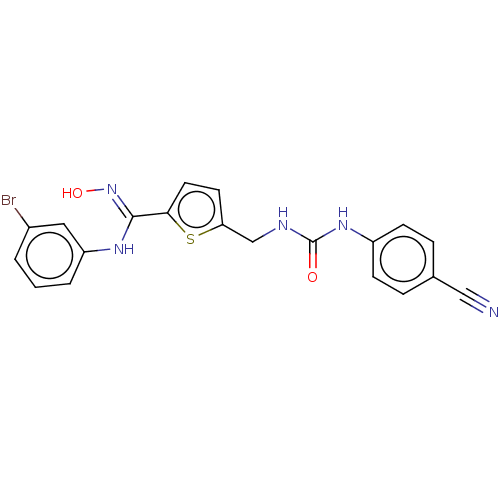
(CHEMBL5074542)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccc(cc2)C#N)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249050
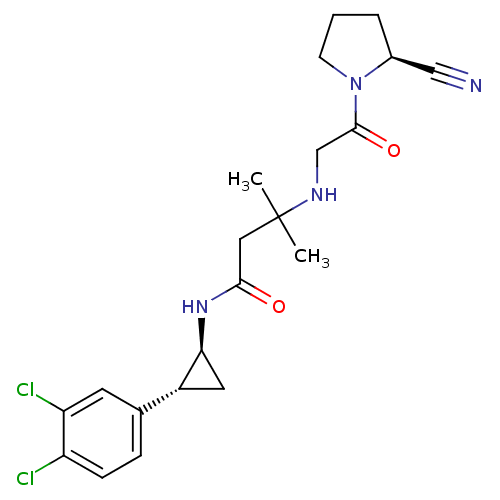
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1ccc(Cl)c(Cl)c1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H26Cl2N4O2/c1-21(2,25-12-20(29)27-7-3-4-14(27)11-24)10-19(28)26-18-9-15(18)13-5-6-16(22)17(23)8-13/h5-6,8,14-15,18,25H,3-4,7,9-10,12H2,1-2H3,(H,26,28)/t14-,15+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584073

(CHEMBL5081219) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330237
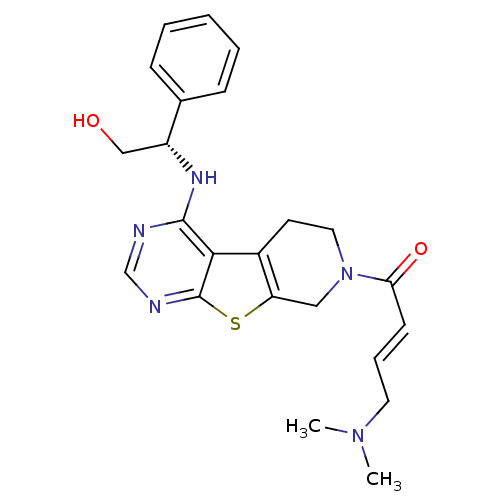
((E)-4-(Dimethylamino)-1-(4-[(1S)-2-hydroxy-1-pheny...)Show SMILES CN(C)C\C=C\C(=O)N1CCc2c(C1)sc1ncnc(N[C@H](CO)c3ccccc3)c21 |r| Show InChI InChI=1S/C23H27N5O2S/c1-27(2)11-6-9-20(30)28-12-10-17-19(13-28)31-23-21(17)22(24-15-25-23)26-18(14-29)16-7-4-3-5-8-16/h3-9,15,18,29H,10-14H2,1-2H3,(H,24,25,26)/b9-6+/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584074
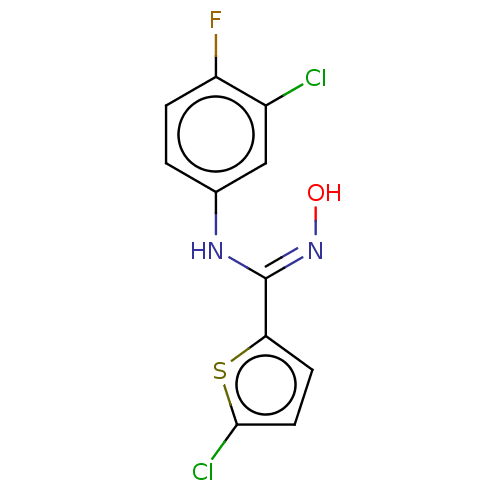
(CHEMBL5075428) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249019
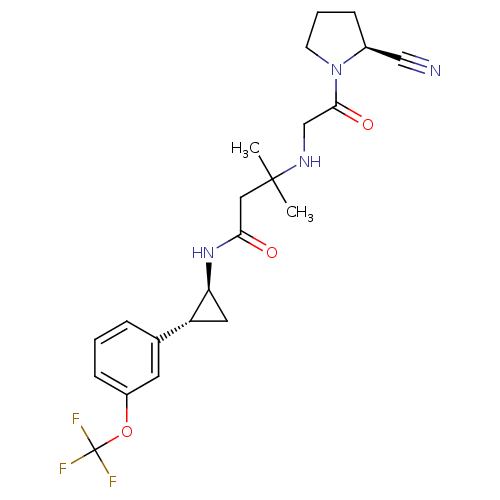
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cccc(OC(F)(F)F)c1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C22H27F3N4O3/c1-21(2,27-13-20(31)29-8-4-6-15(29)12-26)11-19(30)28-18-10-17(18)14-5-3-7-16(9-14)32-22(23,24)25/h3,5,7,9,15,17-18,27H,4,6,8,10-11,13H2,1-2H3,(H,28,30)/t15-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584076
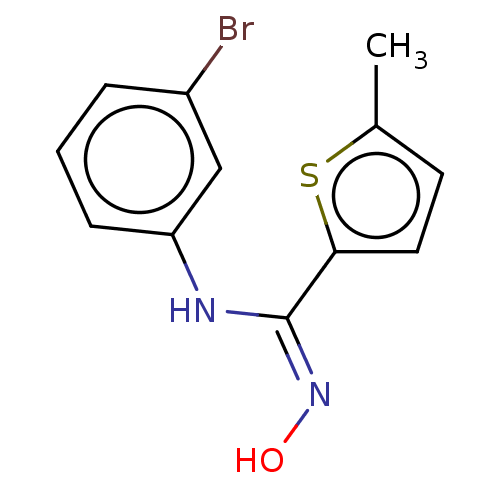
(CHEMBL5083059) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248995
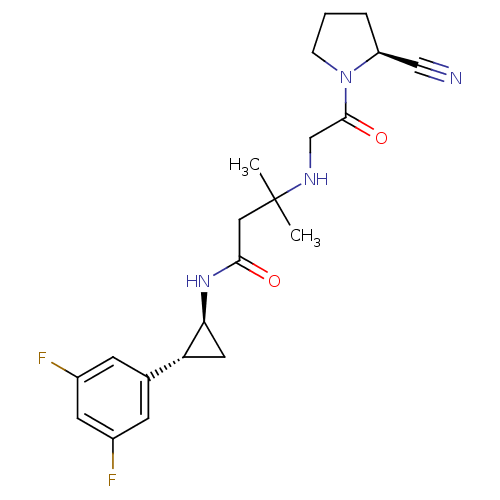
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1cc(F)cc(F)c1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H26F2N4O2/c1-21(2,25-12-20(29)27-5-3-4-16(27)11-24)10-19(28)26-18-9-17(18)13-6-14(22)8-15(23)7-13/h6-8,16-18,25H,3-5,9-10,12H2,1-2H3,(H,26,28)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584086
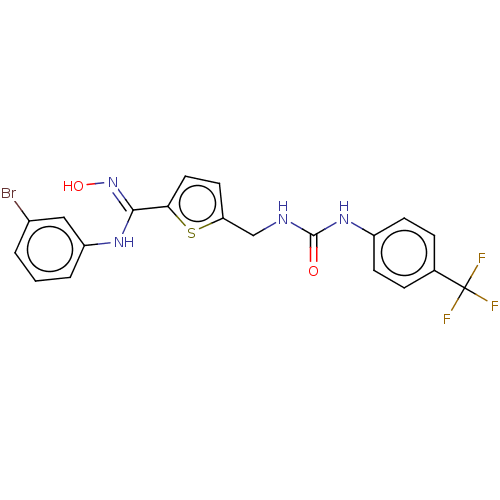
(CHEMBL5094947)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccc(cc2)C(F)(F)F)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248994
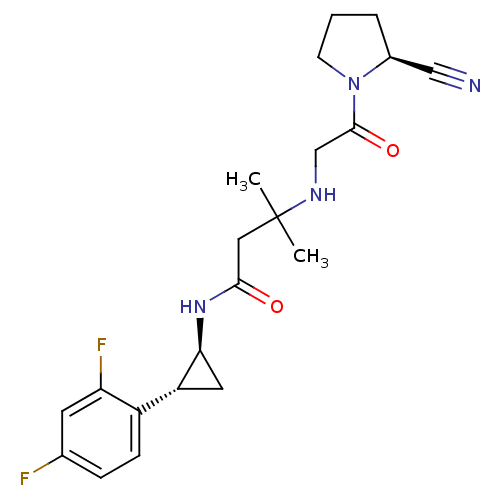
(3-(2-((S)-2-cyanopyrrolidin-1-yl)-2-oxoethylamino)...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1ccc(F)cc1F)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H26F2N4O2/c1-21(2,25-12-20(29)27-7-3-4-14(27)11-24)10-19(28)26-18-9-16(18)15-6-5-13(22)8-17(15)23/h5-6,8,14,16,18,25H,3-4,7,9-10,12H2,1-2H3,(H,26,28)/t14-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248997
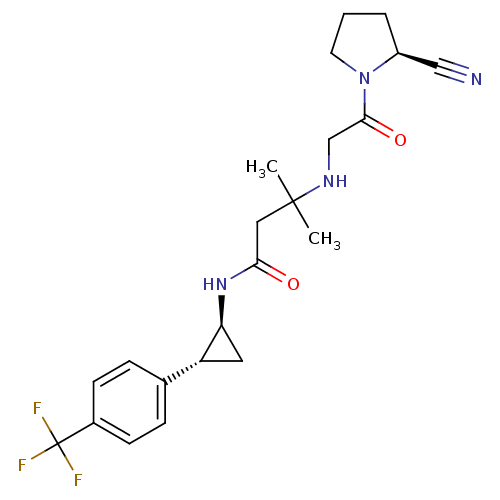
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1ccc(cc1)C(F)(F)F)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C22H27F3N4O2/c1-21(2,27-13-20(31)29-9-3-4-16(29)12-26)11-19(30)28-18-10-17(18)14-5-7-15(8-6-14)22(23,24)25/h5-8,16-18,27H,3-4,9-11,13H2,1-2H3,(H,28,30)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50248974
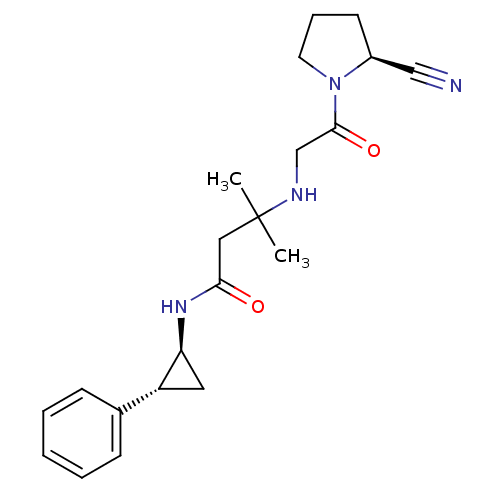
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1ccccc1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H28N4O2/c1-21(2,23-14-20(27)25-10-6-9-16(25)13-22)12-19(26)24-18-11-17(18)15-7-4-3-5-8-15/h3-5,7-8,16-18,23H,6,9-12,14H2,1-2H3,(H,24,26)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50249020
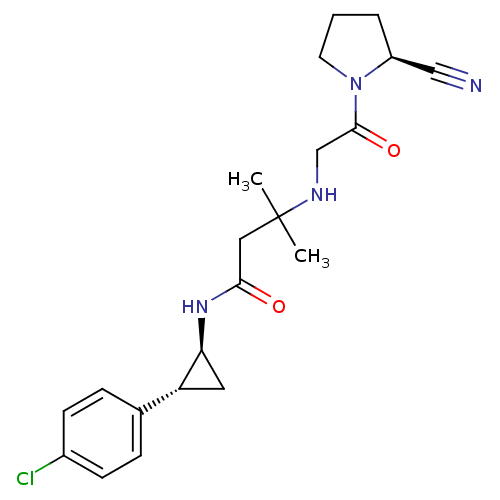
(3-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}ami...)Show SMILES CC(C)(CC(=O)N[C@H]1C[C@@H]1c1ccc(Cl)cc1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H27ClN4O2/c1-21(2,24-13-20(28)26-9-3-4-16(26)12-23)11-19(27)25-18-10-17(18)14-5-7-15(22)8-6-14/h5-8,16-18,24H,3-4,9-11,13H2,1-2H3,(H,25,27)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 2388-99 (2009)
Article DOI: 10.1016/j.bmc.2009.02.020
BindingDB Entry DOI: 10.7270/Q2416WZS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50330240
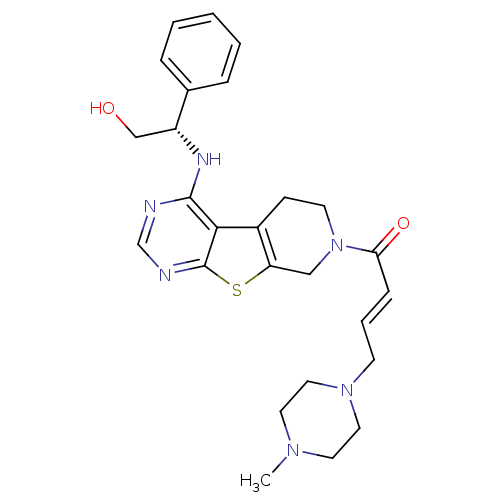
((E)-1-(4-[(1S)-2-Hydroxy-1-phenylethyl]amino-5,6,7...)Show SMILES CN1CCN(C\C=C\C(=O)N2CCc3c(C2)sc2ncnc(N[C@H](CO)c4ccccc4)c32)CC1 |r| Show InChI InChI=1S/C26H32N6O2S/c1-30-12-14-31(15-13-30)10-5-8-23(34)32-11-9-20-22(16-32)35-26-24(20)25(27-18-28-26)29-21(17-33)19-6-3-2-4-7-19/h2-8,18,21,33H,9-17H2,1H3,(H,27,28,29)/b8-5+/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 53: 7316-26 (2010)
Article DOI: 10.1021/jm100607r
BindingDB Entry DOI: 10.7270/Q26D5T64 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584077
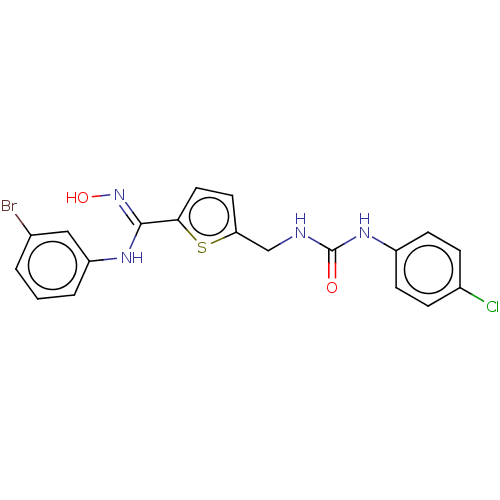
(CHEMBL5091205)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccc(Cl)cc2)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584084
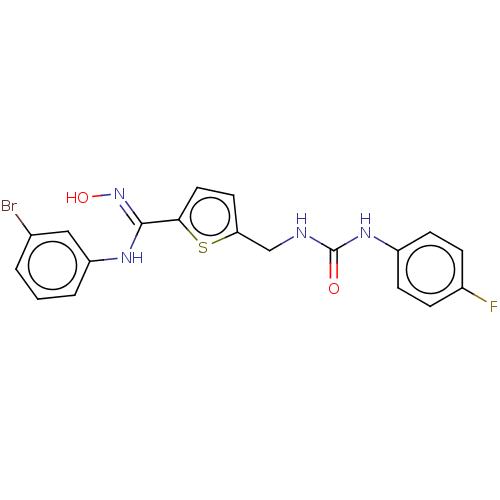
(CHEMBL5080101)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccc(F)cc2)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data