 Found 347 hits with Last Name = 'myers' and Initial = 'sh'
Found 347 hits with Last Name = 'myers' and Initial = 'sh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50248510
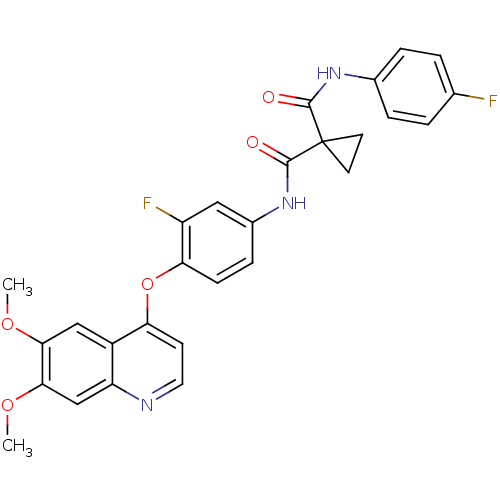
(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length VEGFR2 using poly (Glu,Tyr) as substrate by AlphaScreen assay |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50565800
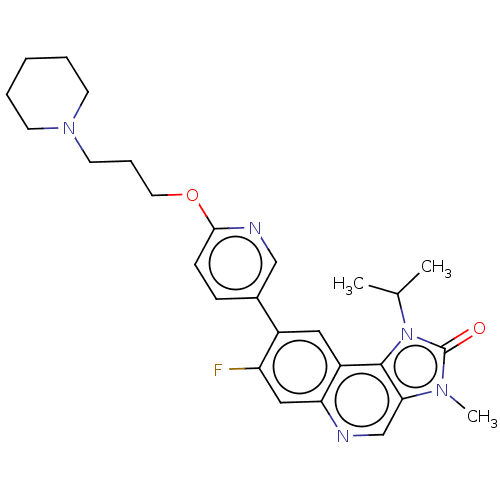
(Azd-1390 | Azd1390)Show SMILES CC(C)n1c2c(cnc3cc(F)c(cc23)-c2ccc(OCCCN3CCCCC3)nc2)n(C)c1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATM (unknown origin) assessed as ATM-dependent phosphorylation using GST-p53 ser15 as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human full length MET by scintillation counting method in presence of 33P-gammaATP |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length AXL (R497 to Y821 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as substr... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM144315
(Gilteritinib | US11512074, Example T-9 | US8969336...)Show SMILES CCc1nc(C(N)=O)c(Nc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1NC1CCOCC1 Show InChI InChI=1S/C29H44N8O3/c1-4-23-28(31-20-9-17-40-18-10-20)34-29(26(33-23)27(30)38)32-21-5-6-24(25(19-21)39-3)37-11-7-22(8-12-37)36-15-13-35(2)14-16-36/h5-6,19-20,22H,4,7-18H2,1-3H3,(H2,30,38)(H2,31,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of AXL (unknown origin) |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human ABL using EAIYAAPFAKKK as substrate after 40 mins by scintillation counting analysis in presence of [gamma-33P-ATP] |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM24498
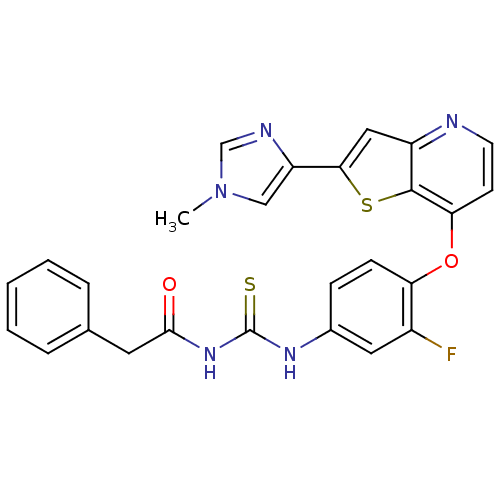
(3-(3-fluoro-4-{[2-(1-methyl-1H-imidazol-4-yl)thien...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=S)NC(=O)Cc4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C26H20FN5O2S2/c1-32-14-20(29-15-32)23-13-19-25(36-23)22(9-10-28-19)34-21-8-7-17(12-18(21)27)30-26(35)31-24(33)11-16-5-3-2-4-6-16/h2-10,12-15H,11H2,1H3,(H2,30,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24498

(3-(3-fluoro-4-{[2-(1-methyl-1H-imidazol-4-yl)thien...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=S)NC(=O)Cc4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C26H20FN5O2S2/c1-32-14-20(29-15-32)23-13-19-25(36-23)22(9-10-28-19)34-21-8-7-17(12-18(21)27)30-26(35)31-24(33)11-16-5-3-2-4-6-16/h2-10,12-15H,11H2,1H3,(H2,30,31,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of MET (unknown origin) |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human SRC (1 to 530 residues) using GGEEEEYFELVKKKK as substrate after 40 mins by scintillation counting analysis in presence of [gamma... |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human BMX using poly(Glu,Tyr) 4:1 as substrate after 40 mins by scintillation counting analysis in presence of [gamma-33P-ATP] |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human MEK1 after 40 mins in presence of MgATP |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MET using poly (Glu,Tyr) as substrate by AlphaScreen assay |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50538983
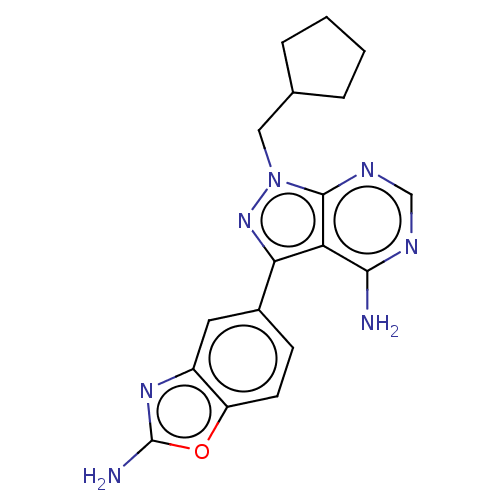
(CHEMBL3797933)Show SMILES Nc1nc2cc(ccc2o1)-c1nn(CC2CCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C18H19N7O/c19-16-14-15(11-5-6-13-12(7-11)23-18(20)26-13)24-25(17(14)22-9-21-16)8-10-3-1-2-4-10/h5-7,9-10H,1-4,8H2,(H2,20,23)(H2,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115215
BindingDB Entry DOI: 10.7270/Q2SN0DGJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 in human MDA-MB-231 cells assessed as receptor phosphorylation after 1 to 3 hrs |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 D835Y mutant (Q580 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172078

(LY-2801653 | Merestinib)Show SMILES Cc1ccc(C(=O)Nc2ccc(Oc3cc4cnn(C)c4cc3-c3cn[nH]c3)c(F)c2)c(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C30H22F2N6O3/c1-17-3-9-23(30(40)38(17)22-7-4-20(31)5-8-22)29(39)36-21-6-10-27(25(32)12-21)41-28-11-18-16-35-37(2)26(18)13-24(28)19-14-33-34-15-19/h3-16H,1-2H3,(H,33,34)(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human full length AXL after 4 hrs followed by stimulation with human recombinant Gas6 for 15 minutes by cell based assay |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Wee1-like protein kinase
(Homo sapiens (Human)) | BDBM50565801
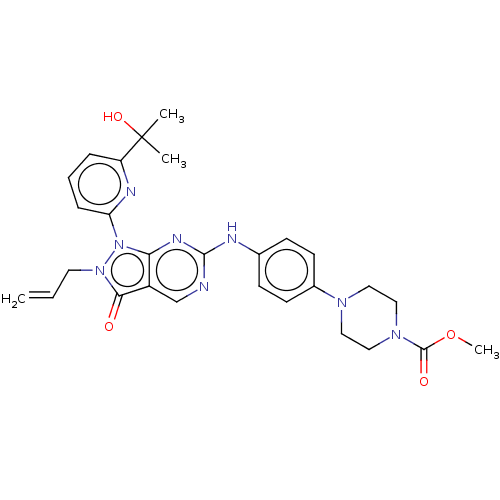
(CHEMBL4780868)Show SMILES COC(=O)N1CCN(CC1)c1ccc(Nc2ncc3c(n2)n(-c2cccc(n2)C(C)(C)O)n(CC=C)c3=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer178 from recombinant WEE1 (unknown origin) incubated for 1 hr by lanthascreen TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384576

(CHEMBL2036808)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(F)cc1 |r,wU:13.12,wD:16.16,(54.06,-16.98,;54.06,-18.52,;55.39,-19.29,;55.39,-20.83,;56.72,-21.6,;58.06,-20.83,;58.06,-19.29,;59.39,-18.52,;60.72,-19.28,;62.2,-18.8,;63.11,-20.06,;62.2,-21.31,;62.67,-22.78,;61.64,-23.92,;62.12,-25.38,;61.08,-26.52,;59.58,-26.2,;58.54,-27.34,;59.11,-24.73,;60.14,-23.59,;60.72,-20.83,;59.39,-21.6,;62.67,-17.34,;64.18,-17.02,;64.66,-15.56,;63.63,-14.41,;64.1,-12.95,;62.11,-14.74,;61.64,-16.2,)| Show InChI InChI=1S/C22H29FN6/c1-2-3-12-25-22-26-13-19-20(16-6-8-17(23)9-7-16)28-29(21(19)27-22)14-15-4-10-18(24)11-5-15/h6-9,13,15,18H,2-5,10-12,14,24H2,1H3,(H,25,26,27)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of MER (unknown origin) |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length RET (E713 to D1014 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50110178
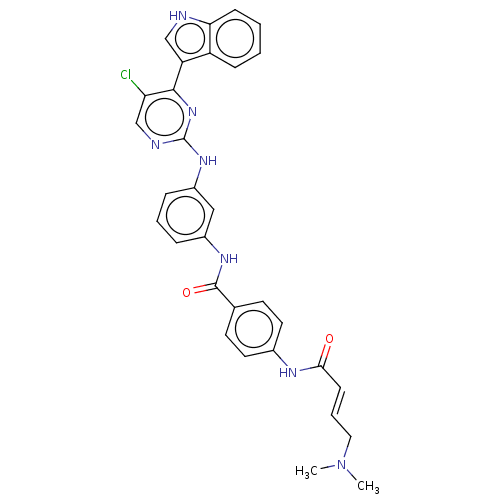
(CHEMBL3603847 | US10787436, Compound I-23)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2ncc(Cl)c(n2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H28ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-15,17-19,33H,16H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CDK7 incubated for 180 mins by LanthaScreen Eu Kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50538983

(CHEMBL3797933)Show SMILES Nc1nc2cc(ccc2o1)-c1nn(CC2CCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C18H19N7O/c19-16-14-15(11-5-6-13-12(7-11)23-18(20)26-13)24-25(17(14)22-9-21-16)8-10-3-1-2-4-10/h5-7,9-10H,1-4,8H2,(H2,20,23)(H2,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115215
BindingDB Entry DOI: 10.7270/Q2SN0DGJ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 D835Y mutant (Q580 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Wee1-like protein kinase
(Homo sapiens (Human)) | BDBM50240826
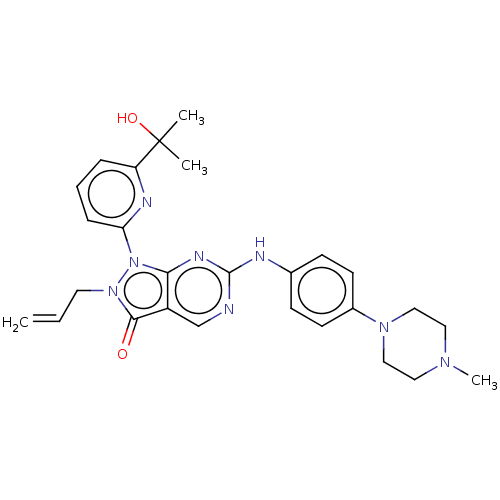
(AZD-1775 | MK-1775 | US11124518, Example AZD1775 |...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3c(n2)n(-c2cccc(n2)C(C)(C)O)n(CC=C)c3=O)cc1 Show InChI InChI=1S/C27H32N8O2/c1-5-13-34-25(36)21-18-28-26(29-19-9-11-20(12-10-19)33-16-14-32(4)15-17-33)31-24(21)35(34)23-8-6-7-22(30-23)27(2,3)37/h5-12,18,37H,1,13-17H2,2-4H3,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-fused human WEE1 (215 to 646 residues) expressed in baculovirus expression system using poly(Lys,Tyr) as substrate incub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length Aurora A (E122 to K401 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as substrate i... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length RET (E713 to D1014 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 ITD mutant expressed in bacterial expression system using poly [Glu, Try] 4:1 as substrate in presence of [ga... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50565799
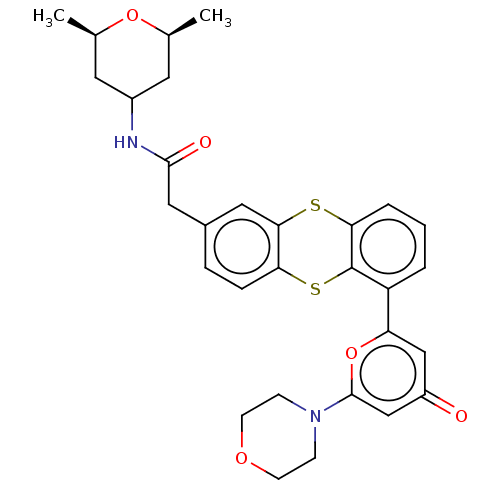
(CHEMBL4780892)Show SMILES C[C@H]1CC(C[C@@H](C)O1)NC(=O)Cc1ccc2Sc3c(Sc2c1)cccc3-c1cc(=O)cc(o1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATM (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
ATR-interacting protein/Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM268079

(2-[(3R)-3-methylmorpholin-4-yl]-4-(1-methyl-1H-pyr...)Show SMILES C[C@@H]1COCCN1c1cc(-c2ccnn2C)c2ccnc(-c3ccn[nH]3)c2n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-TAMRA-labelled Tracer A from human GST-fused/FLAG-tagged full length ATR/human N-terminal FLAG-tagged full length ATRIP expressed i... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172080
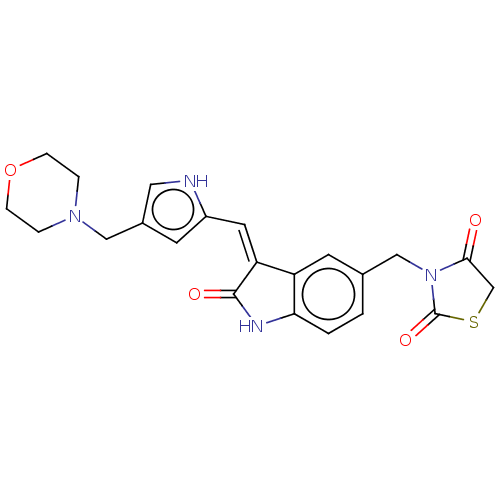
(CHEMBL3808948)Show SMILES O=C1CSC(=O)N1Cc1ccc2NC(=O)\C(=C/c3cc(CN4CCOCC4)c[nH]3)c2c1 Show InChI InChI=1S/C22H22N4O4S/c27-20-13-31-22(29)26(20)12-14-1-2-19-17(8-14)18(21(28)24-19)9-16-7-15(10-23-16)11-25-3-5-30-6-4-25/h1-2,7-10,23H,3-6,11-13H2,(H,24,28)/b18-9- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human AXL by radiometric assay |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length AXL using poly (Glu,Tyr) as substrate by AlphaScreen assay |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50248510

(CHEMBL489326 | N-(4-(6,7-dimethoxyquinolin-4-yloxy...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H23F2N3O5/c1-36-24-14-19-21(15-25(24)37-2)31-12-9-22(19)38-23-8-7-18(13-20(23)30)33-27(35)28(10-11-28)26(34)32-17-5-3-16(29)4-6-17/h3-9,12-15H,10-11H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of MET in human MDA-MB-231 cells assessed as receptor phosphorylation after 1 to 3 hrs |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455354

(CHEMBL4208852)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N8/c1-23-22-25-14-18-20(27-30(21(18)26-22)15-16-5-3-4-6-16)17-7-8-19(24-13-17)29-11-9-28(2)10-12-29/h7-8,13-14,16H,3-6,9-12,15H2,1-2H3,(H,23,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length VEGFR2 (R787 to P1253 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AXL expressed in Escherichia coli BL21 infected with T7 phage by qPCR method |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172077

(CHEMBL3810063)Show SMILES Cc1c(C(=O)Nc2ccc(Oc3ccnc4[nH]cc(-c5ccccc5)c34)c(F)c2)c(=O)n(-c2ccc(F)cc2)n1C Show InChI InChI=1S/C31H23F2N5O3/c1-18-27(31(40)38(37(18)2)22-11-8-20(32)9-12-22)30(39)36-21-10-13-25(24(33)16-21)41-26-14-15-34-29-28(26)23(17-35-29)19-6-4-3-5-7-19/h3-17H,1-2H3,(H,34,35)(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AXL expressed in Escherichia coli BL21 infected with T7 phage by qPCR method |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172078

(LY-2801653 | Merestinib)Show SMILES Cc1ccc(C(=O)Nc2ccc(Oc3cc4cnn(C)c4cc3-c3cn[nH]c3)c(F)c2)c(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C30H22F2N6O3/c1-17-3-9-23(30(40)38(17)22-7-4-20(31)5-8-22)29(39)36-21-6-10-27(25(32)12-21)41-28-11-18-16-35-37(2)26(18)13-24(28)19-14-33-34-15-19/h3-16H,1-2H3,(H,33,34)(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human full length AXL by scintillation counting method in presence of 33P-gammaATP |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 ITD mutant expressed in bacterial expression system using poly [Glu, Try] 4:1 as substrate in presence of [ga... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of AXL (unknown origin) |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455354

(CHEMBL4208852)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N8/c1-23-22-25-14-18-20(27-30(21(18)26-22)15-16-5-3-4-6-16)17-7-8-19(24-13-17)29-11-9-28(2)10-12-29/h7-8,13-14,16H,3-6,9-12,15H2,1-2H3,(H,23,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 D835Y mutant (Q580 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50208517
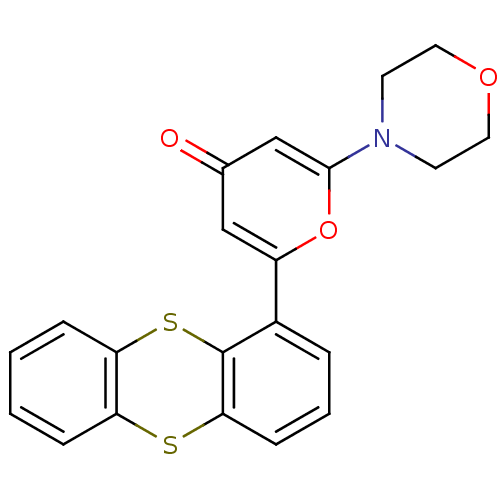
(2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...)Show SMILES O=c1cc(oc(c1)-c1cccc2Sc3ccccc3Sc12)N1CCOCC1 Show InChI InChI=1S/C21H17NO3S2/c23-14-12-16(25-20(13-14)22-8-10-24-11-9-22)15-4-3-7-19-21(15)27-18-6-2-1-5-17(18)26-19/h1-7,12-13H,8-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HeLa nuclear extract derived ATM using glutathioneS-transferase-p53N66 as substrate preincubated for 10 mins followed by ATP addi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50145038

(CHEMBL2143829)Show InChI InChI=1S/C17H15N7O2/c1-25-13-7-10-12(8-14(13)26-2)20-9-21-16(10)24-17(18)22-15(23-24)11-5-3-4-6-19-11/h3-9H,1-2H3,(H2,18,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length Flag-tagged ATM (unknown origin) using GST-p53(1 to 101 residues) as substrates incubated for 90 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length AXL (R497 to Y821 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as substr... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AXL by fluorescence polarization assay |
J Med Chem 59: 3593-608 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01273
BindingDB Entry DOI: 10.7270/Q24M96FS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384600
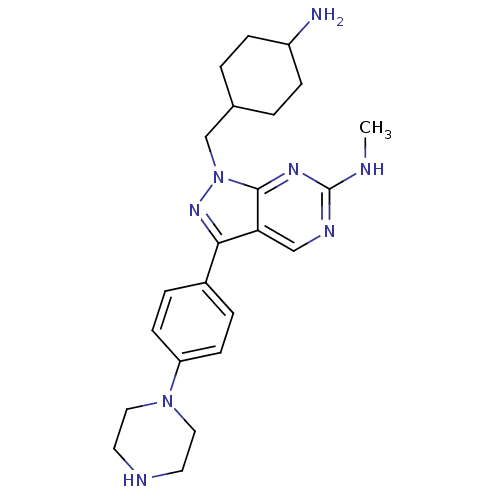
(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of AXL (unknown origin) |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM200300
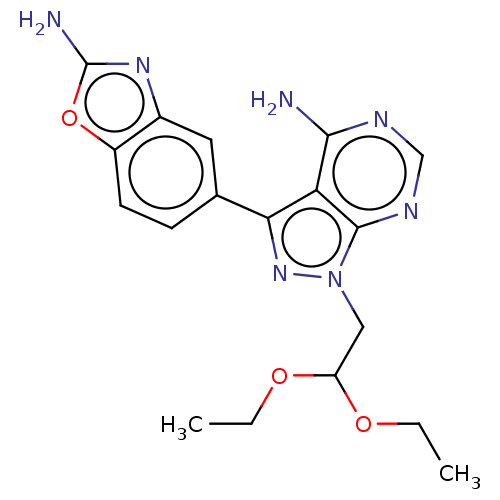
(5-[4-amino-1-(2,2-diethoxyethyl)pyrazolo[3,4-d]pyr...)Show SMILES CCOC(Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)ncnc12)OCC Show InChI InChI=1S/C18H21N7O3/c1-3-26-13(27-4-2)8-25-17-14(16(19)21-9-22-17)15(24-25)10-5-6-12-11(7-10)23-18(20)28-12/h5-7,9,13H,3-4,8H2,1-2H3,(H2,20,23)(H2,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115215
BindingDB Entry DOI: 10.7270/Q2SN0DGJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length MER (R557 to L884 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as substr... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length TYRO3 (S497 to T814 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subs... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data