

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50045936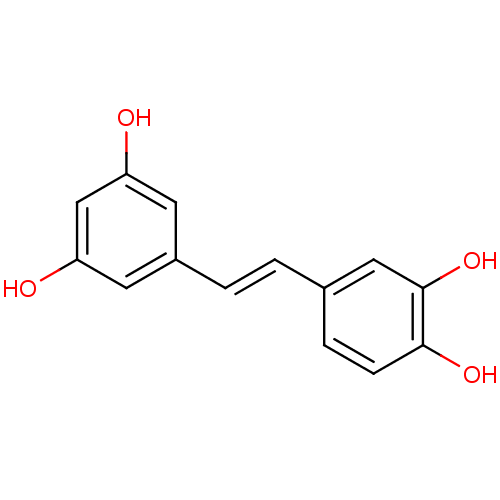 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GLO1 expressed in baculovirus infected sf21 cells assessed as reduction in S-D-lactoylglutathione formatio... | Bioorg Med Chem Lett 27: 1169-1174 (2017) Article DOI: 10.1016/j.bmcl.2017.01.070 BindingDB Entry DOI: 10.7270/Q29W0HQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569120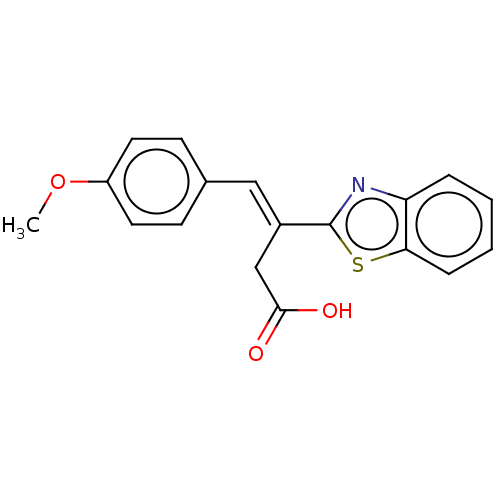 (CHEMBL4861582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569128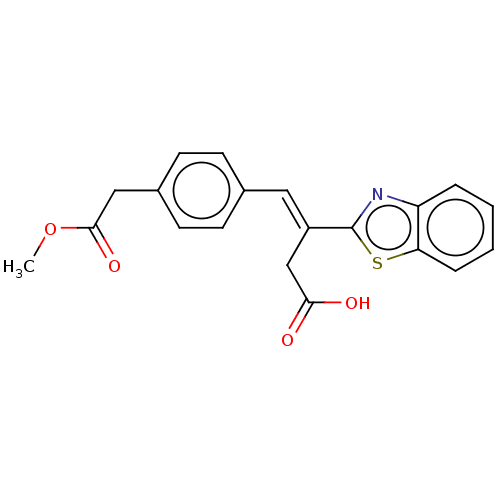 (CHEMBL4853249) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569133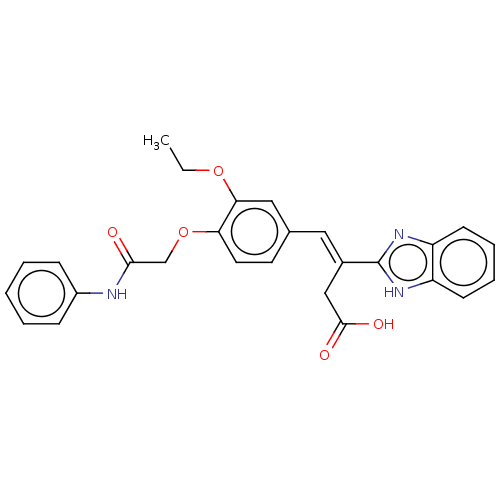 (CHEMBL4875229) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569134 (CHEMBL4863408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569131 (CHEMBL4852940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569129 (CHEMBL4851336) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569125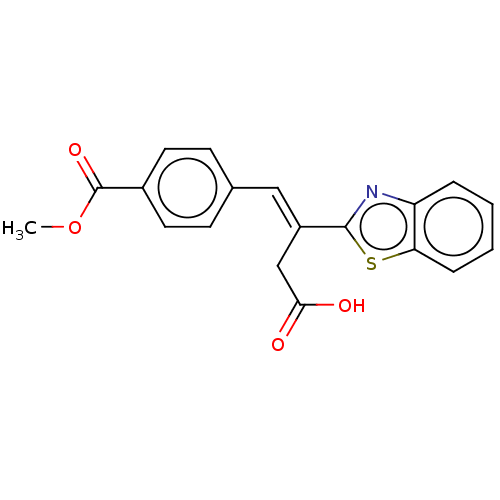 (CHEMBL4871220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569130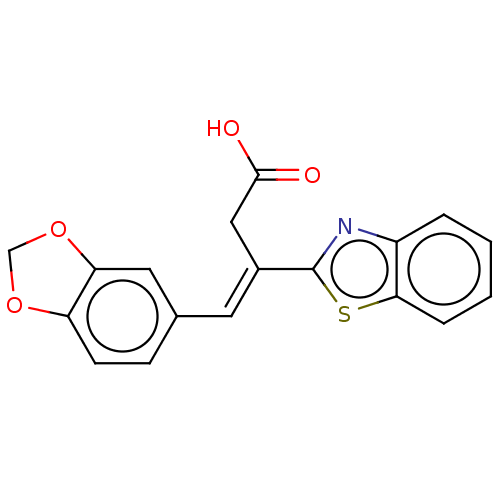 (CHEMBL1303934) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50145829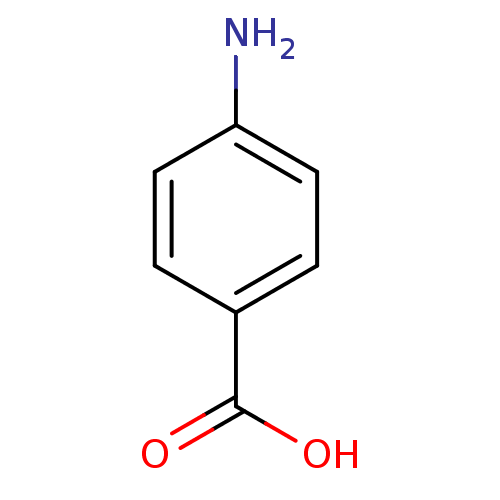 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569127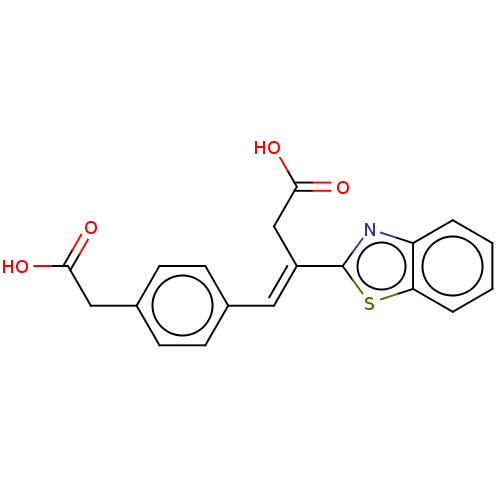 (CHEMBL4877301) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569121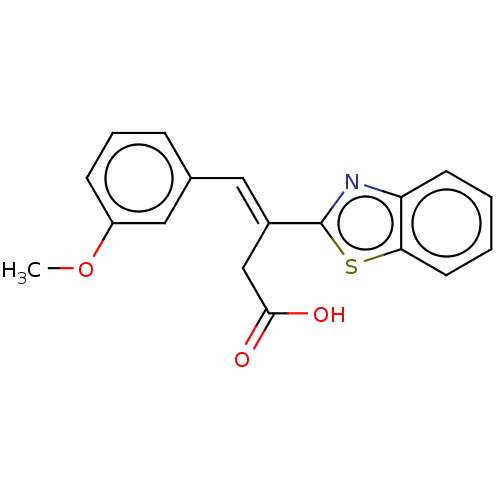 (CHEMBL1162436) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM54054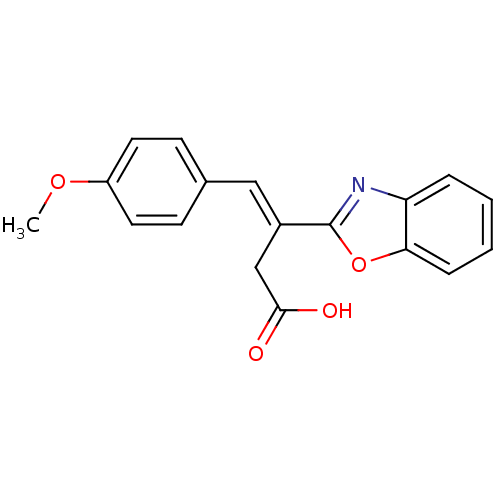 ((E)-3-(1,3-benzoxazol-2-yl)-4-(4-methoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569132 (CHEMBL4848220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569124 (CHEMBL4877073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50195793 (CHEMBL123234) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50195793 (CHEMBL123234) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569123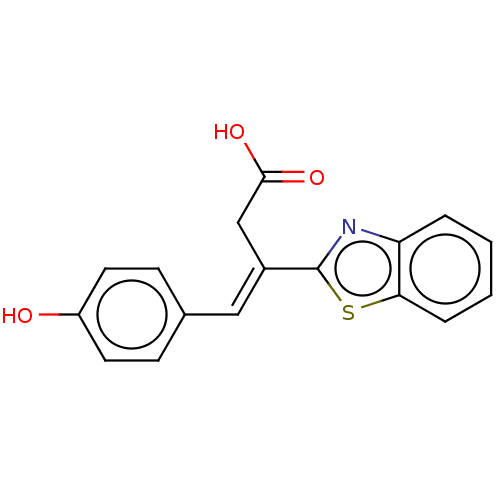 (CHEMBL4874418) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569126 (CHEMBL4849720) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50300847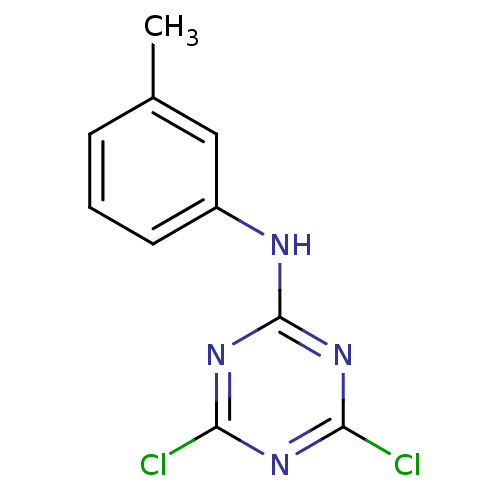 (4,6-dichloro-N-m-tolyl-1,3,5-triazin-2-amine | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of GST-fused human NFkappaB p50 subunit DNA binding activity assessed as shortened diffusion time by fluorescence correlation spectroscopy | Bioorg Med Chem 17: 5293-7 (2009) Article DOI: 10.1016/j.bmc.2009.05.030 BindingDB Entry DOI: 10.7270/Q2222TVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50300846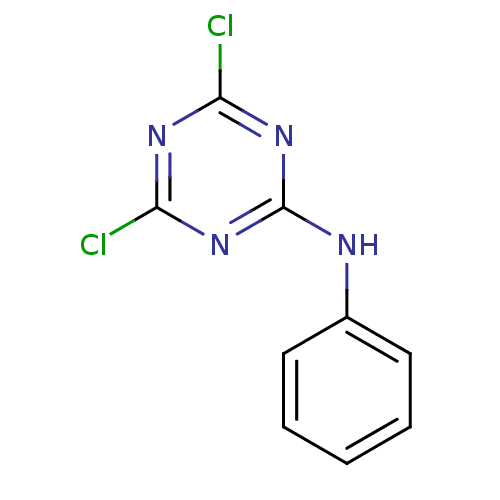 (4,6-dichloro-N-phenyl-1,3,5-triazin-2-amine | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of GST-fused human NFkappaB p50 subunit DNA binding activity assessed as shortened diffusion time by fluorescence correlation spectroscopy | Bioorg Med Chem 17: 5293-7 (2009) Article DOI: 10.1016/j.bmc.2009.05.030 BindingDB Entry DOI: 10.7270/Q2222TVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Homo sapiens (Human)) | BDBM50541116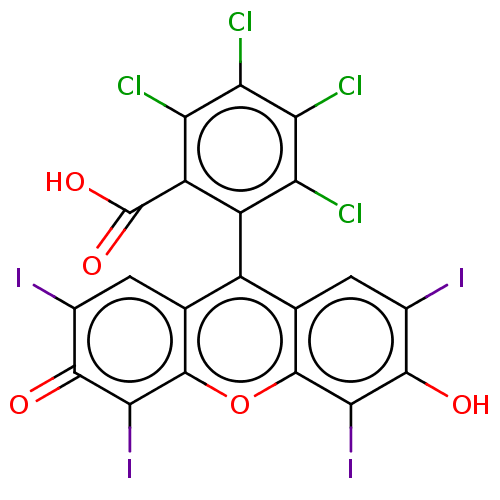 (CHEBI:87202 | Rose bengal) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human PARG assessed as mono(ADP-R) release using PAR as substrate measured after 15 mins by HPLC analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115249 BindingDB Entry DOI: 10.7270/Q2XK8K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50300851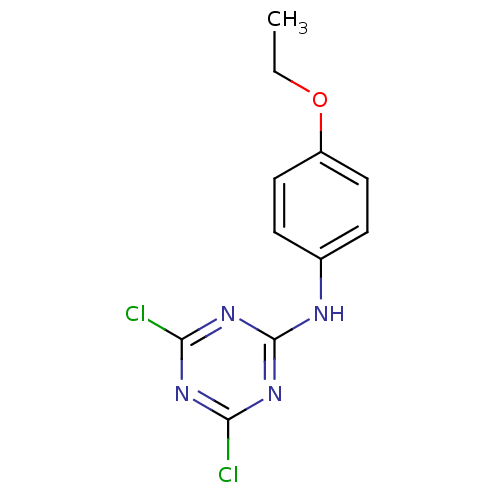 (4,6-dichloro-N-(4-ethoxyphenyl)-1,3,5-triazin-2-am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of GST-fused human NFkappaB p50 subunit DNA binding activity assessed as shortened diffusion time by fluorescence correlation spectroscopy | Bioorg Med Chem 17: 5293-7 (2009) Article DOI: 10.1016/j.bmc.2009.05.030 BindingDB Entry DOI: 10.7270/Q2222TVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50195793 (CHEMBL123234) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase diphenolase activity using L-DOPA as substrate measured every 30 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50195793 (CHEMBL123234) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase diphenolase activity using L-DOPA as substrate measured every 30 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569122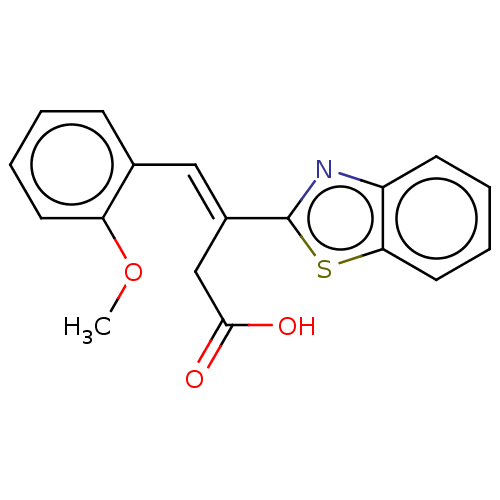 (CHEMBL4870472) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Homo sapiens (Human)) | BDBM50541115 (CHEMBL1254258) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human PARG assessed as mono(ADP-R) release using PAR as substrate measured after 15 mins by HPLC analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115249 BindingDB Entry DOI: 10.7270/Q2XK8K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50060971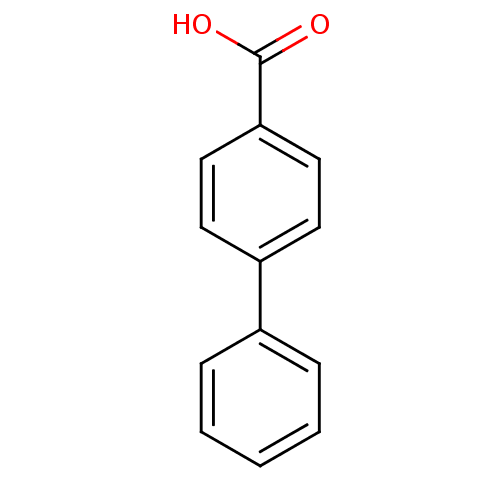 (Biphenyl-4-carboxylic acid | CHEMBL107057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50060971 (Biphenyl-4-carboxylic acid | CHEMBL107057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50300852 (4,6-dichloro-N-(4-isopropylphenyl)-1,3,5-triazin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of GST-fused human NFkappaB p50 subunit DNA binding activity assessed as shortened diffusion time by fluorescence correlation spectroscopy | Bioorg Med Chem 17: 5293-7 (2009) Article DOI: 10.1016/j.bmc.2009.05.030 BindingDB Entry DOI: 10.7270/Q2222TVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Homo sapiens (Human)) | BDBM50058291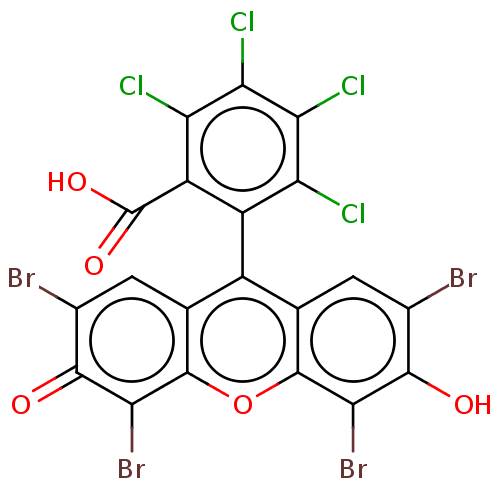 (CHEBI:87193 | CHEMBL1205178) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human PARG assessed as mono(ADP-R) release using PAR as substrate measured after 15 mins by HPLC analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115249 BindingDB Entry DOI: 10.7270/Q2XK8K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50300850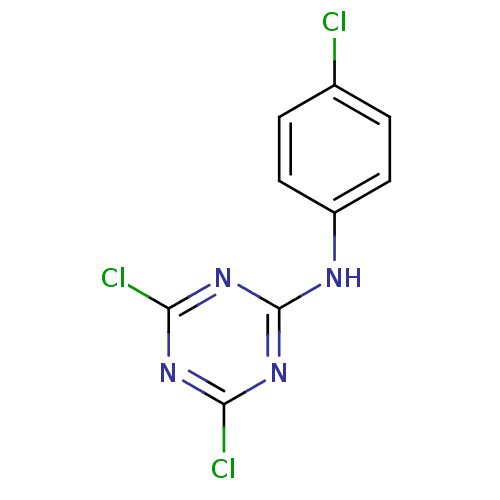 (4,6-dichloro-N-(4-chlorophenyl)-1,3,5-triazin-2-am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of GST-fused human NFkappaB p50 subunit DNA binding activity assessed as shortened diffusion time by fluorescence correlation spectroscopy | Bioorg Med Chem 17: 5293-7 (2009) Article DOI: 10.1016/j.bmc.2009.05.030 BindingDB Entry DOI: 10.7270/Q2222TVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50300848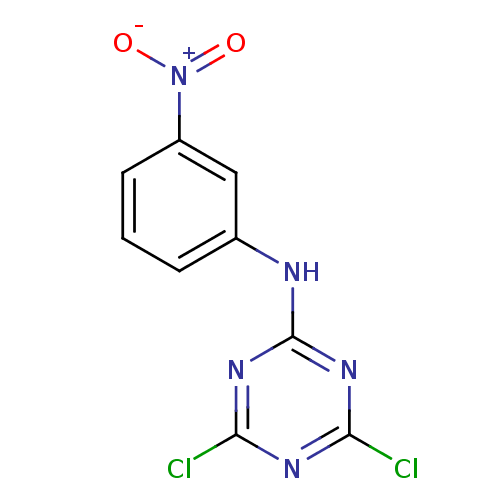 (4,6-dichloro-N-(3-nitrophenyl)-1,3,5-triazin-2-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of GST-fused human NFkappaB p50 subunit DNA binding activity assessed as shortened diffusion time by fluorescence correlation spectroscopy | Bioorg Med Chem 17: 5293-7 (2009) Article DOI: 10.1016/j.bmc.2009.05.030 BindingDB Entry DOI: 10.7270/Q2222TVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Homo sapiens (Human)) | BDBM50541114 (TETRAIDOFLUORESCEIN) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human PARG assessed as mono(ADP-R) release using PAR as substrate measured after 15 mins by HPLC analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115249 BindingDB Entry DOI: 10.7270/Q2XK8K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50060971 (Biphenyl-4-carboxylic acid | CHEMBL107057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase diphenolase activity using L-DOPA as substrate measured every 30 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50060971 (Biphenyl-4-carboxylic acid | CHEMBL107057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase diphenolase activity using L-DOPA as substrate measured every 30 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM197302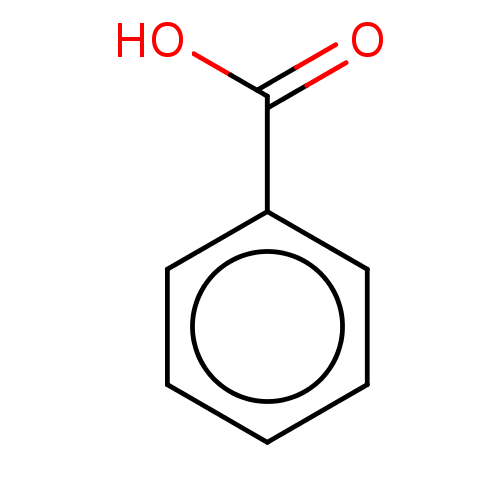 (Benzoic acid | SAMPL4, O1) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM197302 (Benzoic acid | SAMPL4, O1) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50300849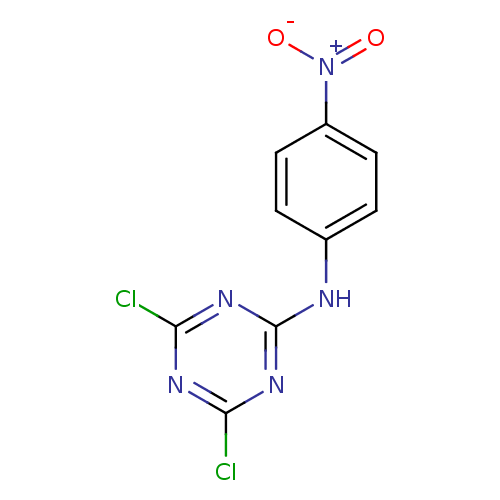 (4,6-dichloro-N-(4-nitrophenyl)-1,3,5-triazin-2-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima Bunri University Curated by ChEMBL | Assay Description Inhibition of GST-fused human NFkappaB p50 subunit DNA binding activity assessed as shortened diffusion time by fluorescence correlation spectroscopy | Bioorg Med Chem 17: 5293-7 (2009) Article DOI: 10.1016/j.bmc.2009.05.030 BindingDB Entry DOI: 10.7270/Q2222TVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly(ADP-ribose) glycohydrolase (Homo sapiens (Human)) | BDBM50206441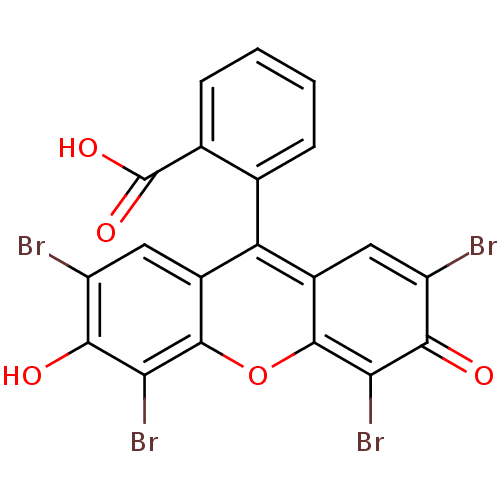 (2-(2,4,5,7-tetrabromo-3-hydroxy-6-oxo-6H-xanthen-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human PARG assessed as mono(ADP-R) release using PAR as substrate measured after 15 mins by HPLC analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115249 BindingDB Entry DOI: 10.7270/Q2XK8K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM243072 (Anisic acid) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM243072 (Anisic acid) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM197302 (Benzoic acid | SAMPL4, O1) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 mins followed by substrate addition measured for 2 mins by spectrophot... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM197302 (Benzoic acid | SAMPL4, O1) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 mins followed by substrate addition measured for 2 mins by spectrophot... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM197302 (Benzoic acid | SAMPL4, O1) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase diphenolase activity using L-DOPA as substrate measured every 30 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM197302 (Benzoic acid | SAMPL4, O1) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase diphenolase activity using L-DOPA as substrate measured every 30 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50533338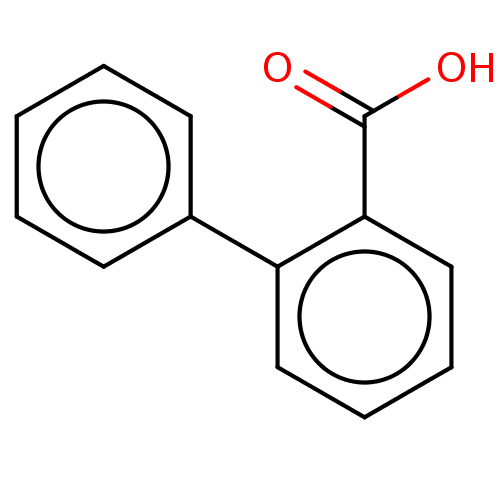 (CHEMBL3092388) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50533338 (CHEMBL3092388) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase diphenolase activity using L-DOPA as substrate measured every 30 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50533338 (CHEMBL3092388) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase diphenolase activity using L-DOPA as substrate measured every 30 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |