

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531908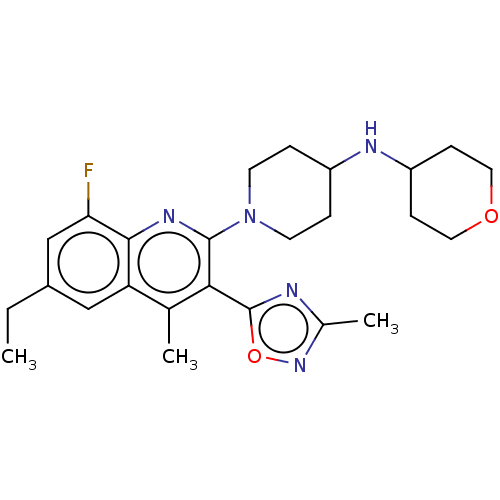 (Btrx-335140 | CYM-53093 | US10676469, Compound 142...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human KOR | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50531908 (Btrx-335140 | CYM-53093 | US10676469, Compound 142...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to human MOR | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185681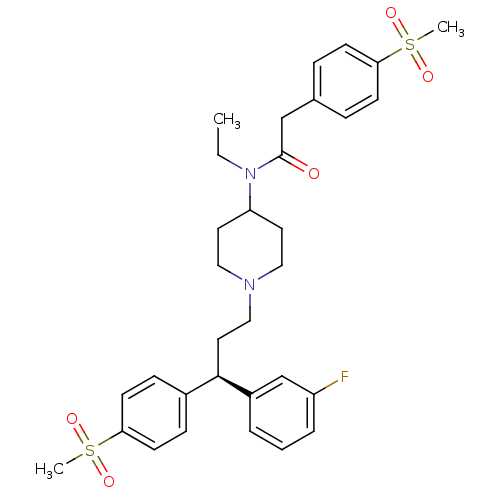 ((R)-N-ethyl-N-(1-(3-(3-fluorophenyl)-3-(4-(methyls...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531921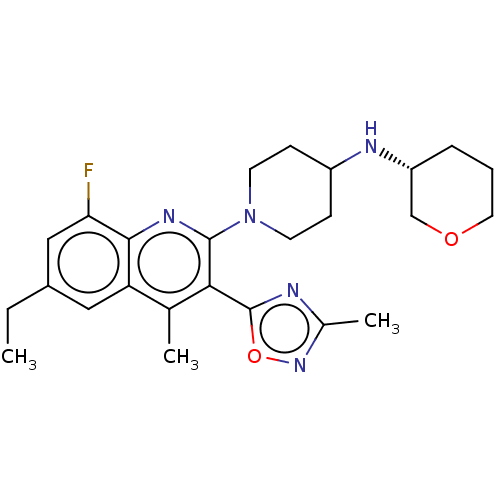 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185666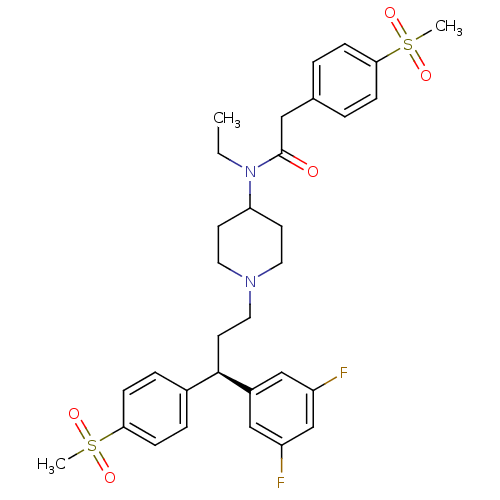 ((R)-N-(1-(3-(3,5-difluorophenyl)-3-(4-(methylsulfo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531915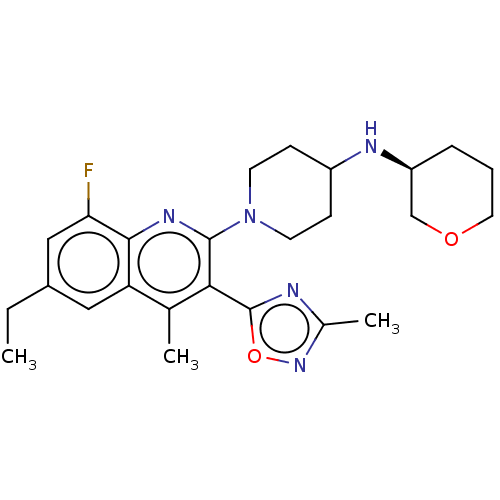 (CHEMBL4440683 | US10676469, Compound 144 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531910 (CHEMBL4576339) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185673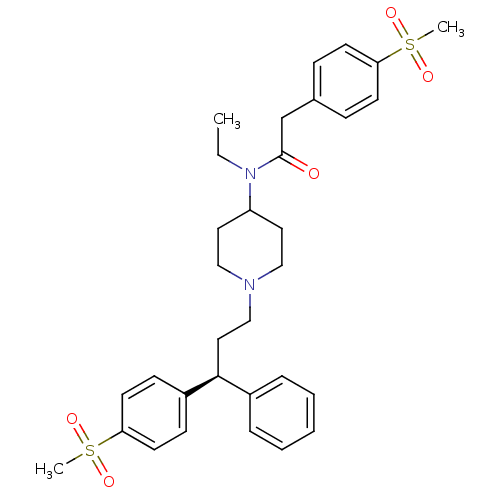 ((S)-N-ethyl-2-(4-(methylsulfonyl)phenyl)-N-(1-(3-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531909 (CHEMBL4523014) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531911 (CHEMBL4459625) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531916 (CHEMBL4456582 | US10676469, Compound 145 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531908 (Btrx-335140 | CYM-53093 | US10676469, Compound 142...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531919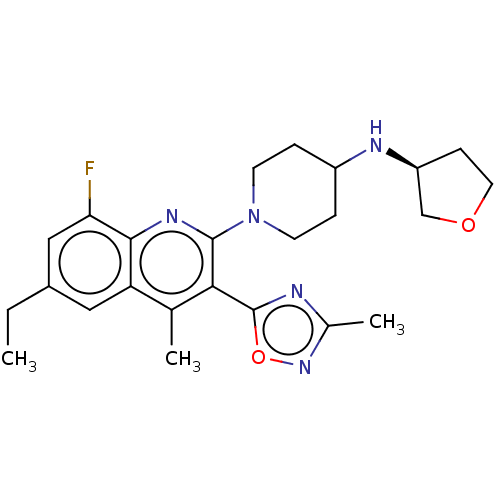 (CHEMBL4549826 | US10676469, Compound 146 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50531908 (Btrx-335140 | CYM-53093 | US10676469, Compound 142...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at 10 uM by Qpatch clamp assay | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185670 ((R)-N-(1-(3-(3-chlorophenyl)-3-(4-(methylsulfonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185667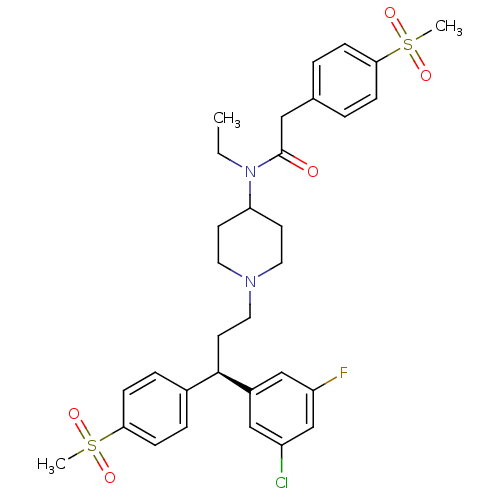 ((R)-N-(1-(3-(3-chloro-5-fluorophenyl)-3-(4-(methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531907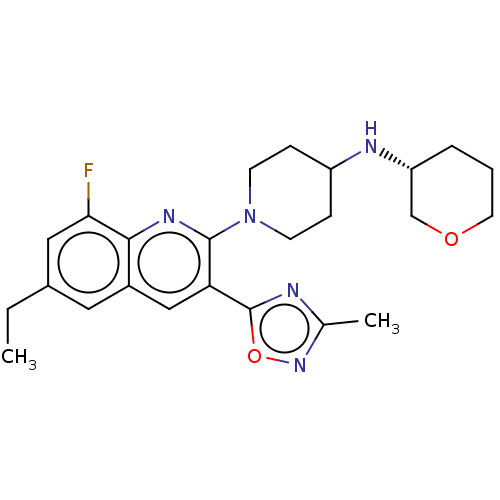 (CHEMBL4583245 | US10676469, Compound 141 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM156575 (US10118915, Compound 355 | US9682966, 355) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM156541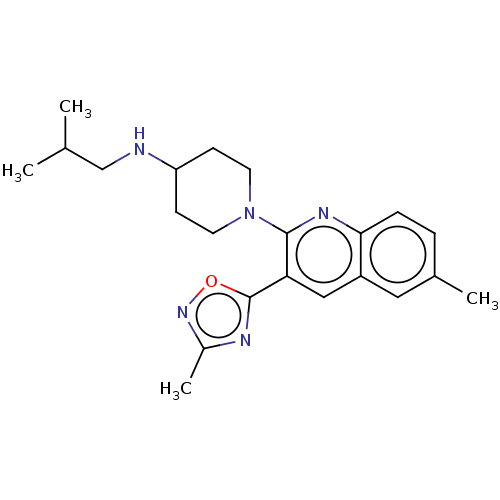 (US10118915, Compound 324 | US9682966, 324) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM159083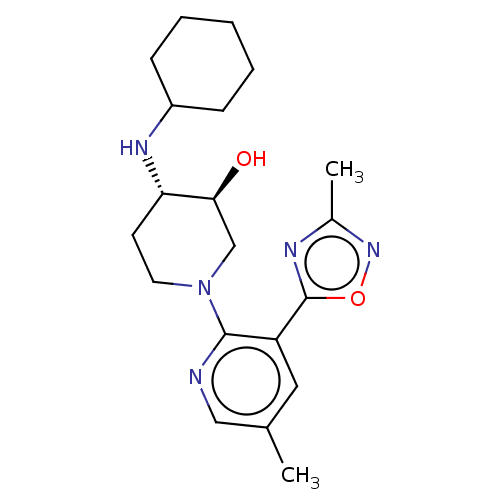 (US10118915, Compound 542 | US9682966, 542) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531912 (CHEMBL4572131) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531904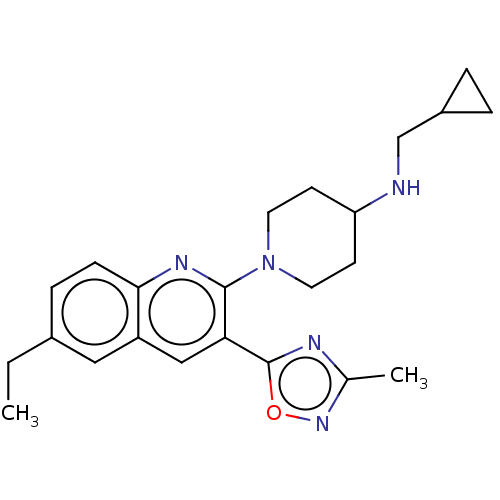 (CHEMBL4540091) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50173389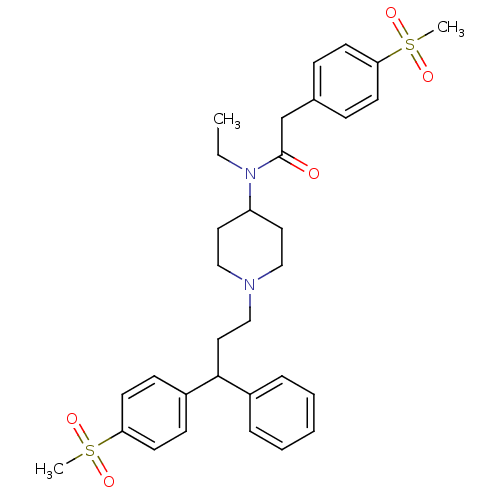 (CHEMBL198150 | N-Ethyl-2-(4-methanesulfonyl-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50029670 (CHEMBL2426277 | US10227342, Example 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531906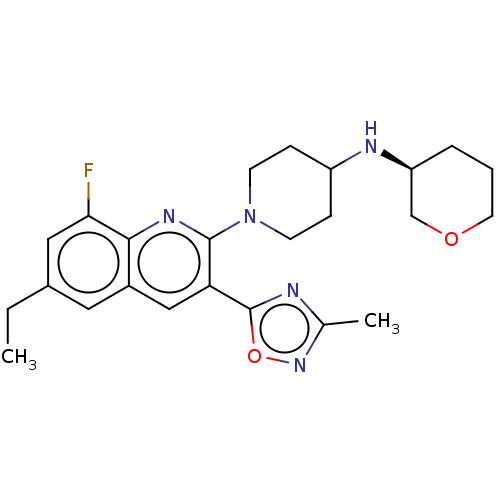 (CHEMBL4443303 | US11124504, Cpd. No. 253) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM157256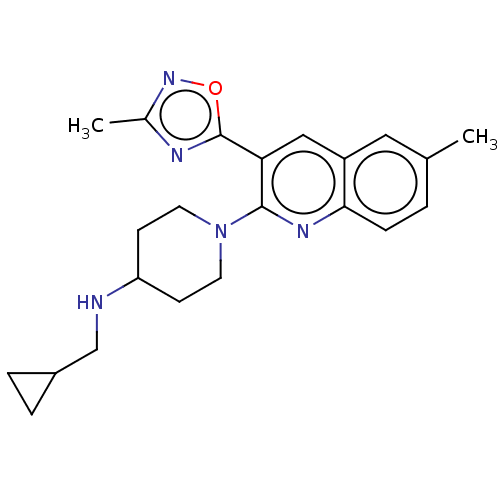 (US10118915, Compound 433 | US9682966, 433) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531905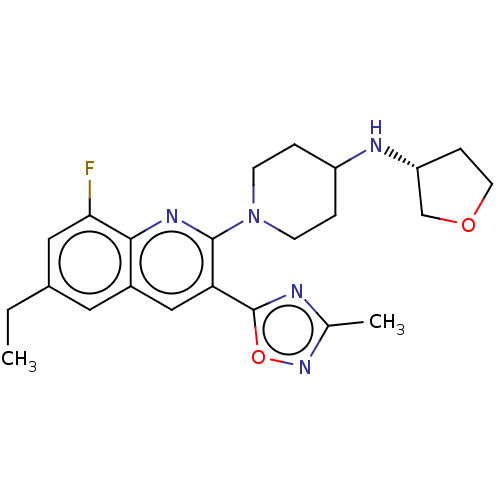 (CHEMBL4566736 | US10676469, Compound 251 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531914 (CHEMBL4525666 | US10676469, Compound 252 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM156439 (US10118915, Compound 233 | US9682966, 233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531913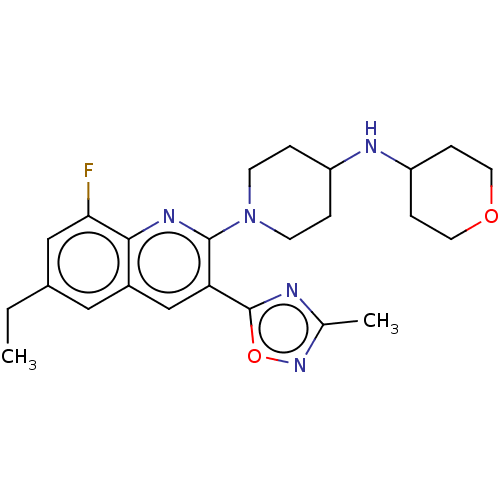 (CHEMBL4584410 | US10676469, Compound 253 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50029667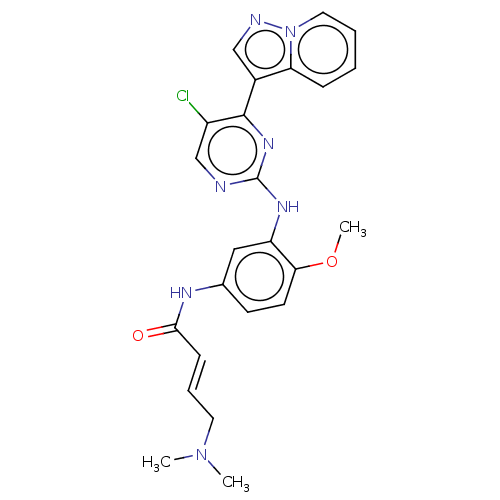 (CHEMBL2426288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531920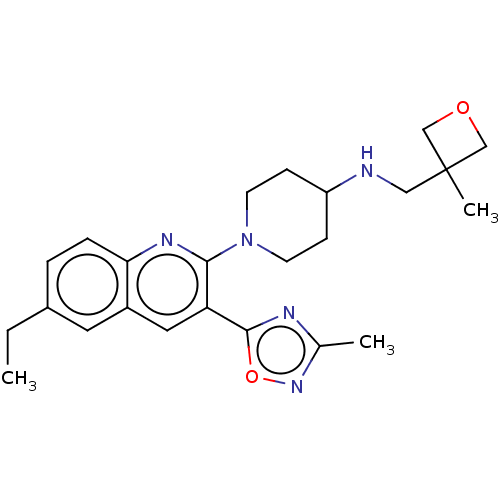 (CHEMBL4434885 | US10676469, Compound A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50029685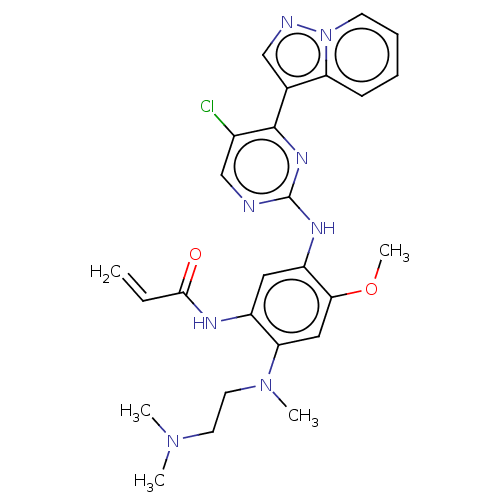 (CHEMBL3353404 | US10227342, Example 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM156498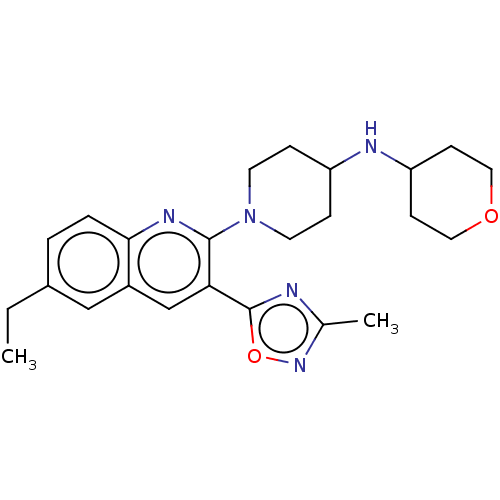 (US10118915, Compound 285 | US9682966, 285) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50029684 (CHEMBL3353403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50029685 (CHEMBL3353404 | US10227342, Example 52) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of INSR (unknown origin) | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531917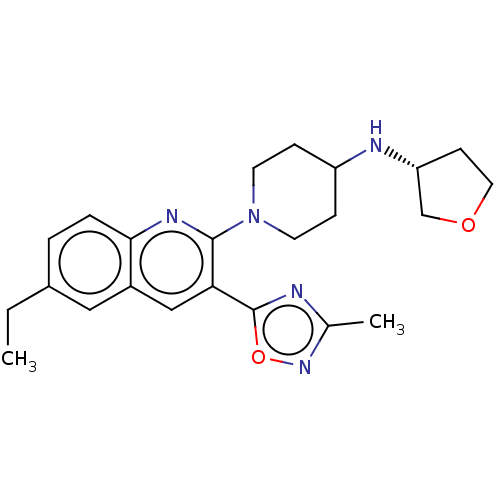 (CHEMBL4441250) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase methylesterase 1 (Homo sapiens (Human)) | BDBM75707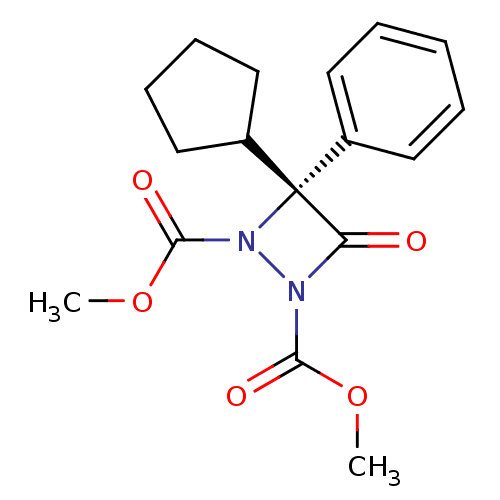 ((3R)-3-cyclopentyl-4-keto-3-phenyl-diazetidine-1,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of PME1 binding to FP-rhodamine in human MDA-MB-231 cells after 30 mins by fluorescence polarization activity-based protein profiling assa... | J Med Chem 54: 5229-36 (2011) Article DOI: 10.1021/jm200502u BindingDB Entry DOI: 10.7270/Q2GX4CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531918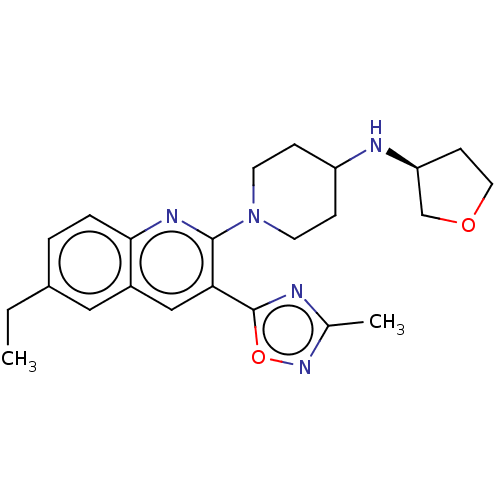 (CHEMBL4444292) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012149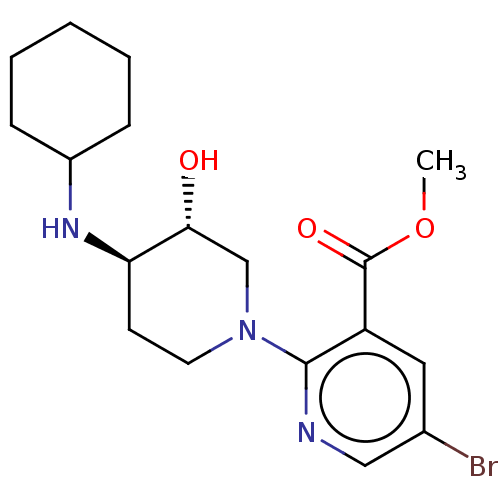 (CHEMBL3264446 | US10118915, Compound 105 | US96829...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029669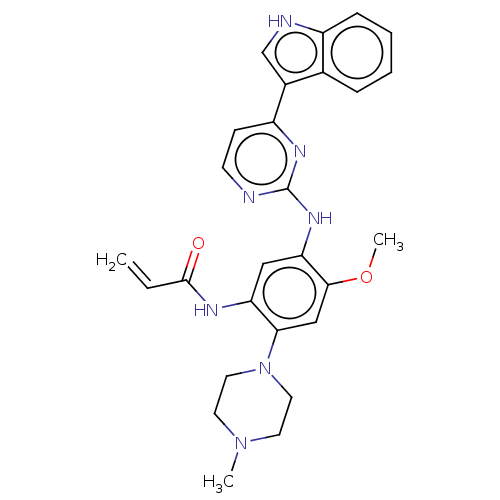 (CHEMBL2426279) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029670 (CHEMBL2426277 | US10227342, Example 26) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185668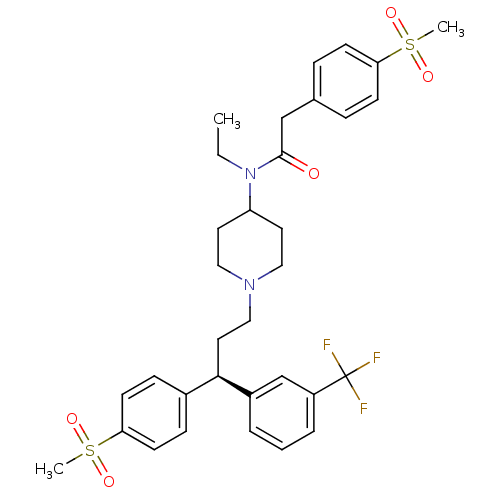 ((R)-N-ethyl-2-(4-(methylsulfonyl)phenyl)-N-(1-(3-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185676 ((R)-N-(1-(3-(2,3-difluorophenyl)-3-(4-(methylsulfo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185672 ((R)-N-(1-(3-(3,4-difluorophenyl)-3-(4-(methylsulfo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50029684 (CHEMBL3353403) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of INSR (unknown origin) | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185669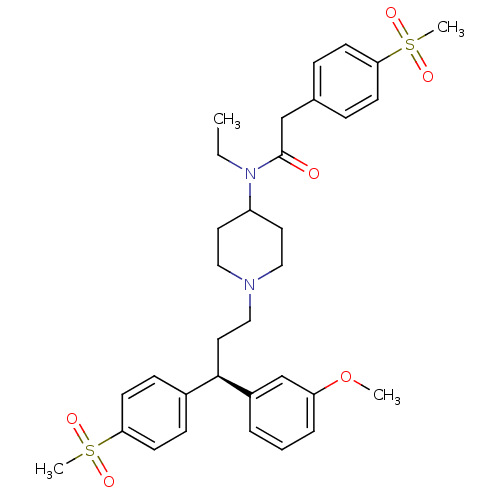 ((R)-N-ethyl-N-(1-(3-(3-methoxyphenyl)-3-(4-(methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50185674 ((R)-N-ethyl-2-(4-(methylsulfonyl)phenyl)-N-(1-(3-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1alpha to human recombinant CCR5 expressed in CHO cells | Bioorg Med Chem Lett 16: 3533-6 (2006) Article DOI: 10.1016/j.bmcl.2006.03.089 BindingDB Entry DOI: 10.7270/Q24B30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50029686 (CHEMBL3353405 | US10227342, Example 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) | J Med Chem 57: 8249-67 (2014) Article DOI: 10.1021/jm500973a BindingDB Entry DOI: 10.7270/Q2ZS2Z4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM159083 (US10118915, Compound 542 | US9682966, 542) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused MOR (unknown origin) expressed in human U2OS cells assessed as inhibition of DAMGO-induced beta-arrestin migra... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 247 total ) | Next | Last >> |