 Found 124 hits with Last Name = 'gallion' and Initial = 'sl'
Found 124 hits with Last Name = 'gallion' and Initial = 'sl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065147
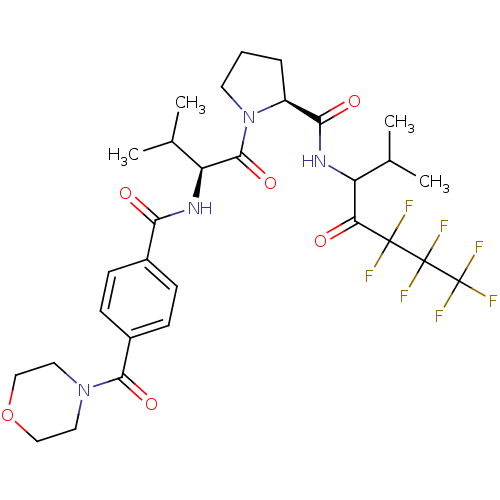
((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)C(F)(F)F Show InChI InChI=1S/C30H37F7N4O6/c1-16(2)21(23(42)28(31,32)29(33,34)30(35,36)37)38-25(44)20-6-5-11-41(20)27(46)22(17(3)4)39-24(43)18-7-9-19(10-8-18)26(45)40-12-14-47-15-13-40/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,38,44)(H,39,43)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065158
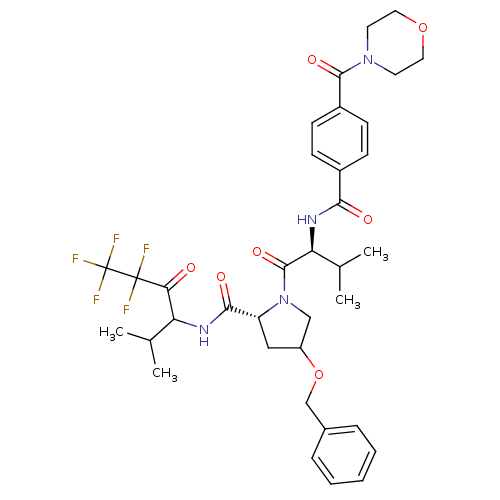
((R)-4-Benzyloxy-1-{(S)-3-methyl-2-[4-(morpholine-4...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CC(C[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F)OCc1ccccc1 Show InChI InChI=1S/C36H43F5N4O7/c1-21(2)28(30(46)35(37,38)36(39,40)41)42-32(48)27-18-26(52-20-23-8-6-5-7-9-23)19-45(27)34(50)29(22(3)4)43-31(47)24-10-12-25(13-11-24)33(49)44-14-16-51-17-15-44/h5-13,21-22,26-29H,14-20H2,1-4H3,(H,42,48)(H,43,47)/t26?,27-,28?,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035495
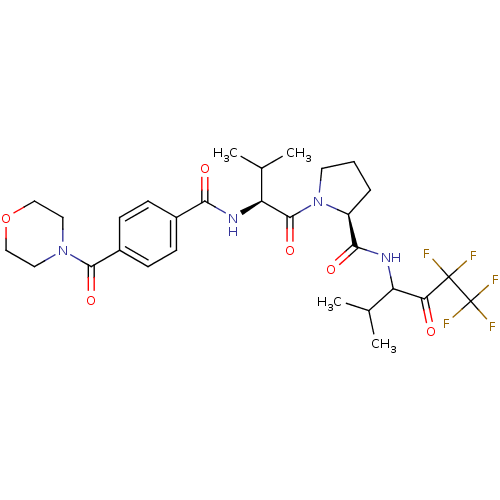
((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C29H37F5N4O6/c1-16(2)21(23(39)28(30,31)29(32,33)34)35-25(41)20-6-5-11-38(20)27(43)22(17(3)4)36-24(40)18-7-9-19(10-8-18)26(42)37-12-14-44-15-13-37/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,35,41)(H,36,40)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065157
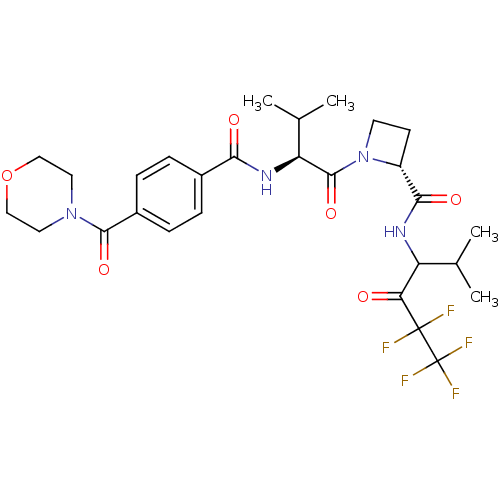
((R)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CC[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C28H35F5N4O6/c1-15(2)20(22(38)27(29,30)28(31,32)33)34-24(40)19-9-10-37(19)26(42)21(16(3)4)35-23(39)17-5-7-18(8-6-17)25(41)36-11-13-43-14-12-36/h5-8,15-16,19-21H,9-14H2,1-4H3,(H,34,40)(H,35,39)/t19-,20?,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065161
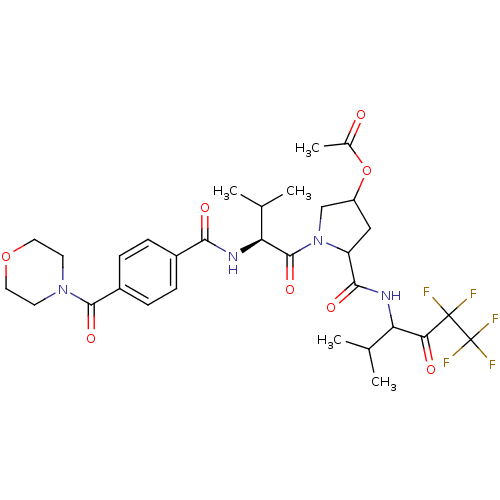
(Acetic acid 1-{(S)-3-methyl-2-[4-(morpholine-4-car...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CC(CC1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F)OC(C)=O Show InChI InChI=1S/C31H39F5N4O8/c1-16(2)23(25(42)30(32,33)31(34,35)36)37-27(44)22-14-21(48-18(5)41)15-40(22)29(46)24(17(3)4)38-26(43)19-6-8-20(9-7-19)28(45)39-10-12-47-13-11-39/h6-9,16-17,21-24H,10-15H2,1-5H3,(H,37,44)(H,38,43)/t21?,22?,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065163
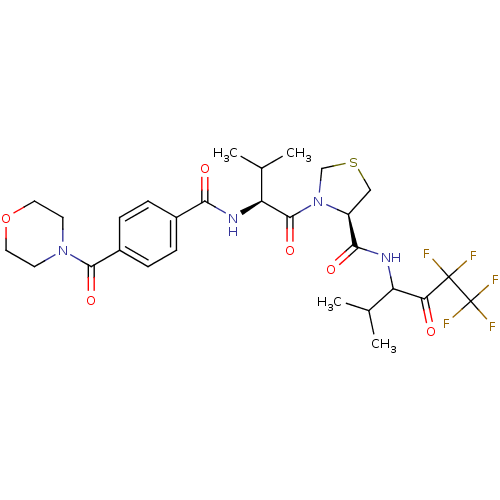
((R)-3-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CSC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C28H35F5N4O6S/c1-15(2)20(22(38)27(29,30)28(31,32)33)34-24(40)19-13-44-14-37(19)26(42)21(16(3)4)35-23(39)17-5-7-18(8-6-17)25(41)36-9-11-43-12-10-36/h5-8,15-16,19-21H,9-14H2,1-4H3,(H,34,40)(H,35,39)/t19-,20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065155
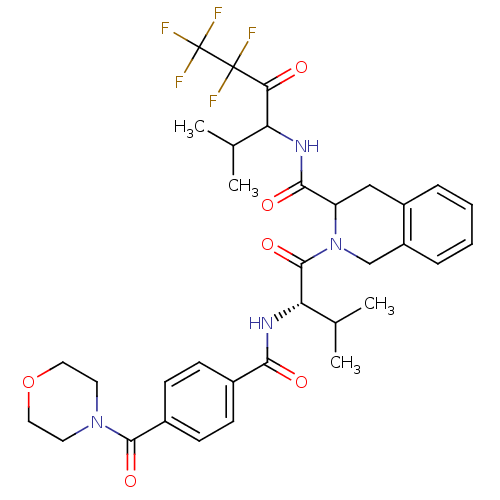
(2-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1Cc2ccccc2CC1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C34H39F5N4O6/c1-19(2)26(28(44)33(35,36)34(37,38)39)40-30(46)25-17-23-7-5-6-8-24(23)18-43(25)32(48)27(20(3)4)41-29(45)21-9-11-22(12-10-21)31(47)42-13-15-49-16-14-42/h5-12,19-20,25-27H,13-18H2,1-4H3,(H,40,46)(H,41,45)/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282635
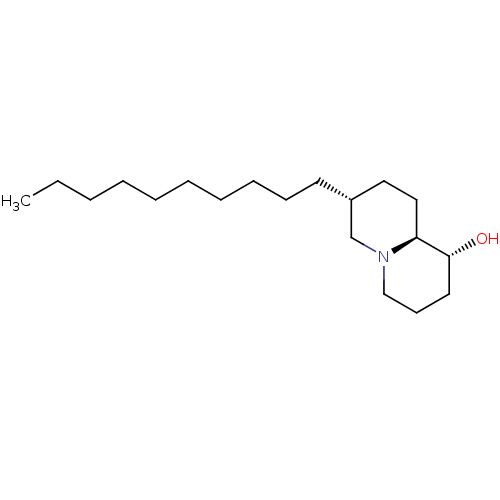
((1R,7R,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065154
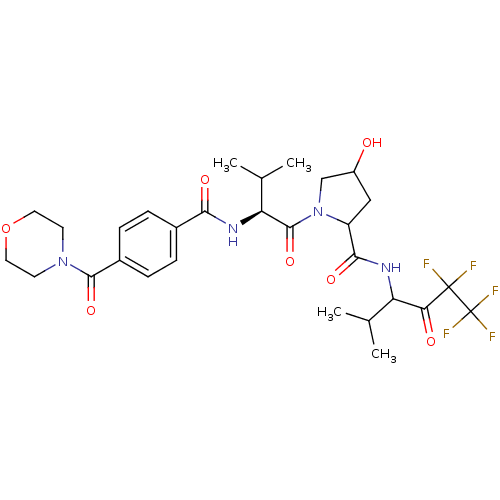
(4-Hydroxy-1-{(S)-3-methyl-2-[4-(morpholine-4-carbo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CC(O)CC1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C29H37F5N4O7/c1-15(2)21(23(40)28(30,31)29(32,33)34)35-25(42)20-13-19(39)14-38(20)27(44)22(16(3)4)36-24(41)17-5-7-18(8-6-17)26(43)37-9-11-45-12-10-37/h5-8,15-16,19-22,39H,9-14H2,1-4H3,(H,35,42)(H,36,41)/t19?,20?,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065146
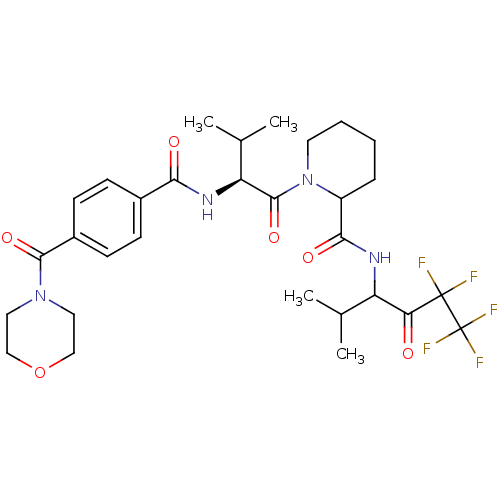
(1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCCCC1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C30H39F5N4O6/c1-17(2)22(24(40)29(31,32)30(33,34)35)36-26(42)21-7-5-6-12-39(21)28(44)23(18(3)4)37-25(41)19-8-10-20(11-9-19)27(43)38-13-15-45-16-14-38/h8-11,17-18,21-23H,5-7,12-16H2,1-4H3,(H,36,42)(H,37,41)/t21?,22?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065164

((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(C)(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C30H39F5N4O6/c1-17(2)21(23(40)29(31,32)30(33,34)35)36-25(42)20-7-6-12-39(20)27(44)22(28(3,4)5)37-24(41)18-8-10-19(11-9-18)26(43)38-13-15-45-16-14-38/h8-11,17,20-22H,6-7,12-16H2,1-5H3,(H,36,42)(H,37,41)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065156
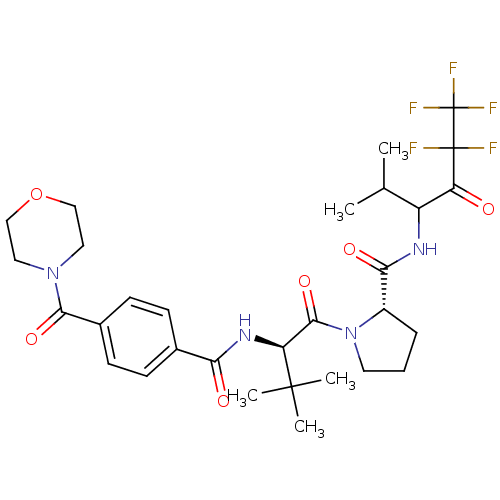
((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...)Show SMILES CC(C)C(NC(=O)[C@@H]1CCCN1C(=O)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(C)(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C30H39F5N4O6/c1-17(2)21(23(40)29(31,32)30(33,34)35)36-25(42)20-7-6-12-39(20)27(44)22(28(3,4)5)37-24(41)18-8-10-19(11-9-18)26(43)38-13-15-45-16-14-38/h8-11,17,20-22H,6-7,12-16H2,1-5H3,(H,36,42)(H,37,41)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282634
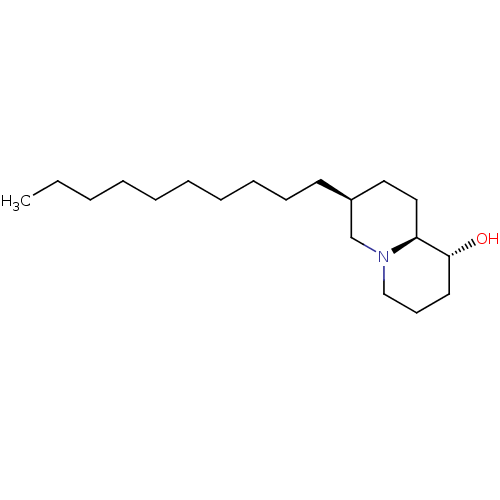
((1R,7S,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065162
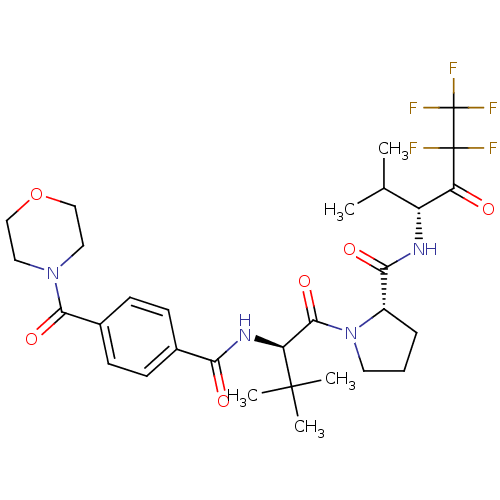
((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(C)(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C30H39F5N4O6/c1-17(2)21(23(40)29(31,32)30(33,34)35)36-25(42)20-7-6-12-39(20)27(44)22(28(3,4)5)37-24(41)18-8-10-19(11-9-18)26(43)38-13-15-45-16-14-38/h8-11,17,20-22H,6-7,12-16H2,1-5H3,(H,36,42)(H,37,41)/t20-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065153
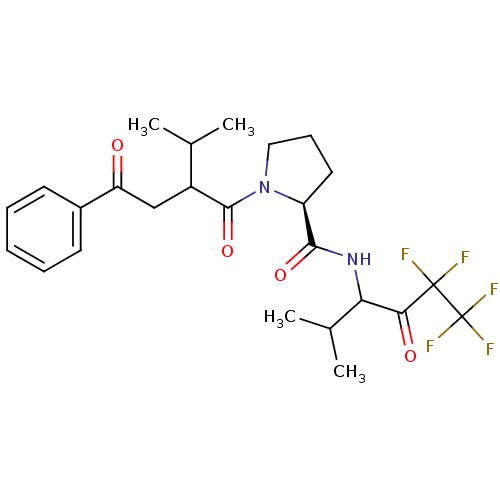
((S)-1-[3-Methyl-2-(2-oxo-2-phenyl-ethyl)-butyryl]-...)Show SMILES CC(C)C(CC(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C25H31F5N2O4/c1-14(2)17(13-19(33)16-9-6-5-7-10-16)23(36)32-12-8-11-18(32)22(35)31-20(15(3)4)21(34)24(26,27)25(28,29)30/h5-7,9-10,14-15,17-18,20H,8,11-13H2,1-4H3,(H,31,35)/t17?,18-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065151
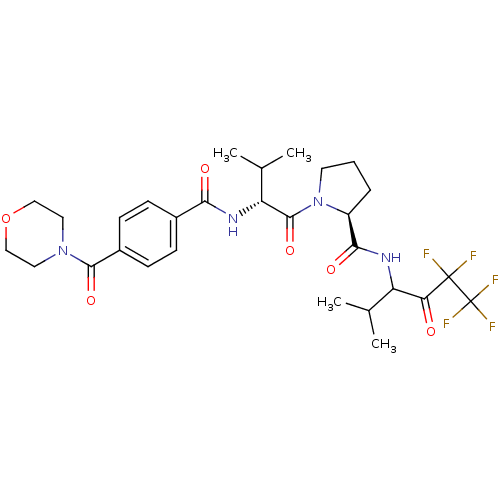
((S)-1-{(R)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C29H37F5N4O6/c1-16(2)21(23(39)28(30,31)29(32,33)34)35-25(41)20-6-5-11-38(20)27(43)22(17(3)4)36-24(40)18-7-9-19(10-8-18)26(42)37-12-14-44-15-13-37/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,35,41)(H,36,40)/t20-,21?,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065150
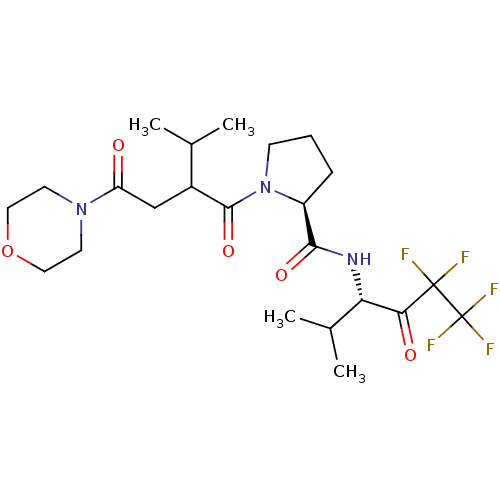
((S)-1-[3-Methyl-2-(2-morpholin-4-yl-2-oxo-ethyl)-b...)Show SMILES CC(C)C(CC(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C23H34F5N3O5/c1-13(2)15(12-17(32)30-8-10-36-11-9-30)21(35)31-7-5-6-16(31)20(34)29-18(14(3)4)19(33)22(24,25)23(26,27)28/h13-16,18H,5-12H2,1-4H3,(H,29,34)/t15?,16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065148

(4-(Morpholine-4-carbonyl)-N-{(S)-2-oxo-1-[(S)-1-(3...)Show SMILES CC(C)C(NC(=O)[C@H](C)N1CC[C@H](NC(=O)c2ccc(cc2)C(=O)N2CCOCC2)C1=O)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C26H31F5N4O6/c1-14(2)19(20(36)25(27,28)26(29,30)31)33-21(37)15(3)35-9-8-18(24(35)40)32-22(38)16-4-6-17(7-5-16)23(39)34-10-12-41-13-11-34/h4-7,14-15,18-19H,8-13H2,1-3H3,(H,32,38)(H,33,37)/t15-,18-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065159
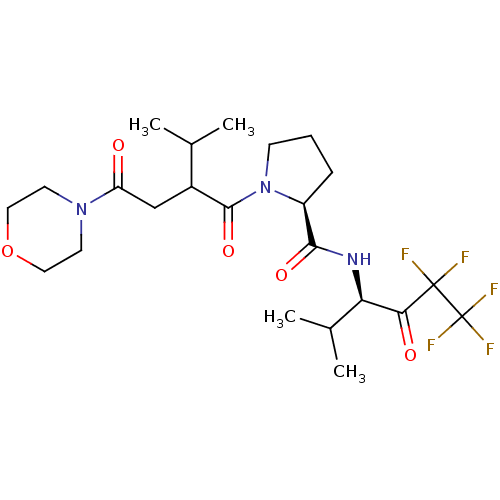
((S)-1-[3-Methyl-2-(2-morpholin-4-yl-2-oxo-ethyl)-b...)Show SMILES CC(C)C(CC(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C23H34F5N3O5/c1-13(2)15(12-17(32)30-8-10-36-11-9-30)21(35)31-7-5-6-16(31)20(34)29-18(14(3)4)19(33)22(24,25)23(26,27)28/h13-16,18H,5-12H2,1-4H3,(H,29,34)/t15?,16-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065160

(4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(3,3,4,4...)Show SMILES CC(C)C(NC(=O)CN1CCC[C@@H](NC(=O)c2ccc(cc2)C(=O)N2CCOCC2)C1=O)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C26H31F5N4O6/c1-15(2)20(21(37)25(27,28)26(29,30)31)33-19(36)14-35-9-3-4-18(24(35)40)32-22(38)16-5-7-17(8-6-16)23(39)34-10-12-41-13-11-34/h5-8,15,18,20H,3-4,9-14H2,1-2H3,(H,32,38)(H,33,36)/t18-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065149

(4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(3,3,4,4...)Show SMILES CC(C)C(NC(=O)CN1CC[C@@H](NC(=O)c2ccc(cc2)C(=O)N2CCOCC2)C1=O)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C25H29F5N4O6/c1-14(2)19(20(36)24(26,27)25(28,29)30)32-18(35)13-34-8-7-17(23(34)39)31-21(37)15-3-5-16(6-4-15)22(38)33-9-11-40-12-10-33/h3-6,14,17,19H,7-13H2,1-2H3,(H,31,37)(H,32,35)/t17-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50065152
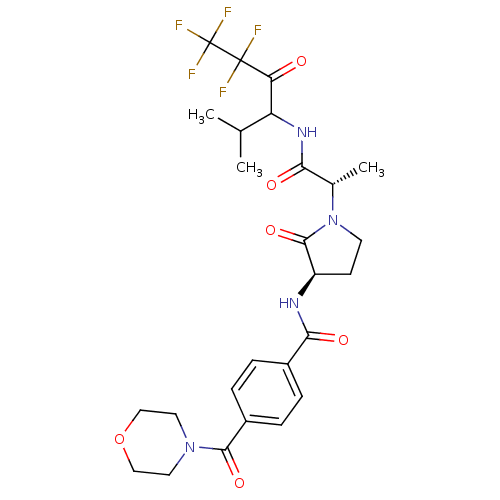
(4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(S)-1-(3...)Show SMILES CC(C)C(NC(=O)[C@H](C)N1CC[C@@H](NC(=O)c2ccc(cc2)C(=O)N2CCOCC2)C1=O)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C26H31F5N4O6/c1-14(2)19(20(36)25(27,28)26(29,30)31)33-21(37)15(3)35-9-8-18(24(35)40)32-22(38)16-4-6-17(7-5-16)23(39)34-10-12-41-13-11-34/h4-7,14-15,18-19H,8-13H2,1-3H3,(H,32,38)(H,33,37)/t15-,18+,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. |
J Med Chem 41: 2461-80 (1998)
Article DOI: 10.1021/jm970812e
BindingDB Entry DOI: 10.7270/Q23X85S8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36516
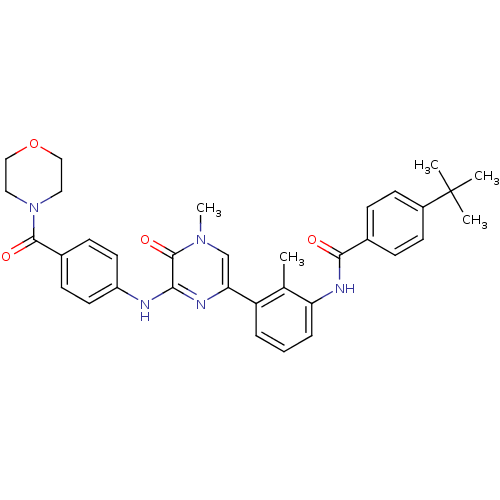
(4-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C34H37N5O4/c1-22-27(7-6-8-28(22)37-31(40)23-9-13-25(14-10-23)34(2,3)4)29-21-38(5)33(42)30(36-29)35-26-15-11-24(12-16-26)32(41)39-17-19-43-20-18-39/h6-16,21H,17-20H2,1-5H3,(H,35,36)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | 1.5 | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312155

(1-(2-methoxy-5-(trifluoromethyl)phenyl)-3-(3-(8-(p...)Show SMILES COc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O2/c1-39-23-6-5-19(27(28,29)30)14-21(23)36-26(38)34-20-4-2-3-18(13-20)22-16-37-12-11-32-25(37)24(35-22)33-15-17-7-9-31-10-8-17/h2-14,16H,15H2,1H3,(H,33,35)(H2,34,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312157
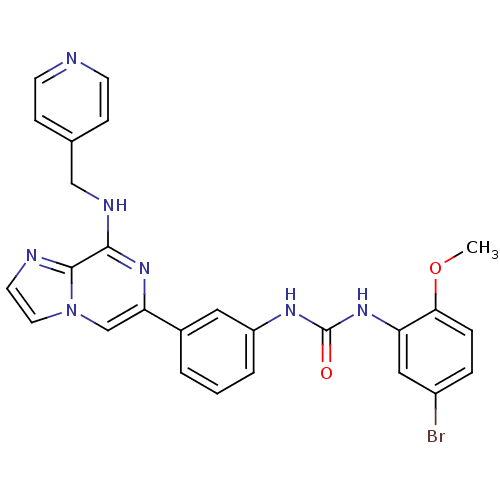
(1-(5-bromo-2-methoxyphenyl)-3-(3-(8-(pyridin-4-ylm...)Show SMILES COc1ccc(Br)cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1 Show InChI InChI=1S/C26H22BrN7O2/c1-36-23-6-5-19(27)14-21(23)33-26(35)31-20-4-2-3-18(13-20)22-16-34-12-11-29-25(34)24(32-22)30-15-17-7-9-28-10-8-17/h2-14,16H,15H2,1H3,(H,30,32)(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312156
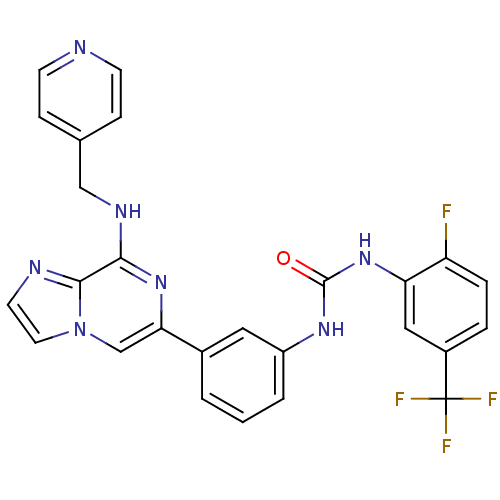
(1-(2-fluoro-5-(trifluoromethyl)phenyl)-3-(3-(8-(py...)Show SMILES Fc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C26H19F4N7O/c27-20-5-4-18(26(28,29)30)13-21(20)36-25(38)34-19-3-1-2-17(12-19)22-15-37-11-10-32-24(37)23(35-22)33-14-16-6-8-31-9-7-16/h1-13,15H,14H2,(H,33,35)(H2,34,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312157

(1-(5-bromo-2-methoxyphenyl)-3-(3-(8-(pyridin-4-ylm...)Show SMILES COc1ccc(Br)cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1 Show InChI InChI=1S/C26H22BrN7O2/c1-36-23-6-5-19(27)14-21(23)33-26(35)31-20-4-2-3-18(13-20)22-16-34-12-11-29-25(34)24(32-22)30-15-17-7-9-28-10-8-17/h2-14,16H,15H2,1H3,(H,30,32)(H2,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312153

(1-(3-(8-(pyridin-4-ylmethylamino)imidazo[1,2-a]pyr...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2cccc(c2)-c2cn3ccnc3c(NCc3ccncc3)n2)c1 Show InChI InChI=1S/C26H20F3N7O/c27-26(28,29)19-4-2-6-21(14-19)34-25(37)33-20-5-1-3-18(13-20)22-16-36-12-11-31-24(36)23(35-22)32-15-17-7-9-30-10-8-17/h1-14,16H,15H2,(H,32,35)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50312155

(1-(2-methoxy-5-(trifluoromethyl)phenyl)-3-(3-(8-(p...)Show SMILES COc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O2/c1-39-23-6-5-19(27(28,29)30)14-21(23)36-26(38)34-20-4-2-3-18(13-20)22-16-37-12-11-32-25(37)24(35-22)33-15-17-7-9-31-10-8-17/h2-14,16H,15H2,1H3,(H,33,35)(H2,34,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50312153

(1-(3-(8-(pyridin-4-ylmethylamino)imidazo[1,2-a]pyr...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2cccc(c2)-c2cn3ccnc3c(NCc3ccncc3)n2)c1 Show InChI InChI=1S/C26H20F3N7O/c27-26(28,29)19-4-2-6-21(14-19)34-25(37)33-20-5-1-3-18(13-20)22-16-36-12-11-31-24(36)23(35-22)32-15-17-7-9-30-10-8-17/h1-14,16H,15H2,(H,32,35)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312155

(1-(2-methoxy-5-(trifluoromethyl)phenyl)-3-(3-(8-(p...)Show SMILES COc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O2/c1-39-23-6-5-19(27(28,29)30)14-21(23)36-26(38)34-20-4-2-3-18(13-20)22-16-37-12-11-32-25(37)24(35-22)33-15-17-7-9-31-10-8-17/h2-14,16H,15H2,1H3,(H,33,35)(H2,34,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312184
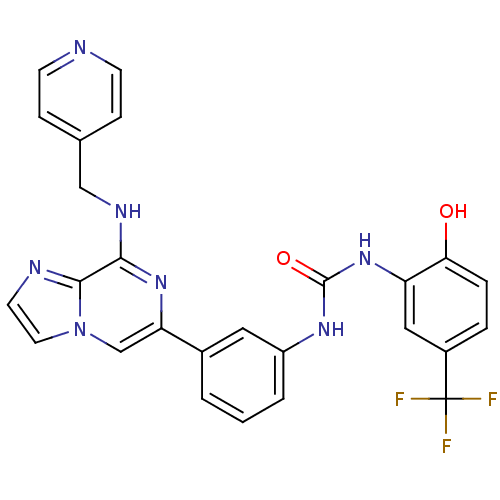
(1-(2-hydroxy-5-(trifluoromethyl)phenyl)-3-(3-(8-(p...)Show SMILES Oc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C26H20F3N7O2/c27-26(28,29)18-4-5-22(37)20(13-18)35-25(38)33-19-3-1-2-17(12-19)21-15-36-11-10-31-24(36)23(34-21)32-14-16-6-8-30-9-7-16/h1-13,15,37H,14H2,(H,32,34)(H2,33,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50312156

(1-(2-fluoro-5-(trifluoromethyl)phenyl)-3-(3-(8-(py...)Show SMILES Fc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C26H19F4N7O/c27-20-5-4-18(26(28,29)30)13-21(20)36-25(38)34-19-3-1-2-17(12-19)22-15-37-11-10-32-24(37)23(35-22)33-14-16-6-8-31-9-7-16/h1-13,15H,14H2,(H,33,35)(H2,34,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312156

(1-(2-fluoro-5-(trifluoromethyl)phenyl)-3-(3-(8-(py...)Show SMILES Fc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C26H19F4N7O/c27-20-5-4-18(26(28,29)30)13-21(20)36-25(38)34-19-3-1-2-17(12-19)22-15-37-11-10-32-24(37)23(35-22)33-14-16-6-8-31-9-7-16/h1-13,15H,14H2,(H,33,35)(H2,34,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312155

(1-(2-methoxy-5-(trifluoromethyl)phenyl)-3-(3-(8-(p...)Show SMILES COc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O2/c1-39-23-6-5-19(27(28,29)30)14-21(23)36-26(38)34-20-4-2-3-18(13-20)22-16-37-12-11-32-25(37)24(35-22)33-15-17-7-9-31-10-8-17/h2-14,16H,15H2,1H3,(H,33,35)(H2,34,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50312155

(1-(2-methoxy-5-(trifluoromethyl)phenyl)-3-(3-(8-(p...)Show SMILES COc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O2/c1-39-23-6-5-19(27(28,29)30)14-21(23)36-26(38)34-20-4-2-3-18(13-20)22-16-37-12-11-32-25(37)24(35-22)33-15-17-7-9-31-10-8-17/h2-14,16H,15H2,1H3,(H,33,35)(H2,34,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312157

(1-(5-bromo-2-methoxyphenyl)-3-(3-(8-(pyridin-4-ylm...)Show SMILES COc1ccc(Br)cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1 Show InChI InChI=1S/C26H22BrN7O2/c1-36-23-6-5-19(27)14-21(23)33-26(35)31-20-4-2-3-18(13-20)22-16-34-12-11-29-25(34)24(32-22)30-15-17-7-9-28-10-8-17/h2-14,16H,15H2,1H3,(H,30,32)(H2,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312153

(1-(3-(8-(pyridin-4-ylmethylamino)imidazo[1,2-a]pyr...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2cccc(c2)-c2cn3ccnc3c(NCc3ccncc3)n2)c1 Show InChI InChI=1S/C26H20F3N7O/c27-26(28,29)19-4-2-6-21(14-19)34-25(37)33-20-5-1-3-18(13-20)22-16-36-12-11-31-24(36)23(35-22)32-15-17-7-9-30-10-8-17/h1-14,16H,15H2,(H,32,35)(H2,33,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312187
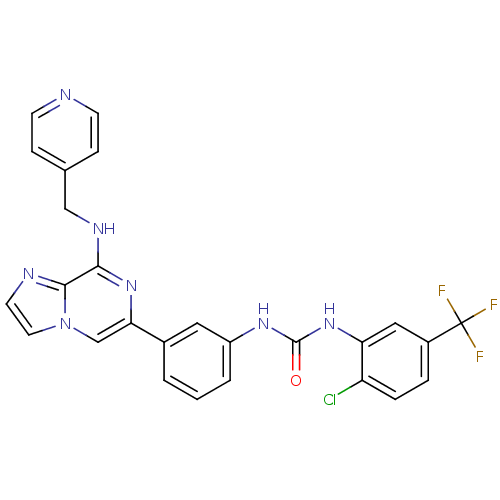
(1-(2-chloro-5-(trifluoromethyl)phenyl)-3-(3-(8-(py...)Show SMILES FC(F)(F)c1ccc(Cl)c(NC(=O)Nc2cccc(c2)-c2cn3ccnc3c(NCc3ccncc3)n2)c1 Show InChI InChI=1S/C26H19ClF3N7O/c27-20-5-4-18(26(28,29)30)13-21(20)36-25(38)34-19-3-1-2-17(12-19)22-15-37-11-10-32-24(37)23(35-22)33-14-16-6-8-31-9-7-16/h1-13,15H,14H2,(H,33,35)(H2,34,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50312153

(1-(3-(8-(pyridin-4-ylmethylamino)imidazo[1,2-a]pyr...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2cccc(c2)-c2cn3ccnc3c(NCc3ccncc3)n2)c1 Show InChI InChI=1S/C26H20F3N7O/c27-26(28,29)19-4-2-6-21(14-19)34-25(37)33-20-5-1-3-18(13-20)22-16-36-12-11-31-24(36)23(35-22)32-15-17-7-9-30-10-8-17/h1-14,16H,15H2,(H,32,35)(H2,33,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312200
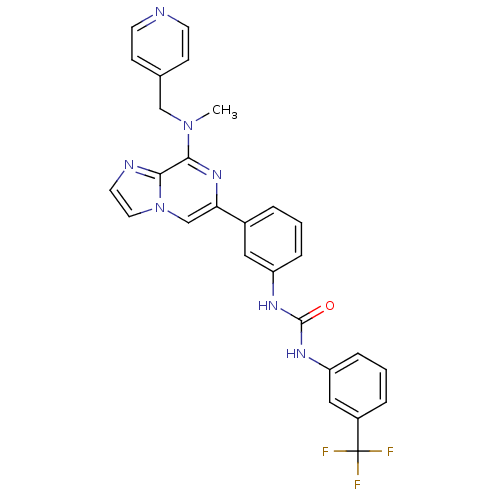
(1-(3-(8-(methyl(pyridin-4-ylmethyl)amino)imidazo[1...)Show SMILES CN(Cc1ccncc1)c1nc(cn2ccnc12)-c1cccc(NC(=O)Nc2cccc(c2)C(F)(F)F)c1 Show InChI InChI=1S/C27H22F3N7O/c1-36(16-18-8-10-31-11-9-18)25-24-32-12-13-37(24)17-23(35-25)19-4-2-6-21(14-19)33-26(38)34-22-7-3-5-20(15-22)27(28,29)30/h2-15,17H,16H2,1H3,(H2,33,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50312153

(1-(3-(8-(pyridin-4-ylmethylamino)imidazo[1,2-a]pyr...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2cccc(c2)-c2cn3ccnc3c(NCc3ccncc3)n2)c1 Show InChI InChI=1S/C26H20F3N7O/c27-26(28,29)19-4-2-6-21(14-19)34-25(37)33-20-5-1-3-18(13-20)22-16-36-12-11-31-24(36)23(35-22)32-15-17-7-9-30-10-8-17/h1-14,16H,15H2,(H,32,35)(H2,33,34,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 after 1 hr |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50312157

(1-(5-bromo-2-methoxyphenyl)-3-(3-(8-(pyridin-4-ylm...)Show SMILES COc1ccc(Br)cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1 Show InChI InChI=1S/C26H22BrN7O2/c1-36-23-6-5-19(27)14-21(23)33-26(35)31-20-4-2-3-18(13-20)22-16-34-12-11-29-25(34)24(32-22)30-15-17-7-9-28-10-8-17/h2-14,16H,15H2,1H3,(H,30,32)(H2,31,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312178

(1-(5-chloro-2-methoxyphenyl)-3-(3-(8-(pyridin-4-yl...)Show SMILES COc1ccc(Cl)cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1 Show InChI InChI=1S/C26H22ClN7O2/c1-36-23-6-5-19(27)14-21(23)33-26(35)31-20-4-2-3-18(13-20)22-16-34-12-11-29-25(34)24(32-22)30-15-17-7-9-28-10-8-17/h2-14,16H,15H2,1H3,(H,30,32)(H2,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312175
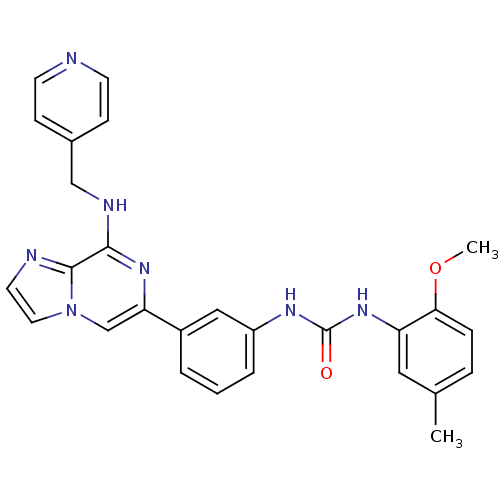
(1-(2-methoxy-5-methylphenyl)-3-(3-(8-(pyridin-4-yl...)Show SMILES COc1ccc(C)cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1 Show InChI InChI=1S/C27H25N7O2/c1-18-6-7-24(36-2)22(14-18)33-27(35)31-21-5-3-4-20(15-21)23-17-34-13-12-29-26(34)25(32-23)30-16-19-8-10-28-11-9-19/h3-15,17H,16H2,1-2H3,(H,30,32)(H2,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312156

(1-(2-fluoro-5-(trifluoromethyl)phenyl)-3-(3-(8-(py...)Show SMILES Fc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C26H19F4N7O/c27-20-5-4-18(26(28,29)30)13-21(20)36-25(38)34-19-3-1-2-17(12-19)22-15-37-11-10-32-24(37)23(35-22)33-14-16-6-8-31-9-7-16/h1-13,15H,14H2,(H,33,35)(H2,34,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312155

(1-(2-methoxy-5-(trifluoromethyl)phenyl)-3-(3-(8-(p...)Show SMILES COc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O2/c1-39-23-6-5-19(27(28,29)30)14-21(23)36-26(38)34-20-4-2-3-18(13-20)22-16-37-12-11-32-25(37)24(35-22)33-15-17-7-9-31-10-8-17/h2-14,16H,15H2,1H3,(H,33,35)(H2,34,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50312157

(1-(5-bromo-2-methoxyphenyl)-3-(3-(8-(pyridin-4-ylm...)Show SMILES COc1ccc(Br)cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1 Show InChI InChI=1S/C26H22BrN7O2/c1-36-23-6-5-19(27)14-21(23)33-26(35)31-20-4-2-3-18(13-20)22-16-34-12-11-29-25(34)24(32-22)30-15-17-7-9-28-10-8-17/h2-14,16H,15H2,1H3,(H,30,32)(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50312156

(1-(2-fluoro-5-(trifluoromethyl)phenyl)-3-(3-(8-(py...)Show SMILES Fc1ccc(cc1NC(=O)Nc1cccc(c1)-c1cn2ccnc2c(NCc2ccncc2)n1)C(F)(F)F Show InChI InChI=1S/C26H19F4N7O/c27-20-5-4-18(26(28,29)30)13-21(20)36-25(38)34-19-3-1-2-17(12-19)22-15-37-11-10-32-24(37)23(35-22)33-14-16-6-8-31-9-7-16/h1-13,15H,14H2,(H,33,35)(H2,34,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50312185

(1-(2,5-dichlorophenyl)-3-(3-(8-(pyridin-4-ylmethyl...)Show SMILES Clc1ccc(Cl)c(NC(=O)Nc2cccc(c2)-c2cn3ccnc3c(NCc3ccncc3)n2)c1 Show InChI InChI=1S/C25H19Cl2N7O/c26-18-4-5-20(27)21(13-18)33-25(35)31-19-3-1-2-17(12-19)22-15-34-11-10-29-24(34)23(32-22)30-14-16-6-8-28-9-7-16/h1-13,15H,14H2,(H,30,32)(H2,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
CGI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation by cell based assay |
Bioorg Med Chem Lett 19: 6991-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.037
BindingDB Entry DOI: 10.7270/Q2DR2VM3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data