

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233601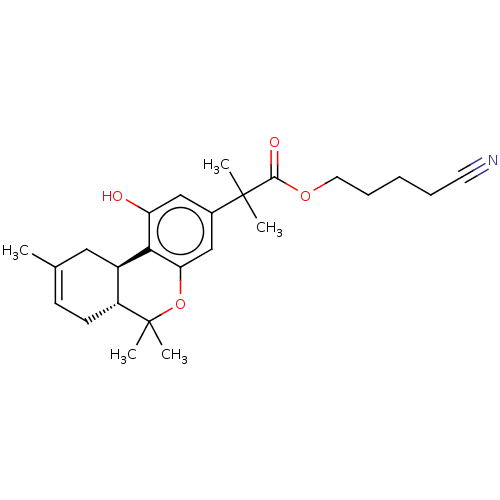 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50532777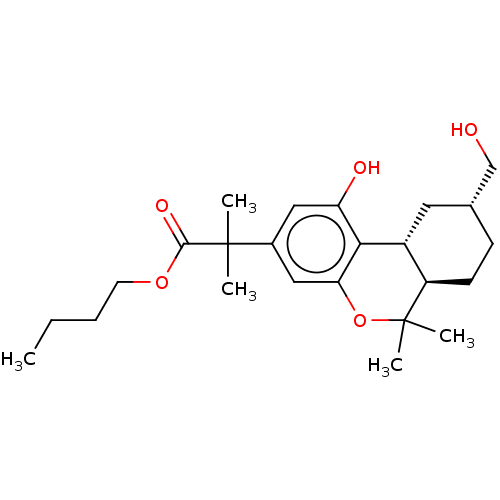 (CHEMBL4470925) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50532777 (CHEMBL4470925) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50328671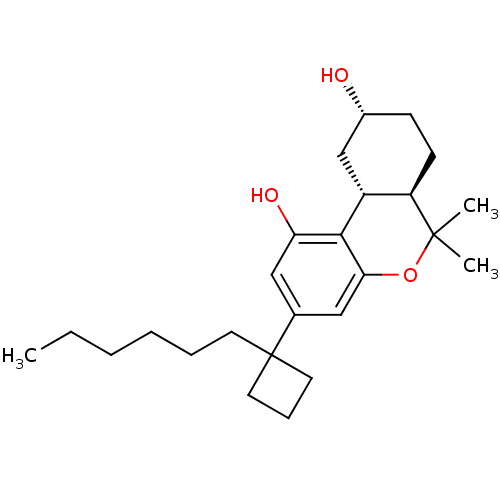 ((6aR,9R,10aR)-3-(1-hexylcyclobutyl)-6,6-dimethyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114027 BindingDB Entry DOI: 10.7270/Q2J96BFX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50328671 ((6aR,9R,10aR)-3-(1-hexylcyclobutyl)-6,6-dimethyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from rat brain CB1 receptor | J Med Chem 53: 6996-7010 (2010) Article DOI: 10.1021/jm100641g BindingDB Entry DOI: 10.7270/Q2BP030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity to mouse CB2 receptor | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067742 (7-((6aR,10aR)-1-Hydroxy-6,6,9-trimethyl-6a,7,10,10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity to mouse CB2 receptor | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50130624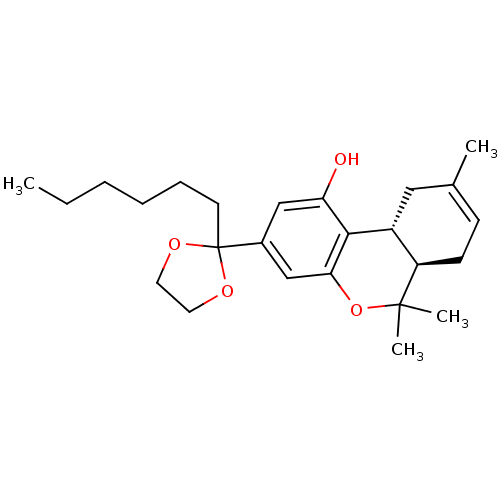 ((6aR,10aR)-3-(2-hexyl-1,3-dioxolan-2-yl)-6,6,9-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Compound was tested for its binding affinity against mouse spleen Cannabinoid receptor 2 using [3H]CP-55,940 as radioligand | J Med Chem 46: 3221-9 (2003) Article DOI: 10.1021/jm020558c BindingDB Entry DOI: 10.7270/Q24M93XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50130624 ((6aR,10aR)-3-(2-hexyl-1,3-dioxolan-2-yl)-6,6,9-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50213605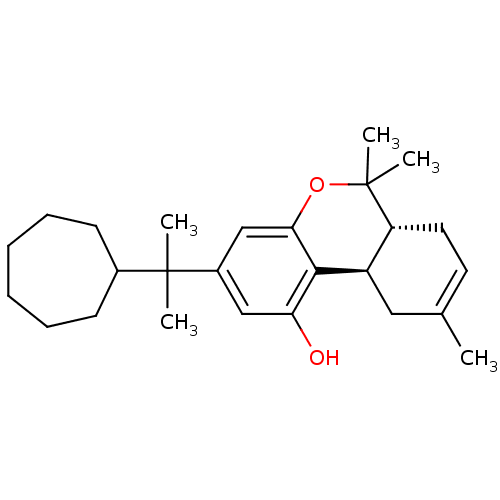 ((6aR,10aR)-3-(2-cycloheptylpropan-2-yl)-6,6,9-trim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50188394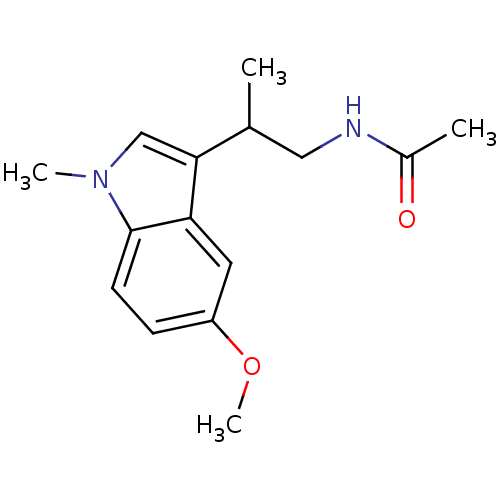 ((+)-N-(2-[5-methoxy-1-methyl-1H-indol-3-yl]propyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human recombinant MT2 receptor expressed in NIH3T3 cells | J Med Chem 49: 3509-19 (2006) Article DOI: 10.1021/jm0512544 BindingDB Entry DOI: 10.7270/Q2JW8DJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50121425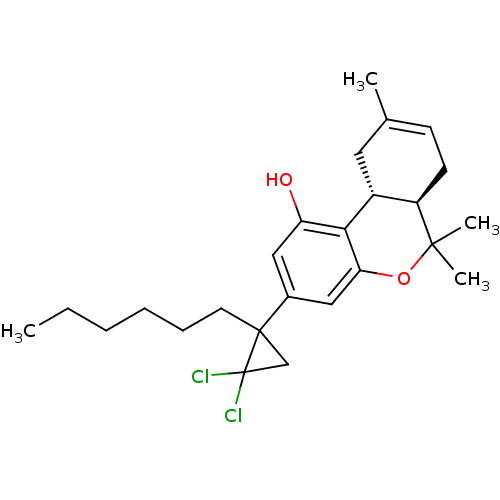 ((6aR,10aR)-3-(2,2-Dichloro-1-hexyl-cyclopropyl)-6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50121425 ((6aR,10aR)-3-(2,2-Dichloro-1-hexyl-cyclopropyl)-6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity for Cannabinoid receptor 2 by the ability to displace radiolabeled CP-55,940 from mouse spleen synaptosomes | Bioorg Med Chem Lett 12: 3583-6 (2002) BindingDB Entry DOI: 10.7270/Q2K936VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582411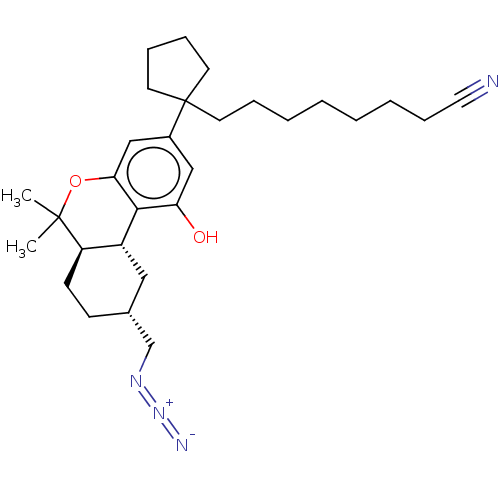 (CHEMBL5084325) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599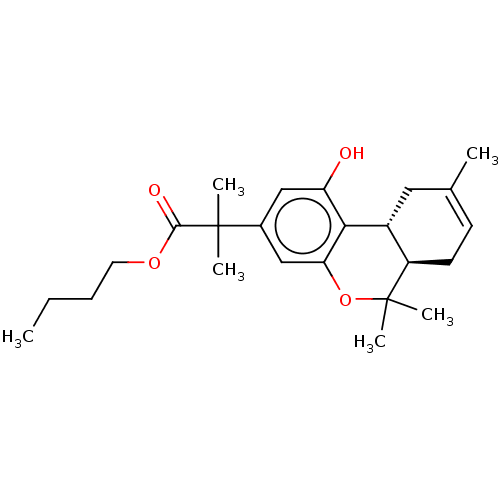 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233619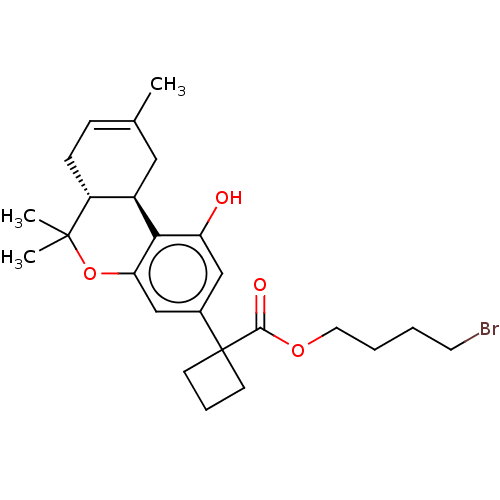 (CHEMBL4085404 | US10882838, Example 1.18) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582411 (CHEMBL5084325) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50063885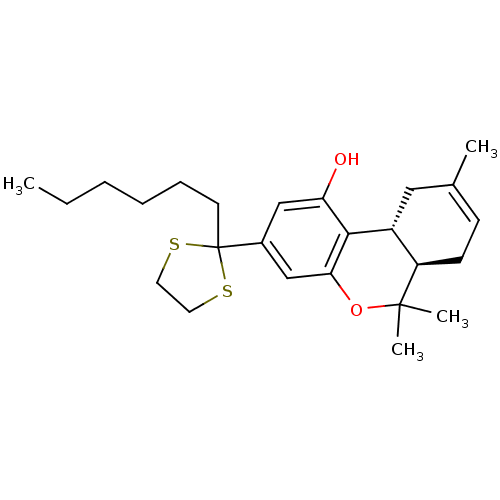 ((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity against rat brain Cannabinoid receptor 1 using [3H]CP-55,940 | J Med Chem 46: 3221-9 (2003) Article DOI: 10.1021/jm020558c BindingDB Entry DOI: 10.7270/Q24M93XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50063885 ((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity for Cannabinoid receptor 1 by the ability to displace radiolabeled CP-55,940 from purified rat forbrain synaptosomes | Bioorg Med Chem Lett 12: 3583-6 (2002) BindingDB Entry DOI: 10.7270/Q2K936VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50063885 ((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50213607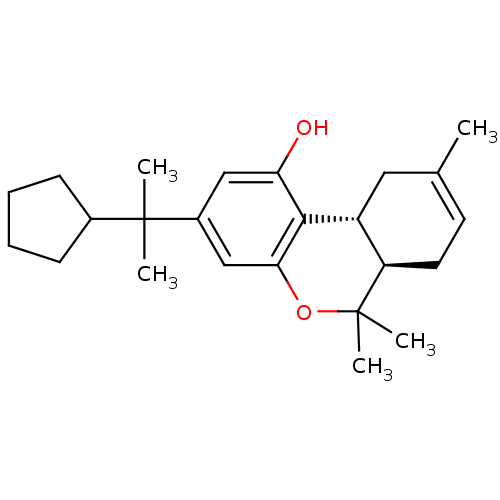 ((6aR,10aR)-3-(2-cyclopentylpropan-2-yl)-6,6,9-trim...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human recombinant MT2 receptor expressed in NIH3T3 cells | J Med Chem 49: 3509-19 (2006) Article DOI: 10.1021/jm0512544 BindingDB Entry DOI: 10.7270/Q2JW8DJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50603275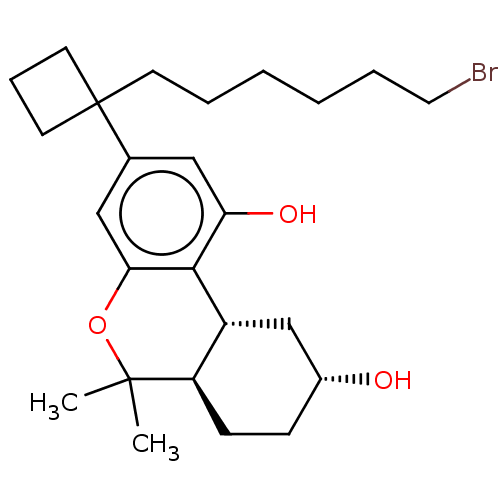 (CHEMBL5187249) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114027 BindingDB Entry DOI: 10.7270/Q2J96BFX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582403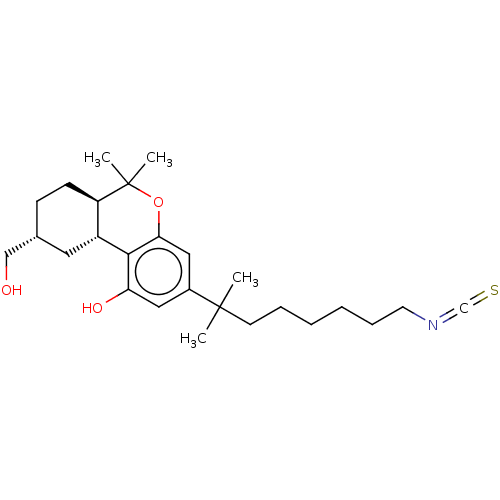 (CHEMBL5085420) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50213607 ((6aR,10aR)-3-(2-cyclopentylpropan-2-yl)-6,6,9-trim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human recombinant MT1 receptor expressed in NIH3T3 cells | J Med Chem 49: 3509-19 (2006) Article DOI: 10.1021/jm0512544 BindingDB Entry DOI: 10.7270/Q2JW8DJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582405 (CHEMBL5074603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582406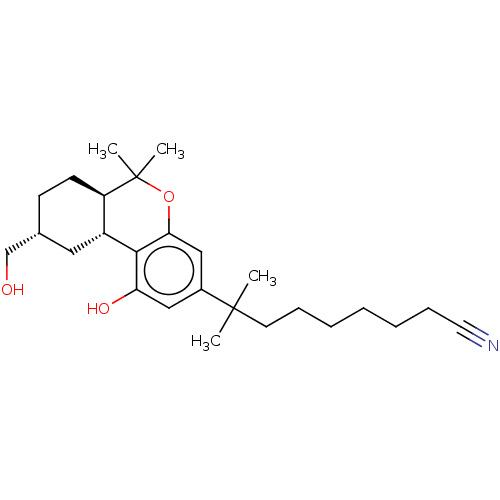 (CHEMBL5091754) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582406 (CHEMBL5091754) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582412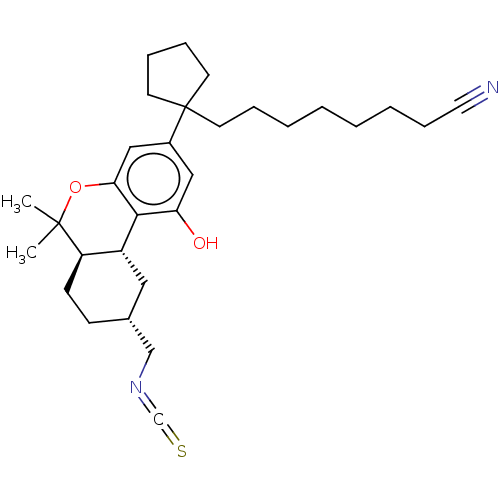 (CHEMBL5087407) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582408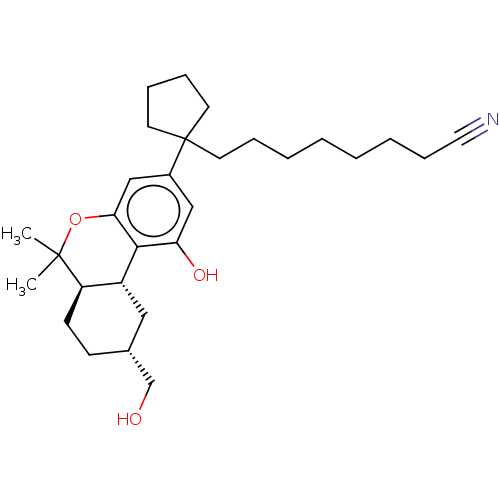 (CHEMBL5080279) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582400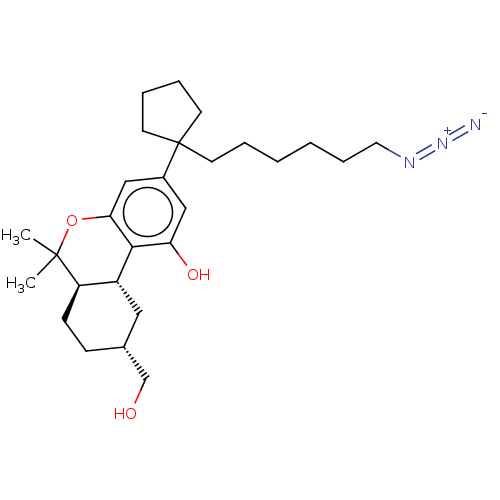 (CHEMBL5077315) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582401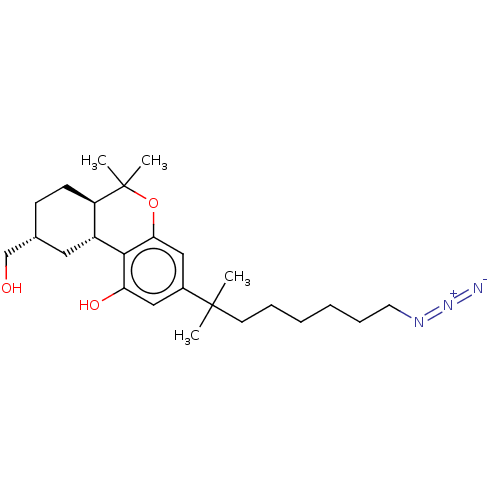 (CHEMBL5081770) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50188394 ((+)-N-(2-[5-methoxy-1-methyl-1H-indol-3-yl]propyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human recombinant MT2 receptor expressed in NIH3T3 cells | J Med Chem 49: 3509-19 (2006) Article DOI: 10.1021/jm0512544 BindingDB Entry DOI: 10.7270/Q2JW8DJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50213599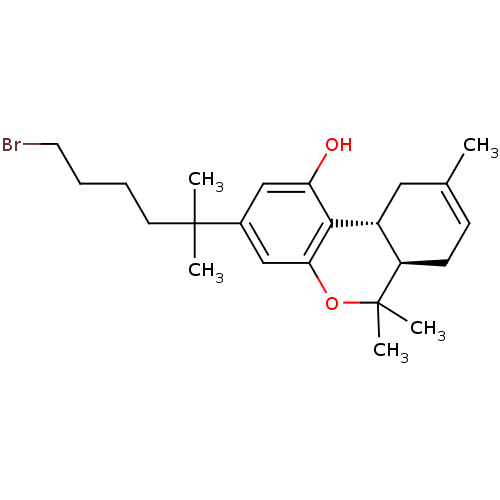 ((6aR,10aR)-3-(6-bromo-2-methylhexan-2-yl)-6,6,9-tr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50063884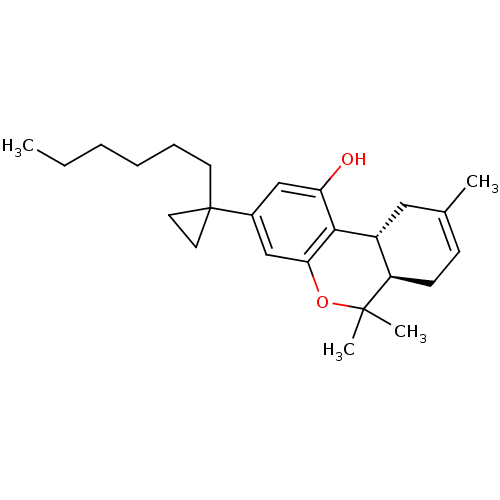 ((6aR,10aR)-3-(1-Hexyl-cyclopropyl)-6,6,9-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity for Cannabinoid receptor 1 by the ability to displace radiolabeled CP-55,940 from purified rat forbrain synaptosomes | Bioorg Med Chem Lett 12: 3583-6 (2002) BindingDB Entry DOI: 10.7270/Q2K936VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50063884 ((6aR,10aR)-3-(1-Hexyl-cyclopropyl)-6,6,9-trimethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50130623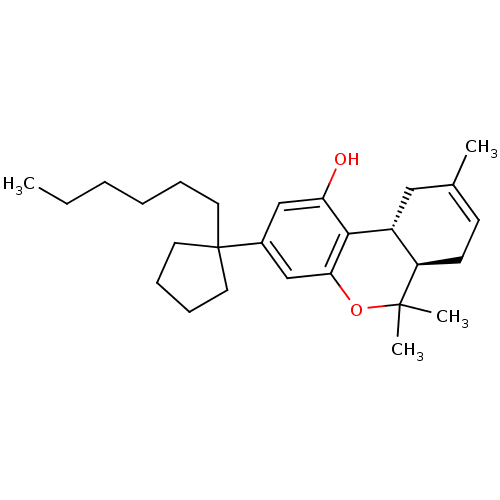 ((6aR,10aR)-3-(1-hexylcyclopentyl)-6,6,9-trimethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 50: 2875-85 (2007) Article DOI: 10.1021/jm0610705 BindingDB Entry DOI: 10.7270/Q2ST7PJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50130623 ((6aR,10aR)-3-(1-hexylcyclopentyl)-6,6,9-trimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity against rat brain Cannabinoid receptor 1 using [3H]CP-55,940 | J Med Chem 46: 3221-9 (2003) Article DOI: 10.1021/jm020558c BindingDB Entry DOI: 10.7270/Q24M93XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50603264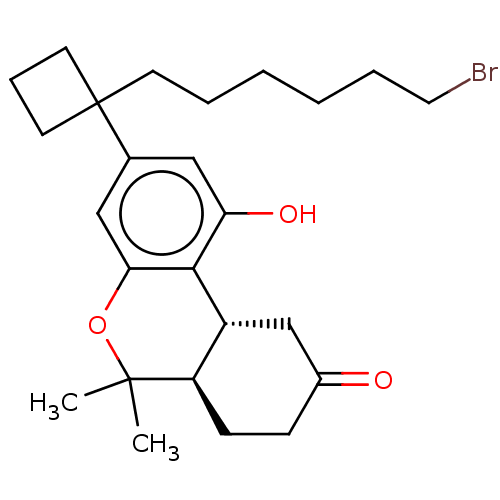 (CHEMBL5206676) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114027 BindingDB Entry DOI: 10.7270/Q2J96BFX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50188417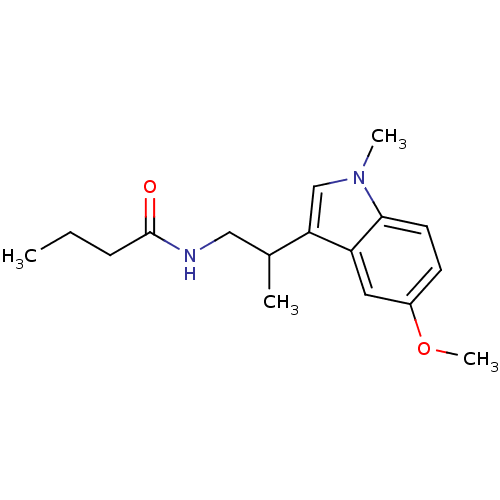 (CHEMBL377101 | N-(2-[5-methoxy-1-methyl-1H-indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human recombinant MT2 receptor expressed in NIH3T3 cells | J Med Chem 49: 3509-19 (2006) Article DOI: 10.1021/jm0512544 BindingDB Entry DOI: 10.7270/Q2JW8DJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50219076 ((6aR-trans)-3-(1-heptylcyclopropyl)-6a,7,10,10a-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in rat brain synaptosomal membrane | J Med Chem 50: 4048-60 (2007) Article DOI: 10.1021/jm070121a BindingDB Entry DOI: 10.7270/Q21N80VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 945 total ) | Next | Last >> |