 Found 685 hits with Last Name = 'gane' and Initial = 't'
Found 685 hits with Last Name = 'gane' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM197
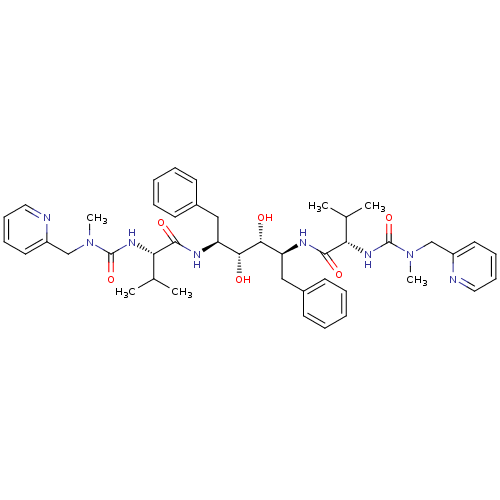
((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36-,37-,38-,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064201
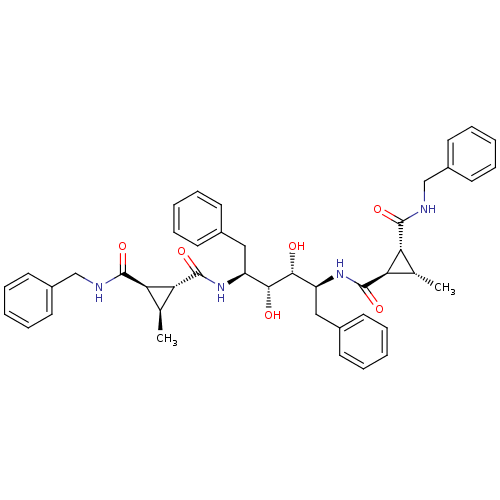
(1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoyl-3-meth...)Show SMILES C[C@H]1[C@H]([C@@H]1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1[C@@H](C)[C@H]1C(=O)NCc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C44H50N4O6/c1-27-35(41(51)45-25-31-19-11-5-12-20-31)37(27)43(53)47-33(23-29-15-7-3-8-16-29)39(49)40(50)34(24-30-17-9-4-10-18-30)48-44(54)38-28(2)36(38)42(52)46-26-32-21-13-6-14-22-32/h3-22,27-28,33-40,49-50H,23-26H2,1-2H3,(H,45,51)(H,46,52)(H,47,53)(H,48,54)/t27-,28-,33-,34-,35+,36+,37+,38+,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064202
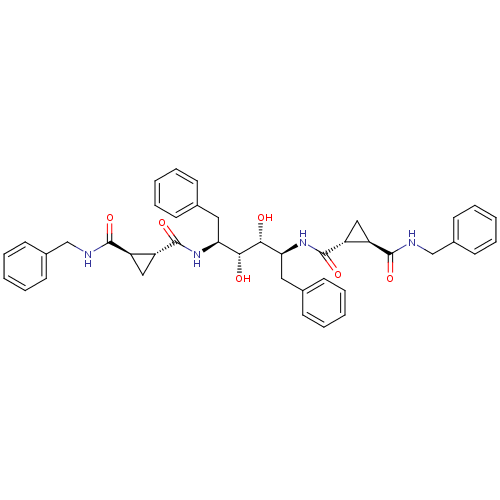
(1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoylcyclopr...)Show SMILES O[C@@H]([C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1C[C@H]1C(=O)NCc1ccccc1)[C@H](Cc1ccccc1)NC(=O)[C@@H]1C[C@H]1C(=O)NCc1ccccc1 Show InChI InChI=1S/C42H46N4O6/c47-37(35(21-27-13-5-1-6-14-27)45-41(51)33-23-31(33)39(49)43-25-29-17-9-3-10-18-29)38(48)36(22-28-15-7-2-8-16-28)46-42(52)34-24-32(34)40(50)44-26-30-19-11-4-12-20-30/h1-20,31-38,47-48H,21-26H2,(H,43,49)(H,44,50)(H,45,51)(H,46,52)/t31-,32-,33-,34-,35+,36+,37-,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064199
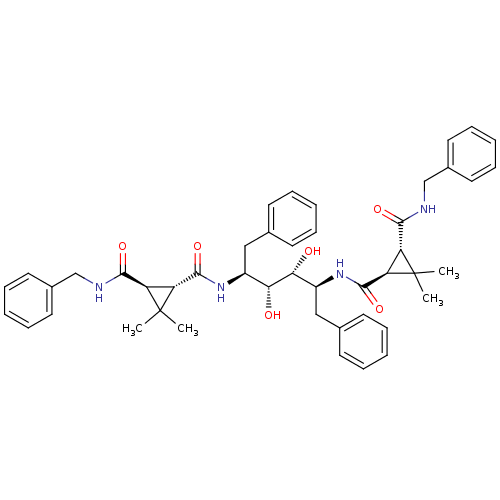
(1N-benzyl-2N-[1-benzyl-4-(3-benzylcarbamoyl-2,2-di...)Show SMILES CC1(C)[C@H]([C@@H]1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H]1[C@H](C(=O)NCc2ccccc2)C1(C)C)C(=O)NCc1ccccc1 Show InChI InChI=1S/C46H54N4O6/c1-45(2)35(41(53)47-27-31-21-13-7-14-22-31)37(45)43(55)49-33(25-29-17-9-5-10-18-29)39(51)40(52)34(26-30-19-11-6-12-20-30)50-44(56)38-36(46(38,3)4)42(54)48-28-32-23-15-8-16-24-32/h5-24,33-40,51-52H,25-28H2,1-4H3,(H,47,53)(H,48,54)(H,49,55)(H,50,56)/t33-,34-,35+,36+,37+,38+,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064203
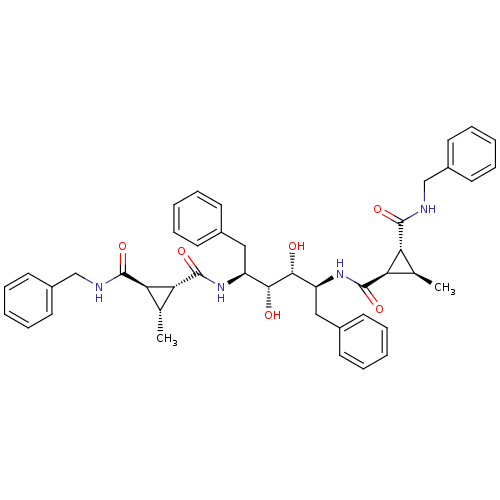
(1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoyl-3-meth...)Show SMILES C[C@@H]1[C@H]([C@@H]1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1[C@H](C)[C@H]1C(=O)NCc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C44H50N4O6/c1-27-35(41(51)45-25-31-19-11-5-12-20-31)37(27)43(53)47-33(23-29-15-7-3-8-16-29)39(49)40(50)34(24-30-17-9-4-10-18-30)48-44(54)38-28(2)36(38)42(52)46-26-32-21-13-6-14-22-32/h3-22,27-28,33-40,49-50H,23-26H2,1-2H3,(H,45,51)(H,46,52)(H,47,53)(H,48,54)/t27-,28-,33+,34+,35-,36-,37-,38-,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50016952
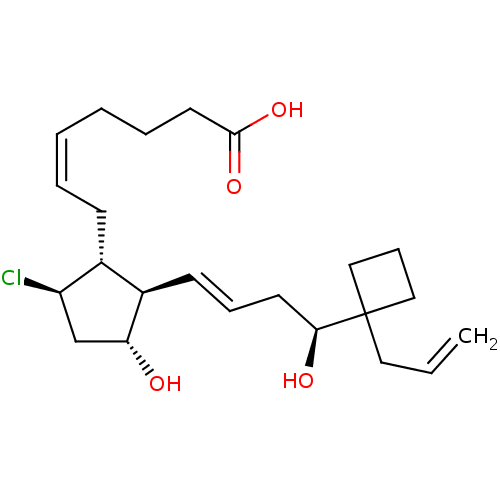
(CHEMBL3286796)Show SMILES O[C@@H](C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O)C1(CC=C)CCC1 |r| Show InChI InChI=1S/C23H35ClO4/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h2-3,5,7,10,17-21,25-26H,1,4,6,8-9,11-16H2,(H,27,28)/b5-3-,10-7+/t17-,18-,19-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50101830
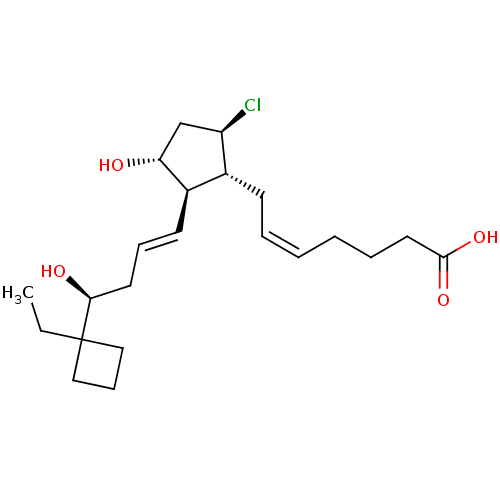
((Z)-7-{(1R,2R,3R,5R)-5-Chloro-2-[(E)-(S)-4-(1-ethy...)Show SMILES CCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H35ClO4/c1-2-22(13-8-14-22)20(25)11-7-10-17-16(18(23)15-19(17)24)9-5-3-4-6-12-21(26)27/h3,5,7,10,16-20,24-25H,2,4,6,8-9,11-15H2,1H3,(H,26,27)/b5-3-,10-7+/t16-,17-,18-,19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50016953
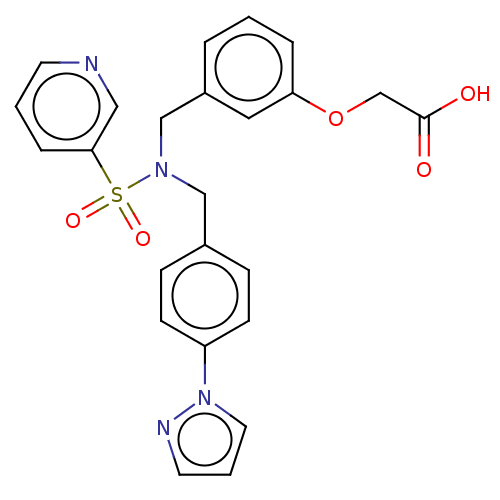
(Taprenepag)Show SMILES OC(=O)COc1cccc(CN(Cc2ccc(cc2)-n2cccn2)S(=O)(=O)c2cccnc2)c1 Show InChI InChI=1S/C24H22N4O5S/c29-24(30)18-33-22-5-1-4-20(14-22)17-27(34(31,32)23-6-2-11-25-15-23)16-19-7-9-21(10-8-19)28-13-3-12-26-28/h1-15H,16-18H2,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50293496
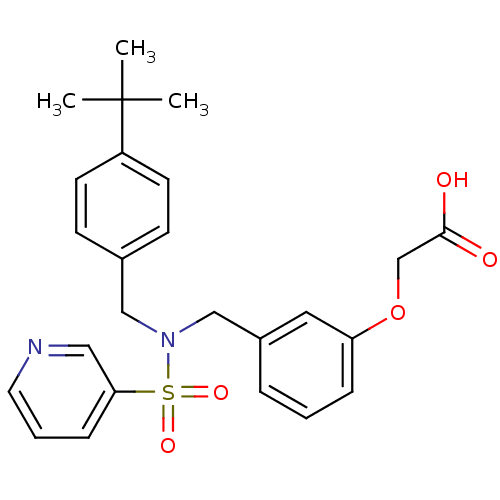
(2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...)Show SMILES CC(C)(C)c1ccc(CN(Cc2cccc(OCC(O)=O)c2)S(=O)(=O)c2cccnc2)cc1 Show InChI InChI=1S/C25H28N2O5S/c1-25(2,3)21-11-9-19(10-12-21)16-27(33(30,31)23-8-5-13-26-15-23)17-20-6-4-7-22(14-20)32-18-24(28)29/h4-15H,16-18H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50016954
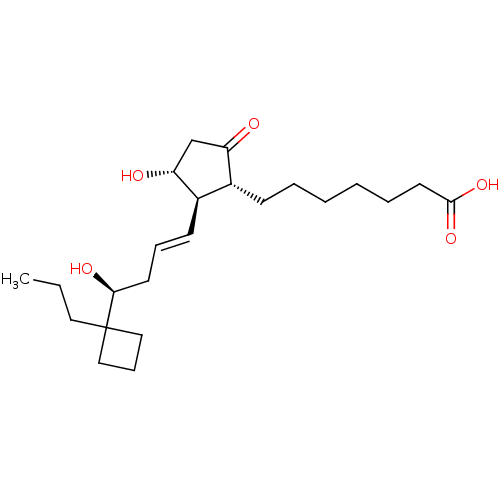
(CHEMBL1628262)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C23H38O5/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h7,10,17-18,20-21,25-26H,2-6,8-9,11-16H2,1H3,(H,27,28)/b10-7+/t17-,18-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50064198
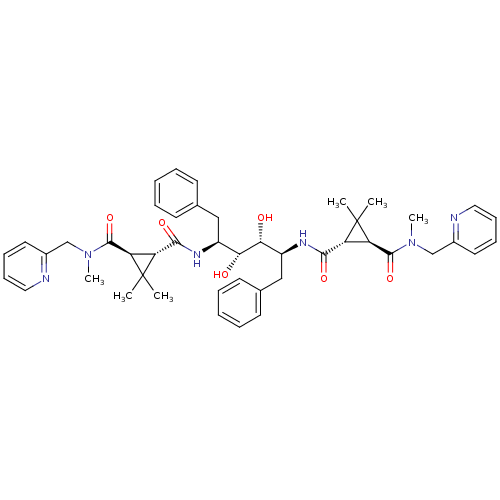
(1N-{1-benzyl-4-[2,2-dimethyl-3-methyl(2-pyridylmet...)Show SMILES CN(Cc1ccccn1)C(=O)[C@H]1[C@H](C(=O)N[C@@H](Cc2ccccc2)[C@@H](O)[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@H]2[C@H](C(=O)N(C)Cc3ccccn3)C2(C)C)C1(C)C Show InChI InChI=1S/C46H56N6O6/c1-45(2)35(37(45)43(57)51(5)27-31-21-13-15-23-47-31)41(55)49-33(25-29-17-9-7-10-18-29)39(53)40(54)34(26-30-19-11-8-12-20-30)50-42(56)36-38(46(36,3)4)44(58)52(6)28-32-22-14-16-24-48-32/h7-24,33-40,53-54H,25-28H2,1-6H3,(H,49,55)(H,50,56)/t33-,34-,35+,36+,37+,38+,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured against wild-type HIV-1 protease |
J Med Chem 41: 1581-97 (1998)
Article DOI: 10.1021/jm980033d
BindingDB Entry DOI: 10.7270/Q27S7MWG |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50016981
(CHEMBL1722929)Show SMILES CCCCCC(O)c1ccc(cc1)[C@H]1CCC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C24H36O4/c1-2-3-6-10-22(25)19-14-12-18(13-15-19)20-16-17-23(26)21(20)9-7-4-5-8-11-24(27)28/h12-15,20-22,25H,2-11,16-17H2,1H3,(H,27,28)/t20-,21-,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50016981

(CHEMBL1722929)Show SMILES CCCCCC(O)c1ccc(cc1)[C@H]1CCC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C24H36O4/c1-2-3-6-10-22(25)19-14-12-18(13-15-19)20-16-17-23(26)21(20)9-7-4-5-8-11-24(27)28/h12-15,20-22,25H,2-11,16-17H2,1H3,(H,27,28)/t20-,21-,22?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Clostridium botulinum BoNT/A using SNAP-25 (141-206) as substrate by HPLC analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01006
BindingDB Entry DOI: 10.7270/Q2WD446P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain |
Citation and Details
Article DOI: 10.1039/d1md00089f
BindingDB Entry DOI: 10.7270/Q24T6P2K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50016954

(CHEMBL1628262)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C23H38O5/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h7,10,17-18,20-21,25-26H,2-6,8-9,11-16H2,1H3,(H,27,28)/b10-7+/t17-,18-,20-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to prostanoid IP receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM85184
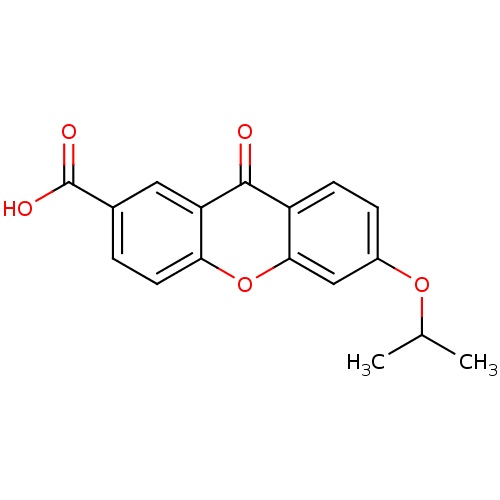
(AH-6809 | CAS_33458-93-4 | NSC_119461)Show InChI InChI=1S/C17H14O5/c1-9(2)21-11-4-5-12-15(8-11)22-14-6-3-10(17(19)20)7-13(14)16(12)18/h3-9H,1-2H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM85184

(AH-6809 | CAS_33458-93-4 | NSC_119461)Show InChI InChI=1S/C17H14O5/c1-9(2)21-11-4-5-12-15(8-11)22-14-6-3-10(17(19)20)7-13(14)16(12)18/h3-9H,1-2H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Antagonist activity at EP2 receptor (unknown origin) by functional cAMP assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM85184

(AH-6809 | CAS_33458-93-4 | NSC_119461)Show InChI InChI=1S/C17H14O5/c1-9(2)21-11-4-5-12-15(8-11)22-14-6-3-10(17(19)20)7-13(14)16(12)18/h3-9H,1-2H3,(H,19,20) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by functional cAMP assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50016952

(CHEMBL3286796)Show SMILES O[C@@H](C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O)C1(CC=C)CCC1 |r| Show InChI InChI=1S/C23H35ClO4/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h2-3,5,7,10,17-21,25-26H,1,4,6,8-9,11-16H2,(H,27,28)/b5-3-,10-7+/t17-,18-,19-,20-,21+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM85184

(AH-6809 | CAS_33458-93-4 | NSC_119461)Show InChI InChI=1S/C17H14O5/c1-9(2)21-11-4-5-12-15(8-11)22-14-6-3-10(17(19)20)7-13(14)16(12)18/h3-9H,1-2H3,(H,19,20) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) by functional cAMP assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85184

(AH-6809 | CAS_33458-93-4 | NSC_119461)Show InChI InChI=1S/C17H14O5/c1-9(2)21-11-4-5-12-15(8-11)22-14-6-3-10(17(19)20)7-13(14)16(12)18/h3-9H,1-2H3,(H,19,20) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Antagonist activity at EP3 receptor (unknown origin) by functional cAMP assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50546871

(CHEMBL4745069)Show SMILES CS(=O)(=O)SCCCCCCN(O)C(=O)\C=C\c1ccc(Cl)cc1Cl | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01006
BindingDB Entry DOI: 10.7270/Q2WD446P |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50016952

(CHEMBL3286796)Show SMILES O[C@@H](C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O)C1(CC=C)CCC1 |r| Show InChI InChI=1S/C23H35ClO4/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h2-3,5,7,10,17-21,25-26H,1,4,6,8-9,11-16H2,(H,27,28)/b5-3-,10-7+/t17-,18-,19-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM85602
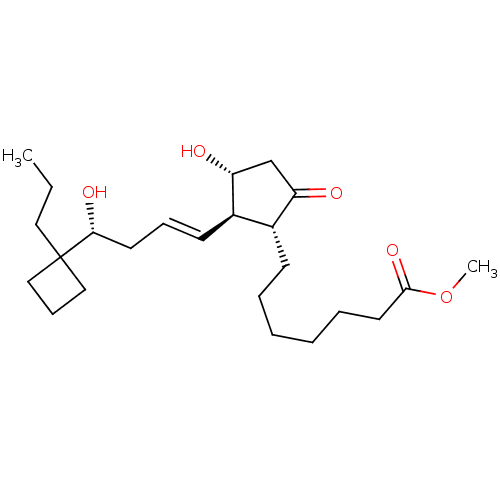
((R)-BUTAPROST | Butaprost (Free Acid) | CAS_69648-...)Show SMILES CCCC1(CCC1)[C@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC |r| Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) by competitive binding assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50016952

(CHEMBL3286796)Show SMILES O[C@@H](C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O)C1(CC=C)CCC1 |r| Show InChI InChI=1S/C23H35ClO4/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h2-3,5,7,10,17-21,25-26H,1,4,6,8-9,11-16H2,(H,27,28)/b5-3-,10-7+/t17-,18-,19-,20-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50293496

(2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...)Show SMILES CC(C)(C)c1ccc(CN(Cc2cccc(OCC(O)=O)c2)S(=O)(=O)c2cccnc2)cc1 Show InChI InChI=1S/C25H28N2O5S/c1-25(2,3)21-11-9-19(10-12-21)16-27(33(30,31)23-8-5-13-26-15-23)17-20-6-4-7-22(14-20)32-18-24(28)29/h4-15H,16-18H2,1-3H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50293496

(2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...)Show SMILES CC(C)(C)c1ccc(CN(Cc2cccc(OCC(O)=O)c2)S(=O)(=O)c2cccnc2)cc1 Show InChI InChI=1S/C25H28N2O5S/c1-25(2,3)21-11-9-19(10-12-21)16-27(33(30,31)23-8-5-13-26-15-23)17-20-6-4-7-22(14-20)32-18-24(28)29/h4-15H,16-18H2,1-3H3,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50293496

(2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...)Show SMILES CC(C)(C)c1ccc(CN(Cc2cccc(OCC(O)=O)c2)S(=O)(=O)c2cccnc2)cc1 Show InChI InChI=1S/C25H28N2O5S/c1-25(2,3)21-11-9-19(10-12-21)16-27(33(30,31)23-8-5-13-26-15-23)17-20-6-4-7-22(14-20)32-18-24(28)29/h4-15H,16-18H2,1-3H3,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to prostanoid IP receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50016953

(Taprenepag)Show SMILES OC(=O)COc1cccc(CN(Cc2ccc(cc2)-n2cccn2)S(=O)(=O)c2cccnc2)c1 Show InChI InChI=1S/C24H22N4O5S/c29-24(30)18-33-22-5-1-4-20(14-22)17-27(34(31,32)23-6-2-11-25-15-23)16-19-7-9-21(10-8-19)28-13-3-12-26-28/h1-15H,16-18H2,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50293496

(2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...)Show SMILES CC(C)(C)c1ccc(CN(Cc2cccc(OCC(O)=O)c2)S(=O)(=O)c2cccnc2)cc1 Show InChI InChI=1S/C25H28N2O5S/c1-25(2,3)21-11-9-19(10-12-21)16-27(33(30,31)23-8-5-13-26-15-23)17-20-6-4-7-22(14-20)32-18-24(28)29/h4-15H,16-18H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50016953

(Taprenepag)Show SMILES OC(=O)COc1cccc(CN(Cc2ccc(cc2)-n2cccn2)S(=O)(=O)c2cccnc2)c1 Show InChI InChI=1S/C24H22N4O5S/c29-24(30)18-33-22-5-1-4-20(14-22)17-27(34(31,32)23-6-2-11-25-15-23)16-19-7-9-21(10-8-19)28-13-3-12-26-28/h1-15H,16-18H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50016953

(Taprenepag)Show SMILES OC(=O)COc1cccc(CN(Cc2ccc(cc2)-n2cccn2)S(=O)(=O)c2cccnc2)c1 Show InChI InChI=1S/C24H22N4O5S/c29-24(30)18-33-22-5-1-4-20(14-22)17-27(34(31,32)23-6-2-11-25-15-23)16-19-7-9-21(10-8-19)28-13-3-12-26-28/h1-15H,16-18H2,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50048539

(CHEMBL3309328)Show InChI InChI=1S/C4H11NO2S2/c1-9(6,7)8-4-2-3-5/h2-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Irreversible inhibition of recombinant Clostridium botulinum N-terminal 6His-tagged BoNT/A (Met1 to Phe425 residues) catalytic domain expressed in Es... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01006
BindingDB Entry DOI: 10.7270/Q2WD446P |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50546870
(CHEMBL4790141)Show SMILES CS(=O)(=O)SCCCCCN(O)C(=O)\C=C\c1ccc(Cl)cc1Cl | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01006
BindingDB Entry DOI: 10.7270/Q2WD446P |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50016954

(CHEMBL1628262)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C23H38O5/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h7,10,17-18,20-21,25-26H,2-6,8-9,11-16H2,1H3,(H,27,28)/b10-7+/t17-,18-,20-,21+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM85602

((R)-BUTAPROST | Butaprost (Free Acid) | CAS_69648-...)Show SMILES CCCC1(CCC1)[C@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC |r| Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50016954

(CHEMBL1628262)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C23H38O5/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h7,10,17-18,20-21,25-26H,2-6,8-9,11-16H2,1H3,(H,27,28)/b10-7+/t17-,18-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM85602

((R)-BUTAPROST | Butaprost (Free Acid) | CAS_69648-...)Show SMILES CCCC1(CCC1)[C@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC |r| Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19-,21-,22-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50016952

(CHEMBL3286796)Show SMILES O[C@@H](C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O)C1(CC=C)CCC1 |r| Show InChI InChI=1S/C23H35ClO4/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h2-3,5,7,10,17-21,25-26H,1,4,6,8-9,11-16H2,(H,27,28)/b5-3-,10-7+/t17-,18-,19-,20-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to prostanoid IP receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50101830

((Z)-7-{(1R,2R,3R,5R)-5-Chloro-2-[(E)-(S)-4-(1-ethy...)Show SMILES CCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H35ClO4/c1-2-22(13-8-14-22)20(25)11-7-10-17-16(18(23)15-19(17)24)9-5-3-4-6-12-21(26)27/h3,5,7,10,16-20,24-25H,2,4,6,8-9,11-15H2,1H3,(H,26,27)/b5-3-,10-7+/t16-,17-,18-,19-,20+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to prostanoid IP receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM85602

((R)-BUTAPROST | Butaprost (Free Acid) | CAS_69648-...)Show SMILES CCCC1(CCC1)[C@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC |r| Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19-,21-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to prostanoid IP receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to prostanoid IP receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50101830

((Z)-7-{(1R,2R,3R,5R)-5-Chloro-2-[(E)-(S)-4-(1-ethy...)Show SMILES CCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H35ClO4/c1-2-22(13-8-14-22)20(25)11-7-10-17-16(18(23)15-19(17)24)9-5-3-4-6-12-21(26)27/h3,5,7,10,16-20,24-25H,2,4,6,8-9,11-15H2,1H3,(H,26,27)/b5-3-,10-7+/t16-,17-,18-,19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50101830

((Z)-7-{(1R,2R,3R,5R)-5-Chloro-2-[(E)-(S)-4-(1-ethy...)Show SMILES CCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H35ClO4/c1-2-22(13-8-14-22)20(25)11-7-10-17-16(18(23)15-19(17)24)9-5-3-4-6-12-21(26)27/h3,5,7,10,16-20,24-25H,2,4,6,8-9,11-15H2,1H3,(H,26,27)/b5-3-,10-7+/t16-,17-,18-,19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85602

((R)-BUTAPROST | Butaprost (Free Acid) | CAS_69648-...)Show SMILES CCCC1(CCC1)[C@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC |r| Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19-,21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data