

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50017721 (1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50097224 (1-Methyl-4-thioxanthen-9-ylidene-piperidine | 1-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50097222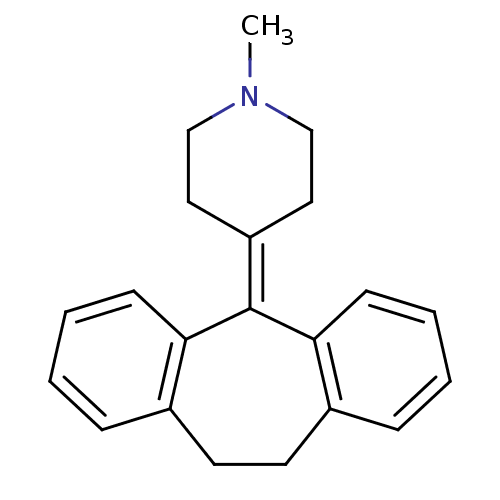 (4-(10,11-Dihydro-dibenzo[a,d]cyclohepten-5-ylidene...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards Vasopressin V1a receptor in rat liver | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50097215 (4-Fluoren-9-ylidene-1-methyl-piperidine | CHEMBL34...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 524 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards Vasopressin V1a receptor in human liver | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50017721 (1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Curated by ChEMBL | Assay Description Inhibition of recombinant Set7/9 (unknown origin) expressed in Escherichia coli BL21 (DE3) using Ac-KRSK-MCA peptide/SAM as substrate preincubated fo... | J Med Chem 59: 3650-60 (2016) Article DOI: 10.1021/acs.jmedchem.5b01732 BindingDB Entry DOI: 10.7270/Q2X068Z4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094076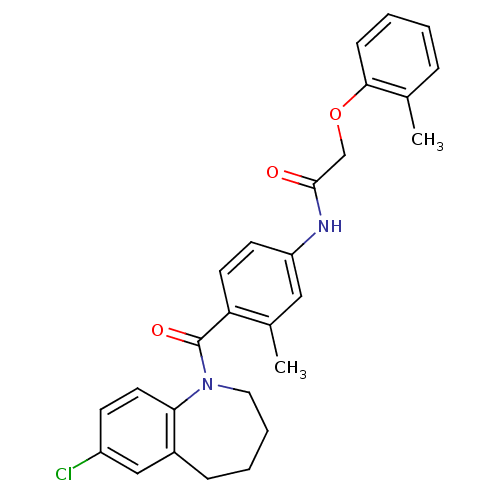 (CHEMBL434654 | N-[4-(7-Chloro-2,3,4,5-tetrahydro-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094079 ((4-Amino-2-methyl-phenyl)-(7-chloro-2,3,4,5-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094068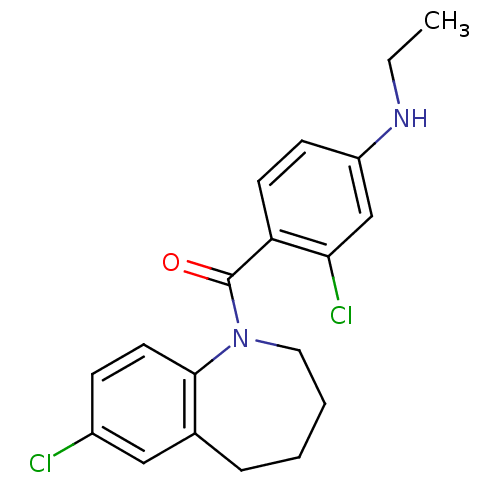 ((2-Chloro-4-ethylamino-phenyl)-(7-chloro-2,3,4,5-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094086 ((4-Allylamino-2-chloro-phenyl)-(2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094082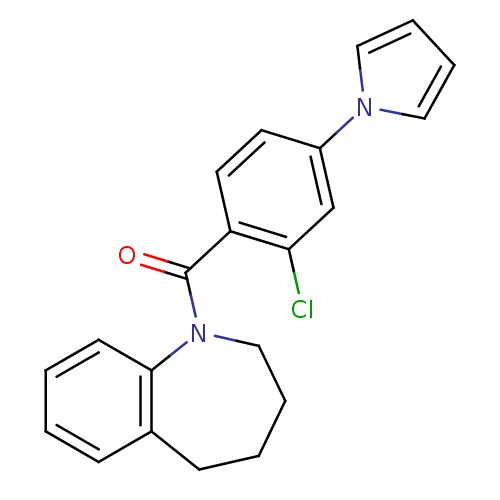 ((2-Chloro-4-pyrrol-1-yl-phenyl)-(2,3,4,5-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094066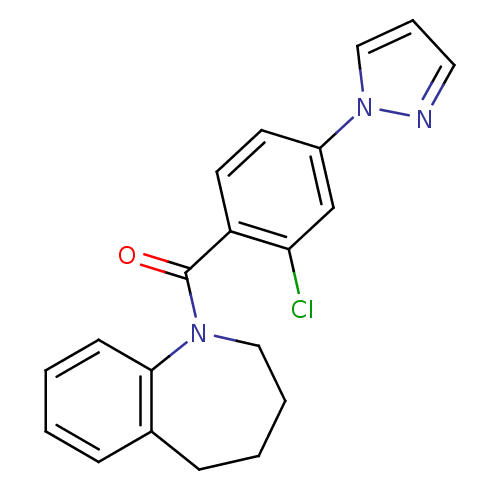 ((2-Chloro-4-pyrazol-1-yl-phenyl)-(2,3,4,5-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094075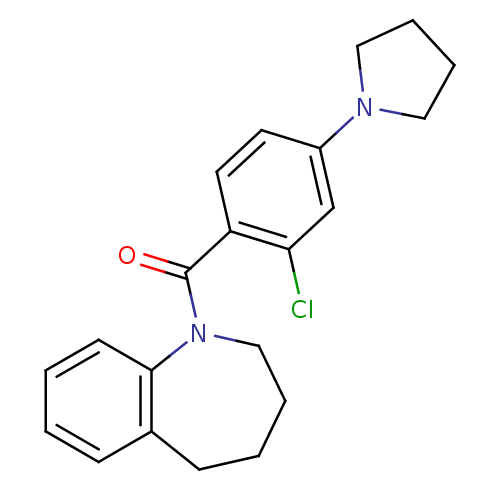 ((2-Chloro-4-pyrrolidin-1-yl-phenyl)-(2,3,4,5-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094075 ((2-Chloro-4-pyrrolidin-1-yl-phenyl)-(2,3,4,5-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity of the compound against human Vasopressin V2 receptor expressed in HeLa cells was determined | J Med Chem 45: 3805-8 (2002) BindingDB Entry DOI: 10.7270/Q2H41QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094070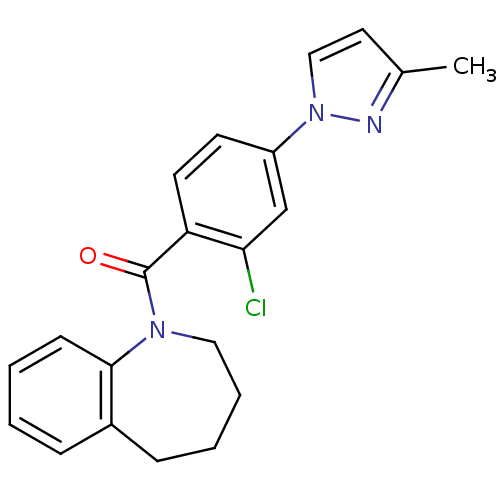 (CHEMBL136109 | [2-Chloro-4-(3-methyl-pyrazol-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094074 ((2-Chloro-4-dimethylamino-phenyl)-(7-chloro-2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094072 ((2-Chloro-4-pyrrolidin-1-yl-phenyl)-(7-chloro-2,3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094089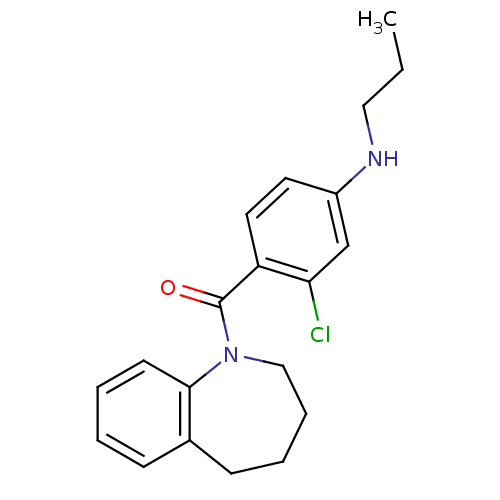 ((2-Chloro-4-propylamino-phenyl)-(2,3,4,5-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094067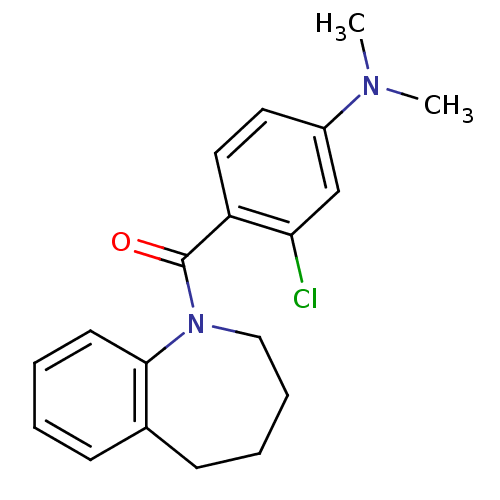 ((2-Chloro-4-dimethylamino-phenyl)-(2,3,4,5-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094077 ((2-Chloro-4-diethylamino-phenyl)-(2,3,4,5-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094078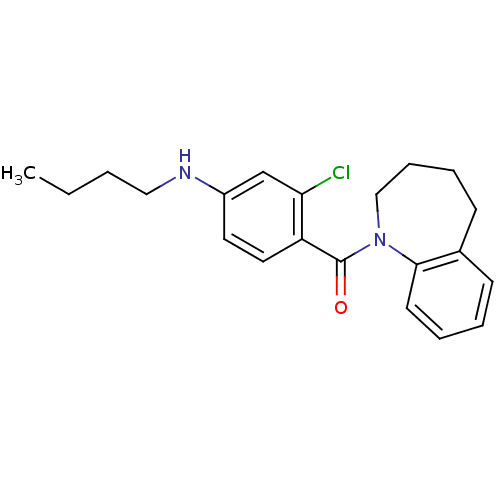 ((4-Butylamino-2-chloro-phenyl)-(2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198670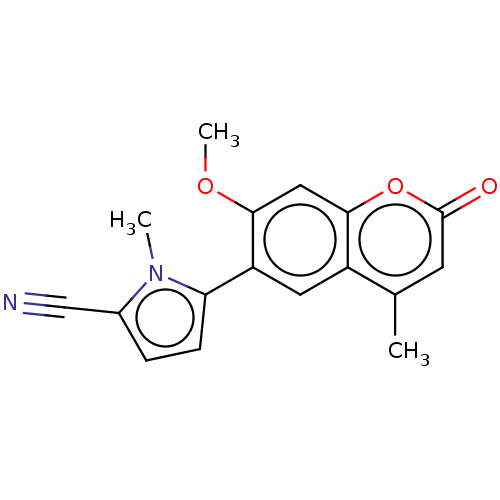 (CHEMBL3960590) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198661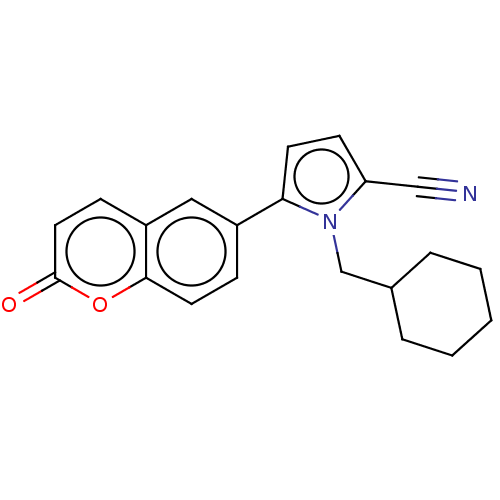 (CHEMBL3955849) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094087 ((2-Chloro-4-pentylamino-phenyl)-(2,3,4,5-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198660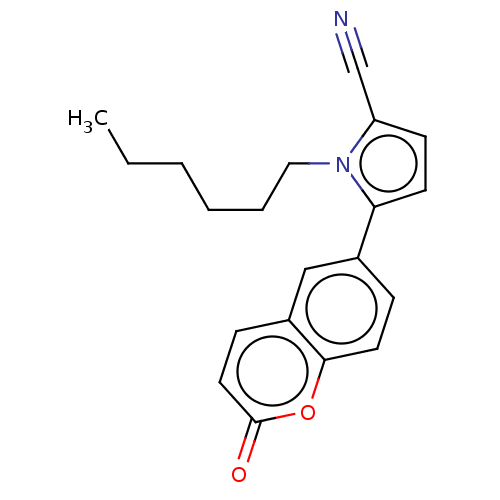 (CHEMBL3928231) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094069 ((2-Chloro-4-ethylamino-phenyl)-(2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094083 ((2-Chloro-4-methylamino-phenyl)-(2,3,4,5-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198677 (CHEMBL3929427) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50094076 (CHEMBL434654 | N-[4-(7-Chloro-2,3,4,5-tetrahydro-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards rat Vasopressin V2 receptor after peroral administration of the compound | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094088 (CHEMBL136790 | [2-Chloro-4-(3-methyl-pyrrolidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198662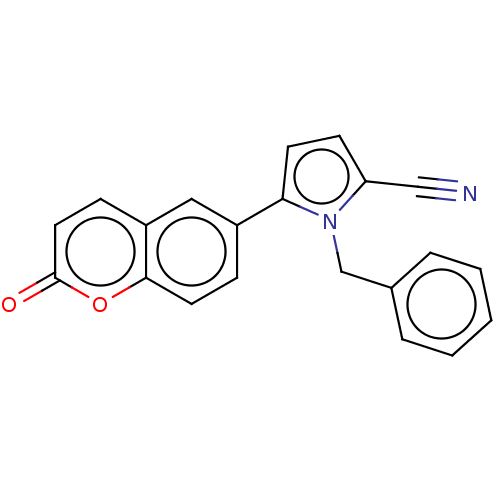 (CHEMBL3966957) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50355243 (CHEMBL1835836) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at human progesterone receptor in T47D cells after 24 hrs by alkaline phosphatase assay | J Med Chem 54: 7055-65 (2011) Article DOI: 10.1021/jm2005404 BindingDB Entry DOI: 10.7270/Q27D2VJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198682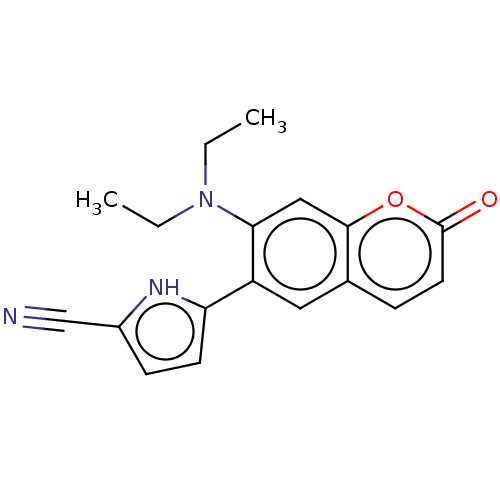 (CHEMBL3918835) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 217 total ) | Next | Last >> |