 Found 429 hits with Last Name = 'kanoh' and Initial = 't'
Found 429 hits with Last Name = 'kanoh' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50143784
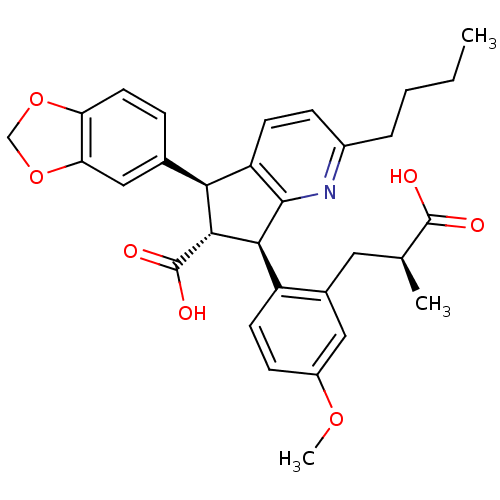
((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...)Show SMILES CCCCc1ccc2[C@@H]([C@H]([C@@H](c2n1)c1ccc(OC)cc1C[C@H](C)C(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H33NO7/c1-4-5-6-20-8-10-23-26(18-7-12-24-25(15-18)39-16-38-24)28(31(35)36)27(29(23)32-20)22-11-9-21(37-3)14-19(22)13-17(2)30(33)34/h7-12,14-15,17,26-28H,4-6,13,16H2,1-3H3,(H,33,34)(H,35,36)/t17-,26-,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1262-70 (1999)
BindingDB Entry DOI: 10.7270/Q2Q52N5Q |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50143784

((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...)Show SMILES CCCCc1ccc2[C@@H]([C@H]([C@@H](c2n1)c1ccc(OC)cc1C[C@H](C)C(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H33NO7/c1-4-5-6-20-8-10-23-26(18-7-12-24-25(15-18)39-16-38-24)28(31(35)36)27(29(23)32-20)22-11-9-21(37-3)14-19(22)13-17(2)30(33)34/h7-12,14-15,17,26-28H,4-6,13,16H2,1-3H3,(H,33,34)(H,35,36)/t17-,26-,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1262-70 (1999)
BindingDB Entry DOI: 10.7270/Q2Q52N5Q |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244370
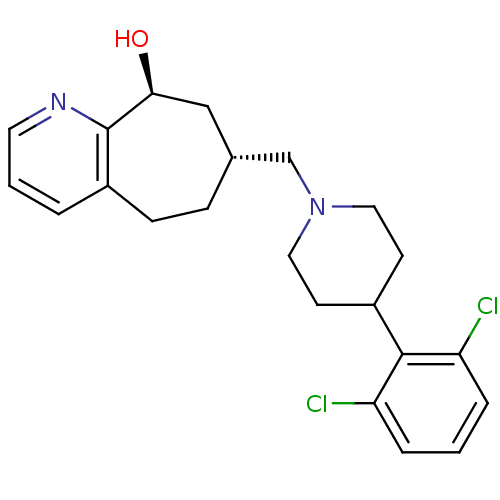
((7R,9S)-7-((4-(2,6-dichlorophenyl)piperidin-1-yl)m...)Show SMILES O[C@H]1C[C@H](CN2CCC(CC2)c2c(Cl)cccc2Cl)CCc2cccnc12 |r| Show InChI InChI=1S/C22H26Cl2N2O/c23-18-4-1-5-19(24)21(18)16-8-11-26(12-9-16)14-15-6-7-17-3-2-10-25-22(17)20(27)13-15/h1-5,10,15-16,20,27H,6-9,11-14H2/t15-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244371
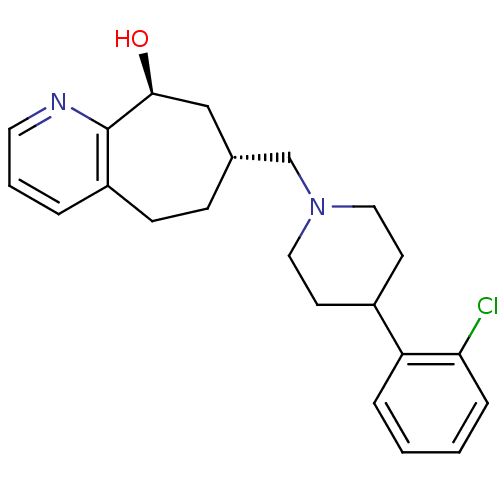
((7R,9S)-7-((4-(2-chlorophenyl)piperidin-1-yl)methy...)Show SMILES O[C@H]1C[C@H](CN2CCC(CC2)c2ccccc2Cl)CCc2cccnc12 |r| Show InChI InChI=1S/C22H27ClN2O/c23-20-6-2-1-5-19(20)17-9-12-25(13-10-17)15-16-7-8-18-4-3-11-24-22(18)21(26)14-16/h1-6,11,16-17,21,26H,7-10,12-15H2/t16-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243726
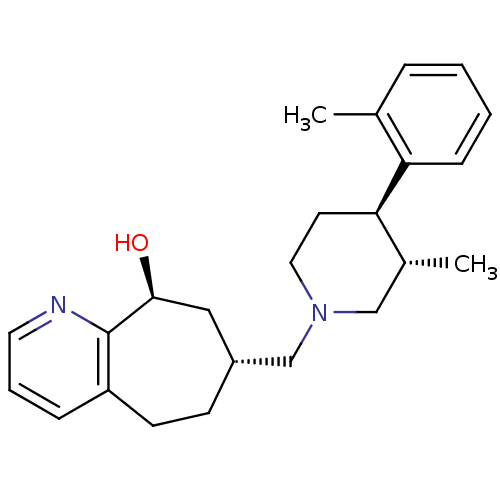
((7R,9S)-7-(((3S,4R)-3-methyl-4-o-tolylpiperidin-1-...)Show SMILES C[C@@H]1CN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC[C@H]1c1ccccc1C |r| Show InChI InChI=1S/C24H32N2O/c1-17-6-3-4-8-21(17)22-11-13-26(15-18(22)2)16-19-9-10-20-7-5-12-25-24(20)23(27)14-19/h3-8,12,18-19,22-23,27H,9-11,13-16H2,1-2H3/t18-,19-,22-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243729
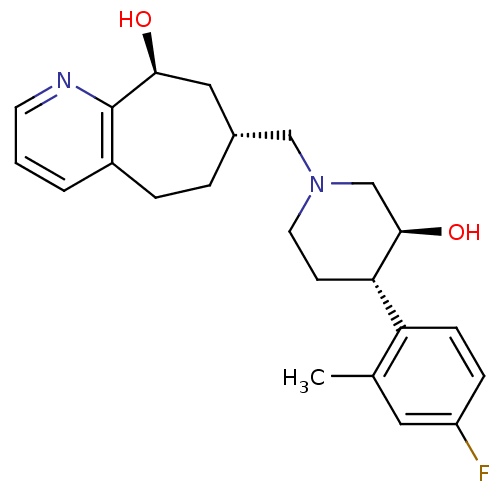
((7R,9S)-7-(((3S,4S)-4-(4-fluoro-2-methylphenyl)-3-...)Show SMILES Cc1cc(F)ccc1[C@@H]1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)C[C@H]1O |r| Show InChI InChI=1S/C23H29FN2O2/c1-15-11-18(24)6-7-19(15)20-8-10-26(14-22(20)28)13-16-4-5-17-3-2-9-25-23(17)21(27)12-16/h2-3,6-7,9,11,16,20-22,27-28H,4-5,8,10,12-14H2,1H3/t16-,20+,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243727
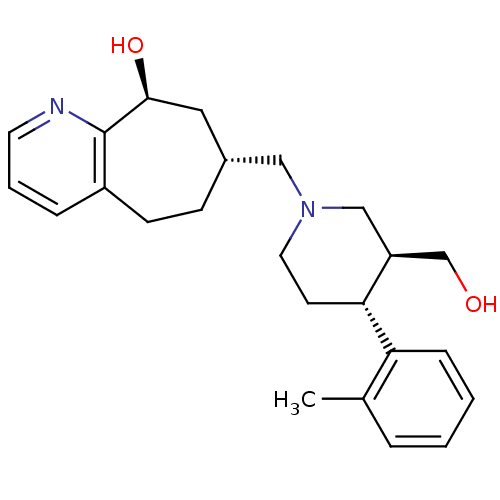
((7R,9S)-7-(((3S,4R)-3-(hydroxymethyl)-4-o-tolylpip...)Show SMILES Cc1ccccc1[C@@H]1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)C[C@H]1CO |r| Show InChI InChI=1S/C24H32N2O2/c1-17-5-2-3-7-21(17)22-10-12-26(15-20(22)16-27)14-18-8-9-19-6-4-11-25-24(19)23(28)13-18/h2-7,11,18,20,22-23,27-28H,8-10,12-16H2,1H3/t18-,20+,22-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243730
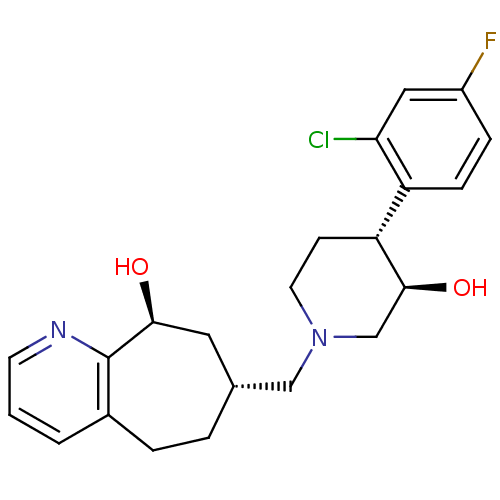
((7R,9S)-7-(((3R,4R)-4-(2-chloro-4-fluorophenyl)-3-...)Show SMILES O[C@H]1CN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC[C@@H]1c1ccc(F)cc1Cl |r| Show InChI InChI=1S/C22H26ClFN2O2/c23-19-11-16(24)5-6-17(19)18-7-9-26(13-21(18)28)12-14-3-4-15-2-1-8-25-22(15)20(27)10-14/h1-2,5-6,8,11,14,18,20-21,27-28H,3-4,7,9-10,12-13H2/t14-,18-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244297
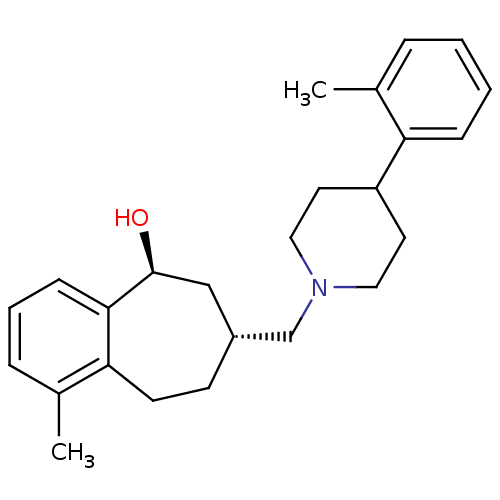
(CHEMBL513585 | cis-1-methyl-7-((4-o-tolylpiperidin...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3c(C)cccc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C25H33NO/c1-18-6-3-4-8-22(18)21-12-14-26(15-13-21)17-20-10-11-23-19(2)7-5-9-24(23)25(27)16-20/h3-9,20-21,25,27H,10-17H2,1-2H3/t20-,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243728
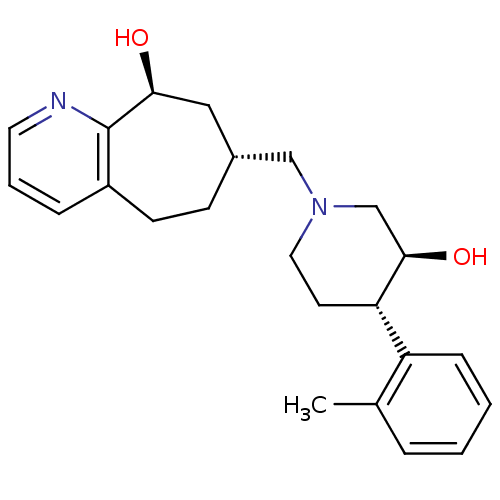
((7R,9S)-7-(((3S,4S)-3-hydroxy-4-o-tolylpiperidin-1...)Show SMILES Cc1ccccc1[C@@H]1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)C[C@H]1O |r| Show InChI InChI=1S/C23H30N2O2/c1-16-5-2-3-7-19(16)20-10-12-25(15-22(20)27)14-17-8-9-18-6-4-11-24-23(18)21(26)13-17/h2-7,11,17,20-22,26-27H,8-10,12-15H2,1H3/t17-,20+,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244336
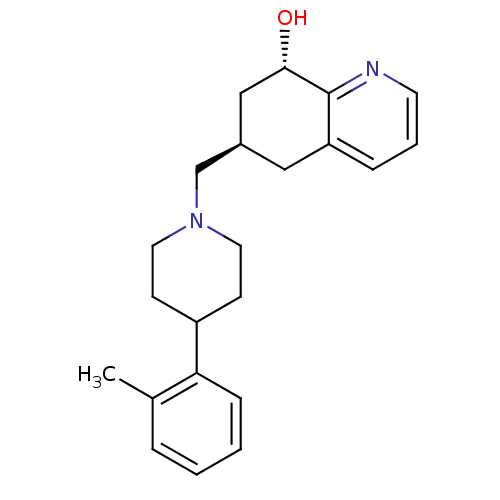
((6R,8S)-6-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,...)Show SMILES Cc1ccccc1C1CCN(C[C@H]2C[C@H](O)c3ncccc3C2)CC1 |r| Show InChI InChI=1S/C22H28N2O/c1-16-5-2-3-7-20(16)18-8-11-24(12-9-18)15-17-13-19-6-4-10-23-22(19)21(25)14-17/h2-7,10,17-18,21,25H,8-9,11-15H2,1H3/t17-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243887
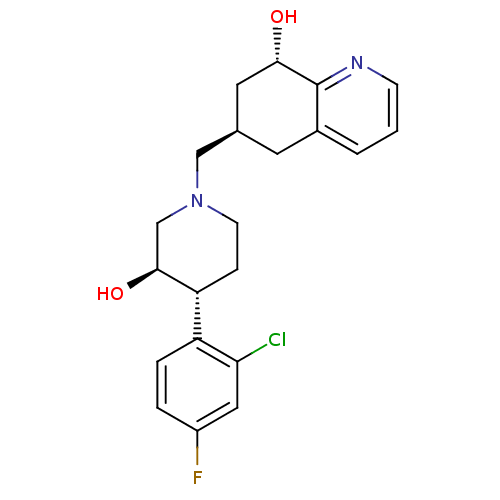
((-)-(3R,4R)-4-(2-Chloro-4-fluorophenyl)-3-hydroxy-...)Show SMILES O[C@H]1CN(C[C@H]2C[C@H](O)c3ncccc3C2)CC[C@@H]1c1ccc(F)cc1Cl |r| Show InChI InChI=1S/C21H24ClFN2O2/c22-18-10-15(23)3-4-16(18)17-5-7-25(12-20(17)27)11-13-8-14-2-1-6-24-21(14)19(26)9-13/h1-4,6,10,13,17,19-20,26-27H,5,7-9,11-12H2/t13-,17-,19+,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244334
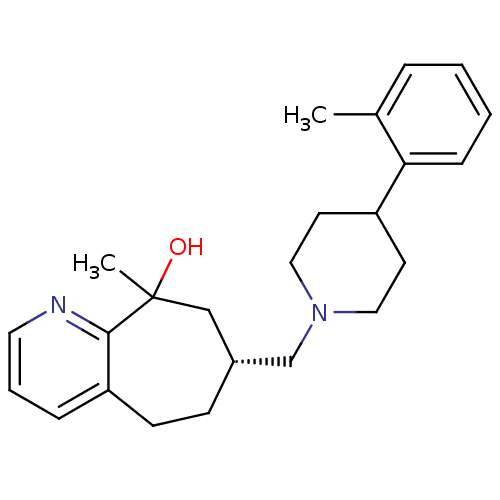
((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3C(C)(O)C2)CC1 |r| Show InChI InChI=1S/C24H32N2O/c1-18-6-3-4-8-22(18)20-11-14-26(15-12-20)17-19-9-10-21-7-5-13-25-23(21)24(2,27)16-19/h3-8,13,19-20,27H,9-12,14-17H2,1-2H3/t19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244334

((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3C(C)(O)C2)CC1 |r| Show InChI InChI=1S/C24H32N2O/c1-18-6-3-4-8-22(18)20-11-14-26(15-12-20)17-19-9-10-21-7-5-13-25-23(21)24(2,27)16-19/h3-8,13,19-20,27H,9-12,14-17H2,1-2H3/t19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244295
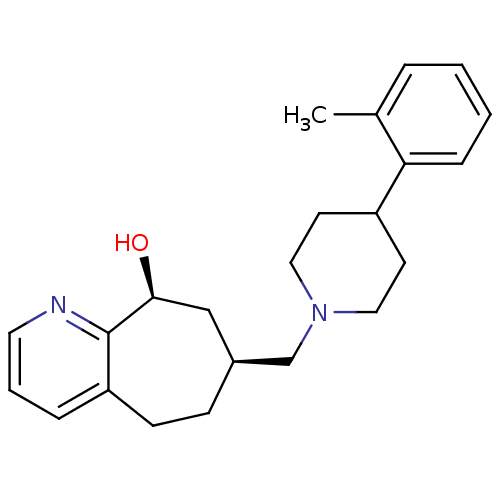
((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...)Show SMILES Cc1ccccc1C1CCN(C[C@H]2CCc3cccnc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C23H30N2O/c1-17-5-2-3-7-21(17)19-10-13-25(14-11-19)16-18-8-9-20-6-4-12-24-23(20)22(26)15-18/h2-7,12,18-19,22,26H,8-11,13-16H2,1H3/t18-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244295

((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...)Show SMILES Cc1ccccc1C1CCN(C[C@H]2CCc3cccnc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C23H30N2O/c1-17-5-2-3-7-21(17)19-10-13-25(14-11-19)16-18-8-9-20-6-4-12-24-23(20)22(26)15-18/h2-7,12,18-19,22,26H,8-11,13-16H2,1H3/t18-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244372
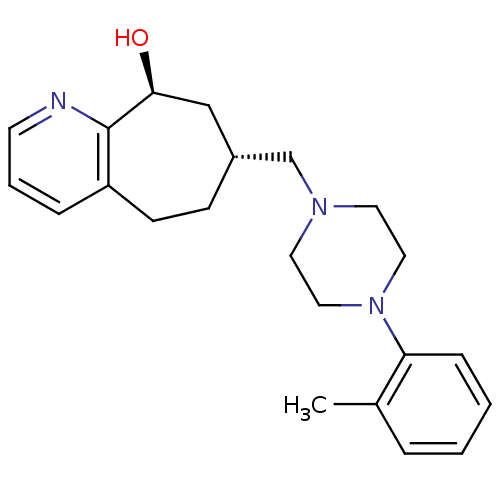
((7R,9S)-7-((4-o-tolylpiperazin-1-yl)methyl)-6,7,8,...)Show SMILES Cc1ccccc1N1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C22H29N3O/c1-17-5-2-3-7-20(17)25-13-11-24(12-14-25)16-18-8-9-19-6-4-10-23-22(19)21(26)15-18/h2-7,10,18,21,26H,8-9,11-16H2,1H3/t18-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243886
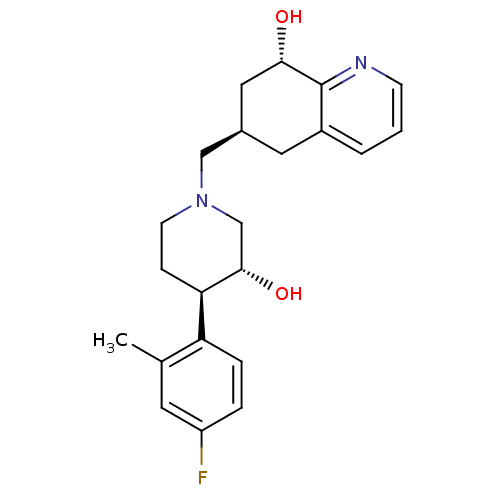
((6R,8S)-6-(((3R,4R)-4-(4-fluoro-2-methylphenyl)-3-...)Show SMILES Cc1cc(F)ccc1[C@H]1CCN(C[C@H]2C[C@H](O)c3ncccc3C2)C[C@@H]1O |r| Show InChI InChI=1S/C22H27FN2O2/c1-14-9-17(23)4-5-18(14)19-6-8-25(13-21(19)27)12-15-10-16-3-2-7-24-22(16)20(26)11-15/h2-5,7,9,15,19-21,26-27H,6,8,10-13H2,1H3/t15-,19-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244335
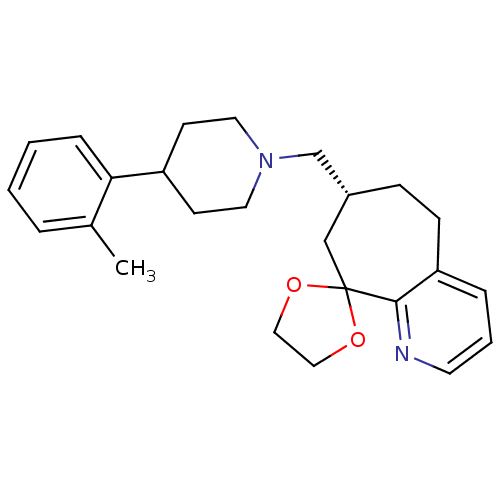
((3R)-3-{[4-(2-methylphenyl)piperidin-1-yl]methyl}-...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3C3(C2)OCCO3)CC1 |r| Show InChI InChI=1S/C25H32N2O2/c1-19-5-2-3-7-23(19)21-10-13-27(14-11-21)18-20-8-9-22-6-4-12-26-24(22)25(17-20)28-15-16-29-25/h2-7,12,20-21H,8-11,13-18H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244373
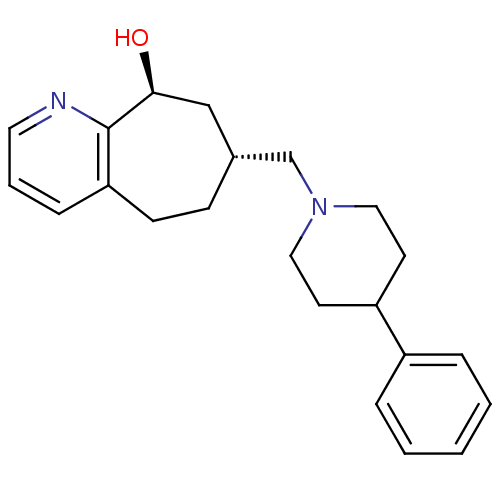
((7R,9S)-7-((4-phenylpiperidin-1-yl)methyl)-6,7,8,9...)Show SMILES O[C@H]1C[C@H](CN2CCC(CC2)c2ccccc2)CCc2cccnc12 |r| Show InChI InChI=1S/C22H28N2O/c25-21-15-17(8-9-20-7-4-12-23-22(20)21)16-24-13-10-19(11-14-24)18-5-2-1-3-6-18/h1-7,12,17,19,21,25H,8-11,13-16H2/t17-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244333
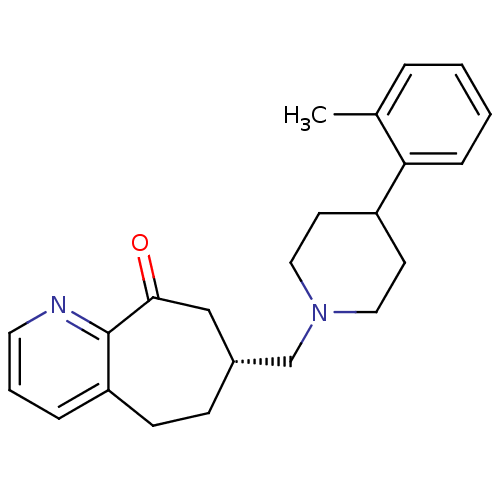
((R)-7-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,8-te...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3C(=O)C2)CC1 |r| Show InChI InChI=1S/C23H28N2O/c1-17-5-2-3-7-21(17)19-10-13-25(14-11-19)16-18-8-9-20-6-4-12-24-23(20)22(26)15-18/h2-7,12,18-19H,8-11,13-16H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243797
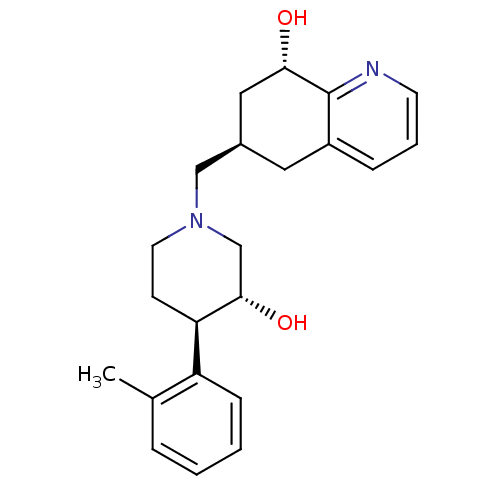
((6R,8S)-6-(((3R,4R)-3-hydroxy-4-o-tolylpiperidin-1...)Show SMILES Cc1ccccc1[C@H]1CCN(C[C@H]2C[C@H](O)c3ncccc3C2)C[C@@H]1O |r| Show InChI InChI=1S/C22H28N2O2/c1-15-5-2-3-7-18(15)19-8-10-24(14-21(19)26)13-16-11-17-6-4-9-23-22(17)20(25)12-16/h2-7,9,16,19-21,25-26H,8,10-14H2,1H3/t16-,19-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244374
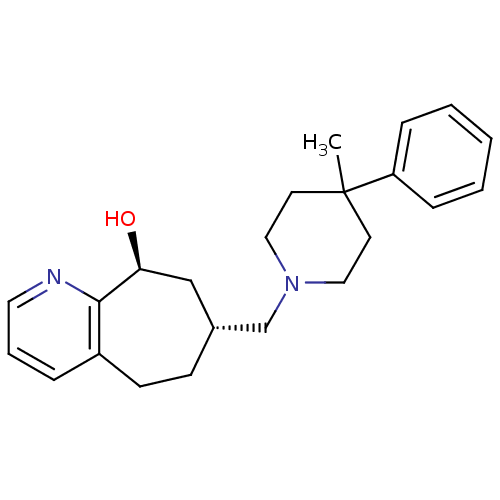
((7R,9S)-7-((4-methyl-4-phenylpiperidin-1-yl)methyl...)Show SMILES CC1(CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C23H30N2O/c1-23(20-7-3-2-4-8-20)11-14-25(15-12-23)17-18-9-10-19-6-5-13-24-22(19)21(26)16-18/h2-8,13,18,21,26H,9-12,14-17H2,1H3/t18-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50061101
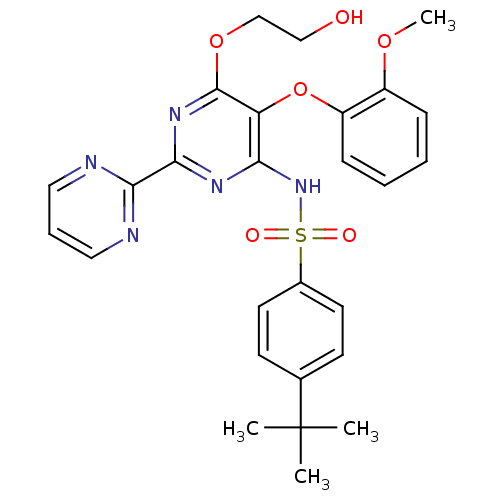
(4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...)Show SMILES COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)nc(nc1OCCO)-c1ncccn1 Show InChI InChI=1S/C27H29N5O6S/c1-27(2,3)18-10-12-19(13-11-18)39(34,35)32-23-22(38-21-9-6-5-8-20(21)36-4)26(37-17-16-33)31-25(30-23)24-28-14-7-15-29-24/h5-15,33H,16-17H2,1-4H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1262-70 (1999)
BindingDB Entry DOI: 10.7270/Q2Q52N5Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244296
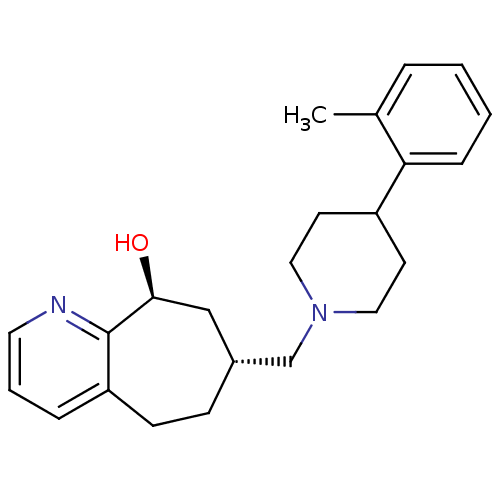
((-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]m...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C23H30N2O/c1-17-5-2-3-7-21(17)19-10-13-25(14-11-19)16-18-8-9-20-6-4-12-24-23(20)22(26)15-18/h2-7,12,18-19,22,26H,8-11,13-16H2,1H3/t18-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM614412
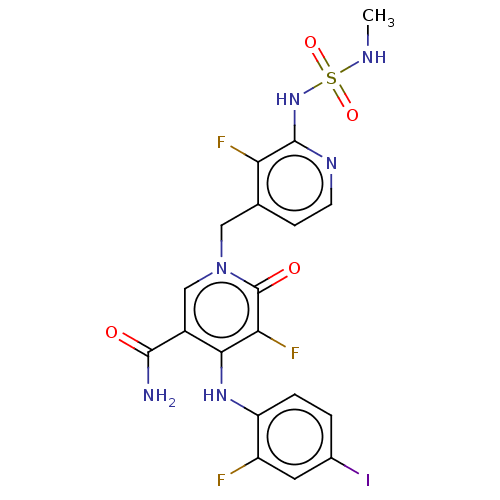
(US20230270730, Compound N-1)Show SMILES CNS(=O)(=O)Nc1nccc(Cn2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2=O)c1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM614413
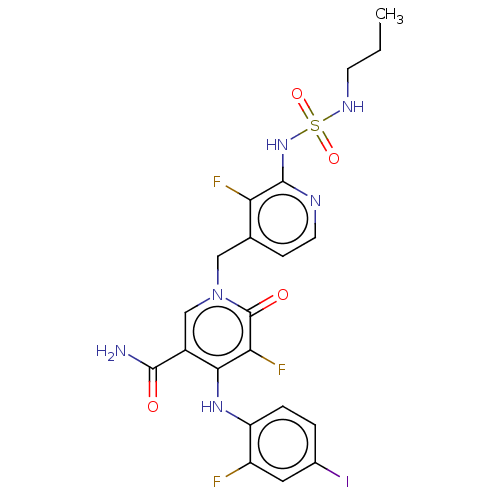
(US20230270730, Compound N-2)Show SMILES CCCNS(=O)(=O)Nc1nccc(Cn2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2=O)c1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614413

(US20230270730, Compound N-2)Show SMILES CCCNS(=O)(=O)Nc1nccc(Cn2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2=O)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614276

(US20230270730, Compound A-29)Show SMILES NC(=O)c1cc(Cc2ccnc(NS(=O)(=O)NC3CCOCC3)c2F)c(F)c(F)c1Nc1ccc(I)cc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614317

(US20230270730, Compound D-1)Show SMILES COCCNS(=O)(=O)Nc1cccc(Cc2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614319

(US20230270730, Compound D-3)Show SMILES NC(=O)c1cc(Cc2cccc(NS(=O)(=O)NC[C@H]3CCCO3)c2F)c(F)c(F)c1Nc1ccc(I)cc1F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614347

(US20230270730, Compound E-13)Show SMILES CS(=O)(=O)Nc1cccc(Cc2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM264072

(US20230270730, Compound A-1)Show SMILES CN(CCc1cnn(c1)-c1cc(ccn1)C(=O)N[N+]#[C-])Cc1ccc(F)cc1 Show InChI InChI=1S/C20H19FN6O/c1-22-25-20(28)17-7-9-23-19(11-17)27-14-16(12-24-27)8-10-26(2)13-15-3-5-18(21)6-4-15/h3-7,9,11-12,14H,8,10,13H2,2H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM350231
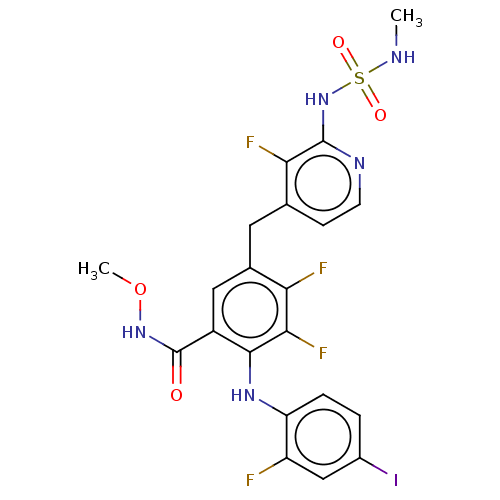
(US20230270730, Compound A-13)Show SMILES COc1ccc(cc1N(C)c1ccc(Oc2nc(O)c3ccncc3n2)cc1)N1CCN(C)CC1 Show InChI InChI=1S/C26H28N6O3/c1-30-12-14-32(15-13-30)19-6-9-24(34-3)23(16-19)31(2)18-4-7-20(8-5-18)35-26-28-22-17-27-11-10-21(22)25(33)29-26/h4-11,16-17H,12-15H2,1-3H3,(H,28,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244370

((7R,9S)-7-((4-(2,6-dichlorophenyl)piperidin-1-yl)m...)Show SMILES O[C@H]1C[C@H](CN2CCC(CC2)c2c(Cl)cccc2Cl)CCc2cccnc12 |r| Show InChI InChI=1S/C22H26Cl2N2O/c23-18-4-1-5-19(24)21(18)16-8-11-26(12-9-16)14-15-6-7-17-3-2-10-25-22(17)20(27)13-15/h1-5,10,15-16,20,27H,6-9,11-14H2/t15-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50293856

(1-(2,2-dimethyl-1,3-dioxan-5-yl)-3-{[1S,3R,6S)-2,1...)Show SMILES OC[C@@H](O)Cn1c2ccccc2n(C2CCN(C[C@H]3[C@H]4CC[C@H](C4)C33CC3)CC2)c1=O |r| Show InChI InChI=1S/C25H35N3O3/c29-16-20(30)14-27-22-3-1-2-4-23(22)28(24(27)31)19-7-11-26(12-8-19)15-21-17-5-6-18(13-17)25(21)9-10-25/h1-4,17-21,29-30H,5-16H2/t17-,18+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells |
J Med Chem 52: 4091-4 (2009)
Article DOI: 10.1021/jm900581g
BindingDB Entry DOI: 10.7270/Q2V98835 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614373
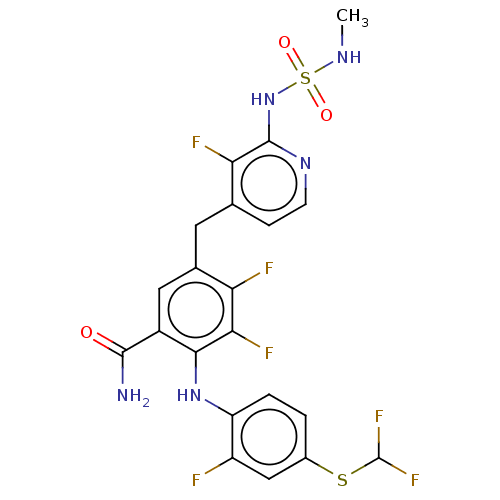
(US20230270730, Compound H-1)Show SMILES CNS(=O)(=O)Nc1nccc(Cc2cc(C(N)=O)c(Nc3ccc(SC(F)F)cc3F)c(F)c2F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614374
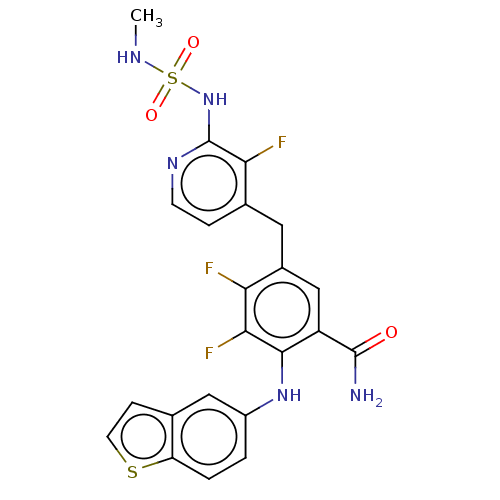
(US20230270730, Compound H-2)Show SMILES CNS(=O)(=O)Nc1nccc(Cc2cc(C(N)=O)c(Nc3ccc4sccc4c3)c(F)c2F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM614375
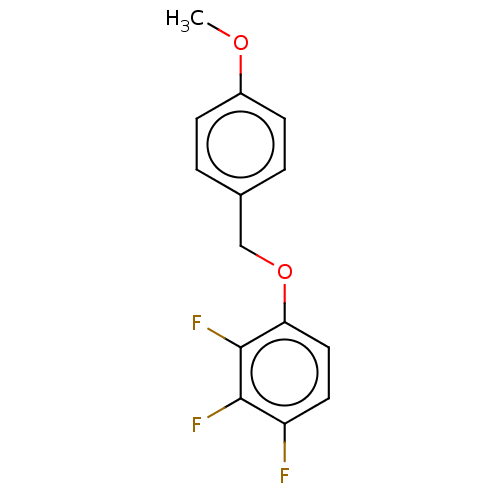
(US20230270730, Compound H-3) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614377

(US20230270730, Compound H-5)Show SMILES CNS(=O)(=O)Nc1cccc(Cc2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2F)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614380
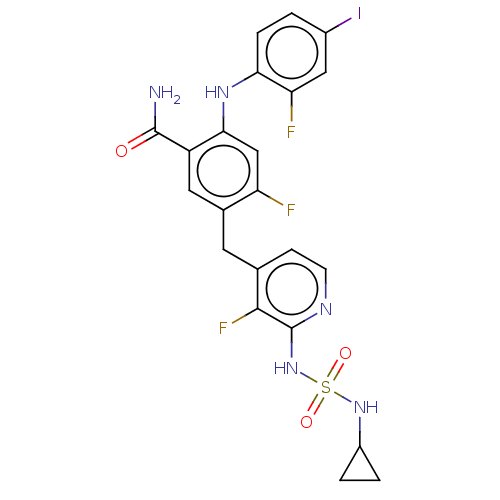
(US20230270730, Compound I-3)Show SMILES NC(=O)c1cc(Cc2ccnc(NS(=O)(=O)NC3CC3)c2F)c(F)cc1Nc1ccc(I)cc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614410
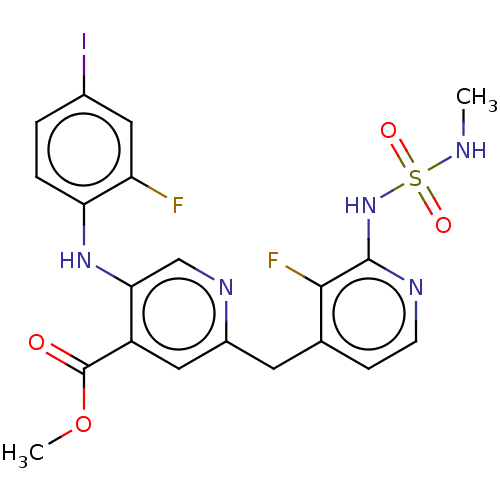
(US20230270730, Compound L-1)Show SMILES CNS(=O)(=O)Nc1nccc(Cc2cc(C(=O)OC)c(Nc3ccc(I)cc3F)cn2)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614412

(US20230270730, Compound N-1)Show SMILES CNS(=O)(=O)Nc1nccc(Cn2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2=O)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM614415
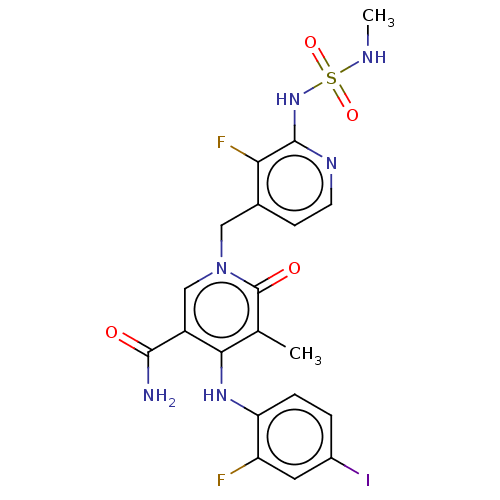
(US20230270730, Compound P-1)Show SMILES CNS(=O)(=O)Nc1nccc(Cn2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(C)c2=O)c1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614389
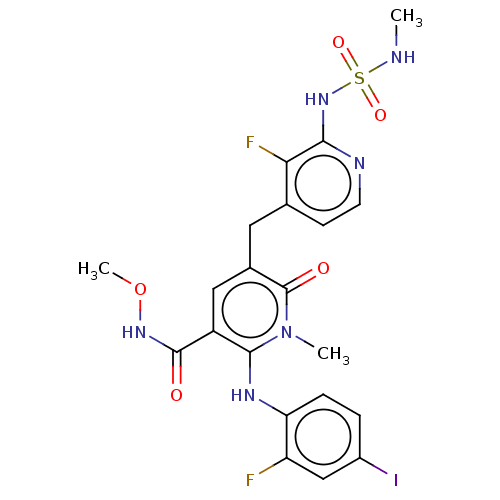
(US20230270730, Compound J-8)Show SMILES CNS(=O)(=O)Nc1nccc(Cc2cc(C(=O)NOC)c(Nc3ccc(I)cc3F)n(C)c2=O)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614272

(US20230270730, Compound A-25)Show SMILES CNS(=O)(=O)Nc1nccc(Cc2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614280
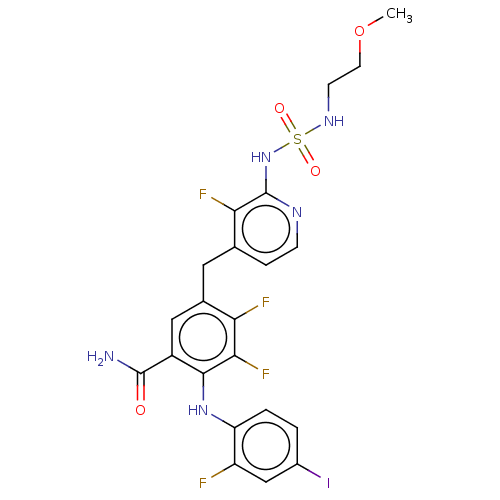
(US20230270730, Compound A-33)Show SMILES COCCNS(=O)(=O)Nc1nccc(Cc2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614287
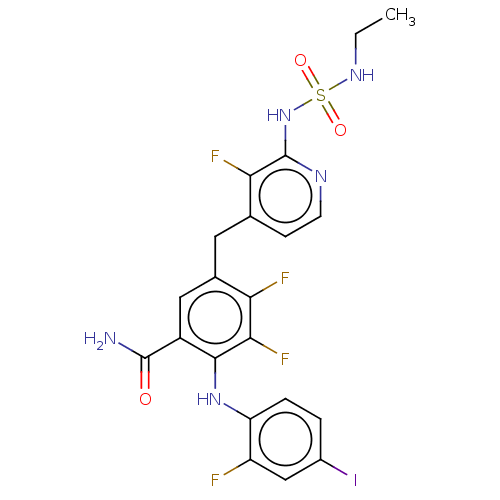
(US20230270730, Compound A-40)Show SMILES CCNS(=O)(=O)Nc1nccc(Cc2cc(C(N)=O)c(Nc3ccc(I)cc3F)c(F)c2F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM614303
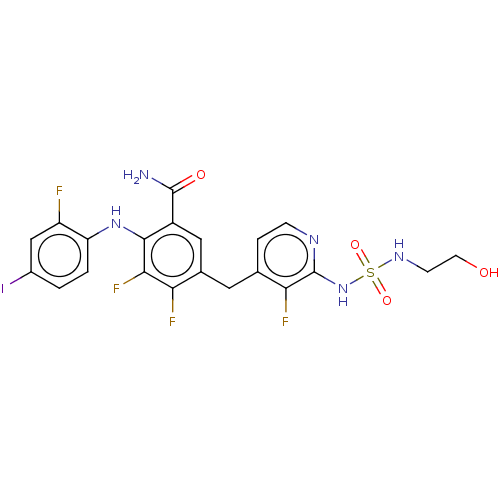
(US20230270730, Compound B-9)Show SMILES NC(=O)c1cc(Cc2ccnc(NS(=O)(=O)NCCO)c2F)c(F)c(F)c1Nc1ccc(I)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM614342

(US20230270730, Compound E-8)Show SMILES NC(=O)c1cc(Cc2cccc(NS(N)(=O)=O)c2F)c(F)c(F)c1Nc1ccc(I)cc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10040779 (2018)
BindingDB Entry DOI: 10.7270/Q2736W19 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data