 Found 15352 hits with Last Name = 'lin' and Initial = 't'
Found 15352 hits with Last Name = 'lin' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059510

(8-Methoxy-3-phenyl-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C19H17NO3/c1-22-14-7-8-16-15-9-10-20(13-5-3-2-4-6-13)12-17(15)19(21)23-18(16)11-14/h2-8,11H,9-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271367
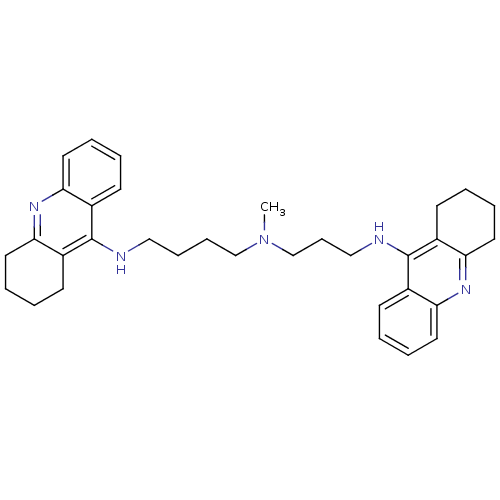
(CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H43N5/c1-39(24-12-22-36-34-27-15-4-8-19-31(27)38-32-20-9-5-16-28(32)34)23-11-10-21-35-33-25-13-2-6-17-29(25)37-30-18-7-3-14-26(30)33/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50271556
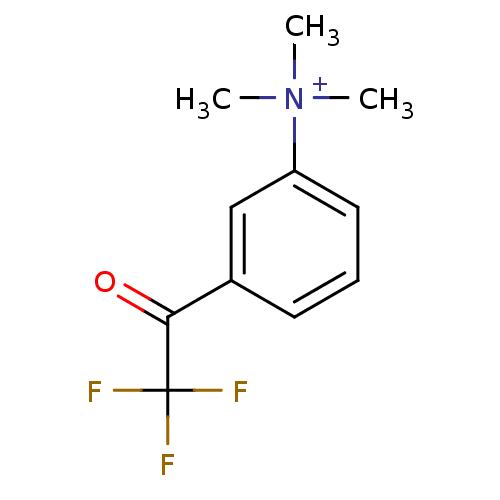
(CHEMBL525622 | N,N,N-trimethyl-3-(2,2,2-trifluoroa...)Show InChI InChI=1S/C11H13F3NO/c1-15(2,3)9-6-4-5-8(7-9)10(16)11(12,13)14/h4-7H,1-3H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10597
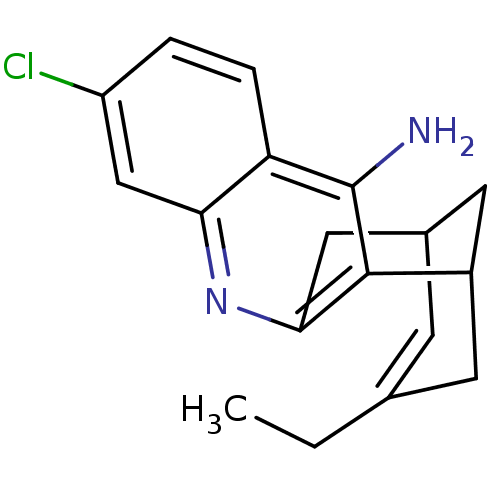
((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:11:9:5:3.2.7,19:8:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... |
J Med Chem 49: 3421-5 (2006)
Article DOI: 10.1021/jm060257t
BindingDB Entry DOI: 10.7270/Q2WW7FWB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0269 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity against Rat 5-hydroxytryptamine 7 receptor using [3H]-5-HT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50253328
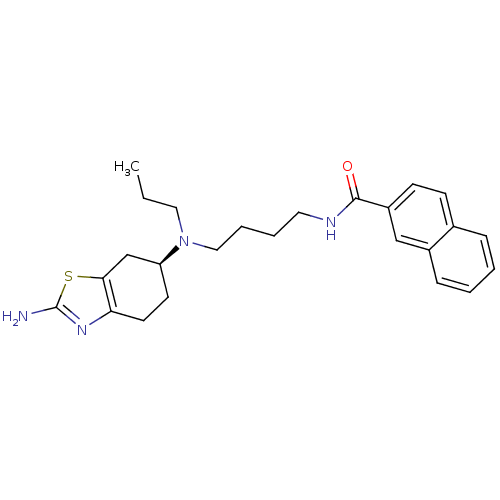
((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...)Show SMILES CCCN(CCCCNC(=O)c1ccc2ccccc2c1)[C@H]1CCc2nc(N)sc2C1 |r| Show InChI InChI=1S/C25H32N4OS/c1-2-14-29(21-11-12-22-23(17-21)31-25(26)28-22)15-6-5-13-27-24(30)20-10-9-18-7-3-4-8-19(18)16-20/h3-4,7-10,16,21H,2,5-6,11-15,17H2,1H3,(H2,26,28)(H,27,30)/t21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum |
J Med Chem 51: 5905-8 (2008)
Article DOI: 10.1021/jm800471h
BindingDB Entry DOI: 10.7270/Q2FN161H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50199865
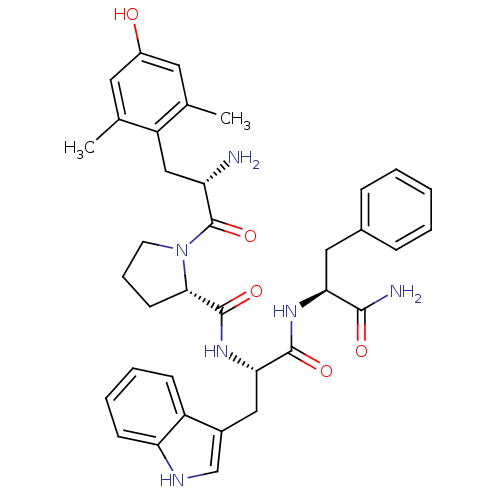
((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(18-24-20-39-29-12-7-6-11-26(24)29)34(45)40-30(33(38)44)17-23-9-4-3-5-10-23/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8965
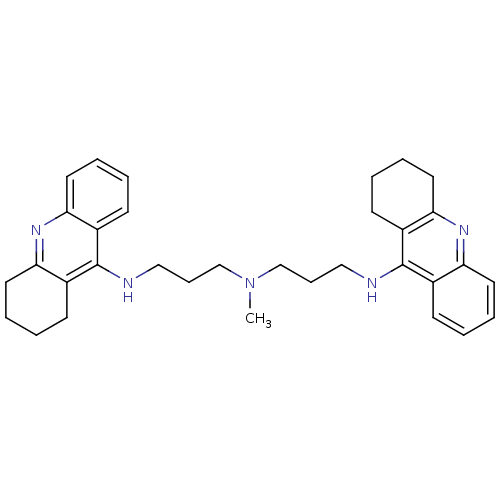
(CHEMBL338755 | Tacrine Dimer 4a | methylbis[3-(1,2...)Show SMILES CN(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H41N5/c1-38(22-10-20-34-32-24-12-2-6-16-28(24)36-29-17-7-3-13-25(29)32)23-11-21-35-33-26-14-4-8-18-30(26)37-31-19-9-5-15-27(31)33/h2,4,6,8,12,14,16,18H,3,5,7,9-11,13,15,17,19-23H2,1H3,(H,34,36)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | -58.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena
| Assay Description
Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... |
J Med Chem 48: 1919-29 (2005)
Article DOI: 10.1021/jm049510k
BindingDB Entry DOI: 10.7270/Q27P8WMJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199865

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(18-24-20-39-29-12-7-6-11-26(24)29)34(45)40-30(33(38)44)17-23-9-4-3-5-10-23/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50149201
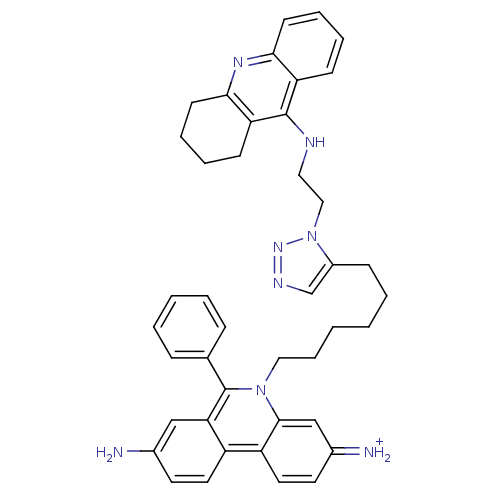
(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50272031

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES CCCCCCC(N)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C44H57N7O6/c1-4-5-6-10-17-34(45)41(54)50-38(25-33-27(2)21-31(52)22-28(33)3)44(57)51-20-13-19-39(51)43(56)49-37(24-30-26-47-35-18-12-11-16-32(30)35)42(55)48-36(40(46)53)23-29-14-8-7-9-15-29/h7-9,11-12,14-16,18,21-22,26,34,36-39,47,52H,4-6,10,13,17,19-20,23-25,45H2,1-3H3,(H2,46,53)(H,48,55)(H,49,56)(H,50,54)/t34?,36-,37-,38-,39-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in human SHSY5Y cells |
Bioorg Med Chem 16: 6286-96 (2008)
Article DOI: 10.1016/j.bmc.2008.04.020
BindingDB Entry DOI: 10.7270/Q2ZG6S2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271469
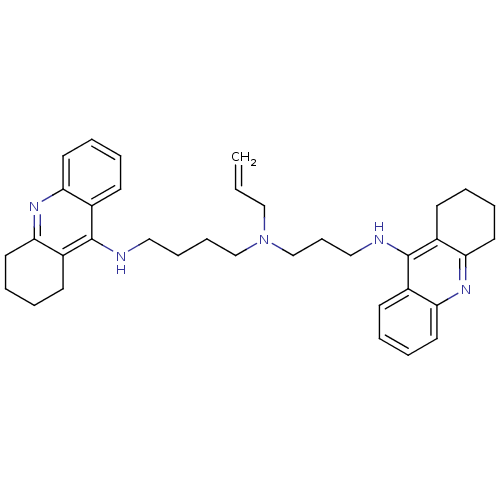
(CHEMBL507174 | N-Allyl-N-(1,2,3,4-tetrahydroacridi...)Show SMILES C=CCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C36H45N5/c1-2-24-41(26-13-23-38-36-29-16-5-9-20-33(29)40-34-21-10-6-17-30(34)36)25-12-11-22-37-35-27-14-3-7-18-31(27)39-32-19-8-4-15-28(32)35/h2-3,5,7,9,14,16,18,20H,1,4,6,8,10-13,15,17,19,21-26H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(RAT) | BDBM86689
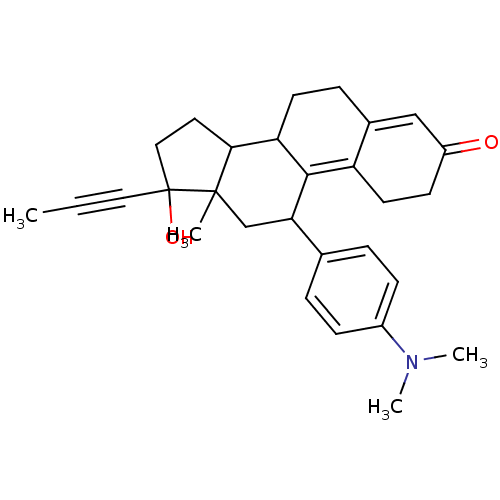
(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8977
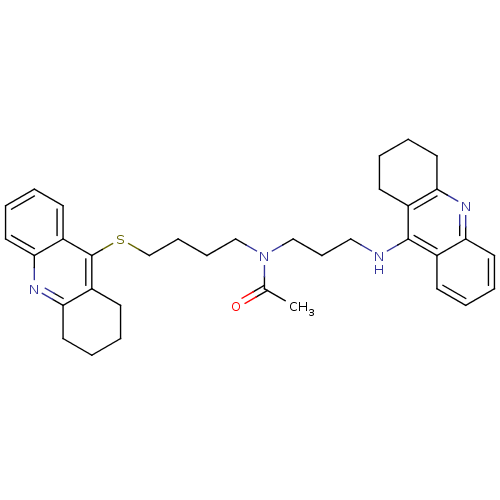
(CHEMBL175949 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...)Show SMILES CC(=O)N(CCCCSc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H42N4OS/c1-25(40)39(23-12-21-36-34-26-13-2-6-17-30(26)37-31-18-7-3-14-27(31)34)22-10-11-24-41-35-28-15-4-8-19-32(28)38-33-20-9-5-16-29(33)35/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,37) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena
| Assay Description
Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... |
J Med Chem 48: 1919-29 (2005)
Article DOI: 10.1021/jm049510k
BindingDB Entry DOI: 10.7270/Q27P8WMJ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151078
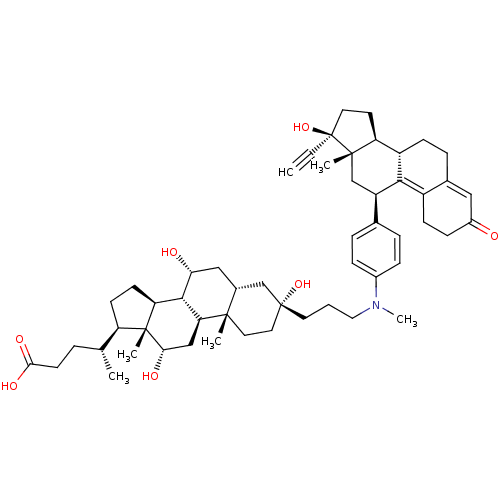
((4R)-4-[(1S,2S,5S,7R,9R,10R,11S,14R,15R,16S)-5-[3-...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@@](O)(CCCN(C)c5ccc(cc5)[C@H]5C[C@@]6(C)[C@@H](CC[C@@]6(O)C#C)[C@@H]6CCC7=CC(=O)CCC7=C56)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C |t:46,53| Show InChI InChI=1S/C54H75NO7/c1-7-54(62)23-21-42-39-16-12-34-27-37(56)15-17-38(34)48(39)40(31-51(42,54)4)33-10-13-36(14-11-33)55(6)26-8-22-53(61)25-24-50(3)35(30-53)28-45(57)49-43-19-18-41(32(2)9-20-47(59)60)52(43,5)46(58)29-44(49)50/h1,10-11,13-14,27,32,35,39-46,49,57-58,61-62H,8-9,12,15-26,28-31H2,2-6H3,(H,59,60)/t32-,35-,39+,40-,41-,42+,43+,44+,45-,46+,49+,50+,51+,52-,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271470
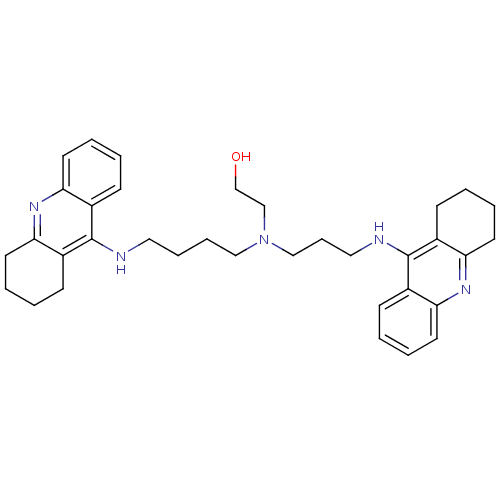
(CHEMBL499224 | N-(2-Hydroxyethyl)-N-(1,2,3,4-tetra...)Show SMILES OCCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H45N5O/c41-25-24-40(23-11-21-37-35-28-14-3-7-18-32(28)39-33-19-8-4-15-29(33)35)22-10-9-20-36-34-26-12-1-5-16-30(26)38-31-17-6-2-13-27(31)34/h1,3,5,7,12,14,16,18,41H,2,4,6,8-11,13,15,17,19-25H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50369748
(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271471
(1,4-bis[3-(1,2,3,4-Tetrahydroacridin-9-yl)aminopro...)Show SMILES C(CNc1c2CCCCc2nc2ccccc12)CN1CCN(CCCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C36H46N6/c1-5-15-31-27(11-1)35(28-12-2-6-16-32(28)39-31)37-19-9-21-41-23-25-42(26-24-41)22-10-20-38-36-29-13-3-7-17-33(29)40-34-18-8-4-14-30(34)36/h1,3,5,7,11,13,15,17H,2,4,6,8-10,12,14,16,18-26H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM50090697
(7-Chloro-2-phenyl-[1,8]naphthyridin-4-ol | 7-chlor...)Show InChI InChI=1S/C14H9ClN2O/c15-13-7-6-10-12(18)8-11(16-14(10)17-13)9-4-2-1-3-5-9/h1-8H,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor in bovine cortical membranes using [3H]-CHA as radioligand |
J Med Chem 47: 3019-31 (2004)
Article DOI: 10.1021/jm030977p
BindingDB Entry DOI: 10.7270/Q2BC3Z07 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-quinpirole from human dopamine D2A receptors expressed in LtK cells |
Bioorg Med Chem Lett 9: 2167-72 (1999)
BindingDB Entry DOI: 10.7270/Q2CF9P97 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151076
((4R)-4-[(1S,2S,7R,10R,11S,14R,15R,16S)-5-[2-({4-[(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-23-44-41-16-11-34-28-37(55)15-18-39(34)49(41)42(31-51(44,53)4)33-9-13-36(14-10-33)54(6)26-27-60-38-22-24-50(3)35(29-38)12-17-40-45-20-19-43(32(2)8-21-48(57)58)52(45,5)47(56)30-46(40)50/h1,9-10,13-14,28,32,35,38,40-47,56,59H,8,11-12,15-27,29-31H2,2-6H3,(H,57,58)/t32-,35-,38?,40+,41+,42-,43-,44+,45+,46+,47+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190
(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271468
(CHEMBL490060 | N-Ethyl-N-(1,2,3,4-tetrahydroacridi...)Show SMILES CCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H45N5/c1-2-40(25-13-23-37-35-28-16-5-9-20-32(28)39-33-21-10-6-17-29(33)35)24-12-11-22-36-34-26-14-3-7-18-30(26)38-31-19-8-4-15-27(31)34/h3,5,7,9,14,16,18,20H,2,4,6,8,10-13,15,17,19,21-25H2,1H3,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50410188
(CHEMBL2096708)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39+,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151077
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R)-5-[2-({4-[(1...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-22-43-40-15-11-34-28-37(55)14-16-39(34)48(40)41(31-52(43,53)5)33-9-12-36(13-10-33)54(6)26-27-60-38-20-23-50(3)35(29-38)30-46(56)49-44-18-17-42(32(2)8-19-47(57)58)51(44,4)24-21-45(49)50/h1,9-10,12-13,28,32,35,38,40-46,49,56,59H,8,11,14-27,29-31H2,2-6H3,(H,57,58)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,49+,50+,51-,52+,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190

(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of rhesus monkey plasma renin. |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394157
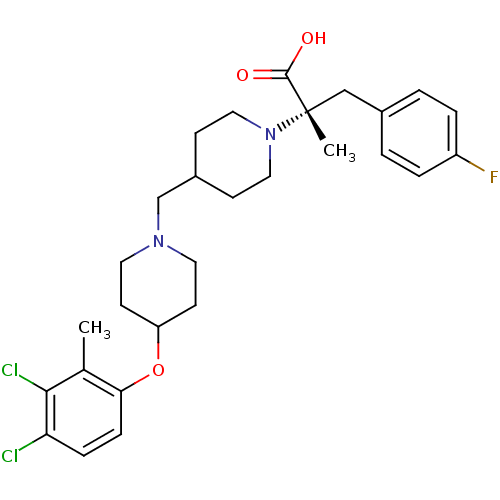
(CHEMBL2158790)Show SMILES Cc1c(Cl)c(Cl)ccc1OC1CCN(CC2CCN(CC2)[C@@](C)(Cc2ccc(F)cc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C28H35Cl2FN2O3/c1-19-25(8-7-24(29)26(19)30)36-23-11-13-32(14-12-23)18-21-9-15-33(16-10-21)28(2,27(34)35)17-20-3-5-22(31)6-4-20/h3-8,21,23H,9-18H2,1-2H3,(H,34,35)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50410187
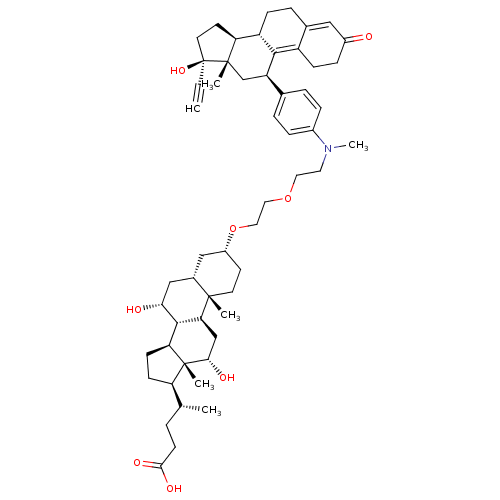
(CHEMBL2096804)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@@H](CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCOCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C55H77NO8/c1-7-55(62)23-21-44-41-15-11-35-28-38(57)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)56(6)24-25-63-26-27-64-39-20-22-52(3)36(29-39)30-47(58)51-45-18-17-43(33(2)8-19-49(60)61)54(45,5)48(59)31-46(51)52/h1,9-10,12-13,28,33,36,39,41-48,51,58-59,62H,8,11,14-27,29-32H2,2-6H3,(H,60,61)/t33-,36+,39-,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151064
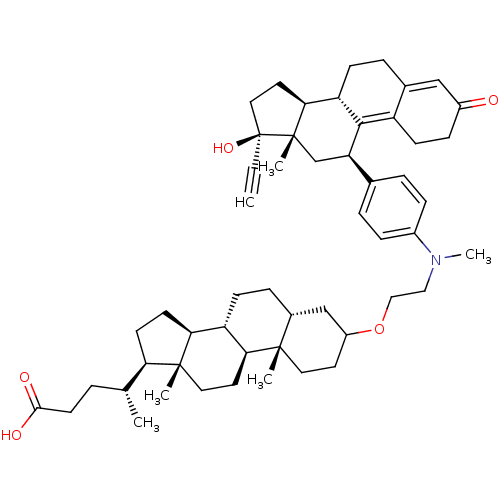
((4R)-4-[(1S,2S,7R,10R,11S,14R,15R)-5-[2-({4-[(10S,...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:57,64| Show InChI InChI=1S/C53H73NO5/c1-7-53(58)27-24-47-42-16-11-35-30-38(55)15-18-40(35)49(42)43(32-52(47,53)5)34-9-13-37(14-10-34)54(6)28-29-59-39-22-25-50(3)36(31-39)12-17-41-45-20-19-44(33(2)8-21-48(56)57)51(45,4)26-23-46(41)50/h1,9-10,13-14,30,33,36,39,41-47,58H,8,11-12,15-29,31-32H2,2-6H3,(H,56,57)/t33-,36-,39?,41+,42+,43-,44-,45+,46+,47+,50+,51-,52+,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151068
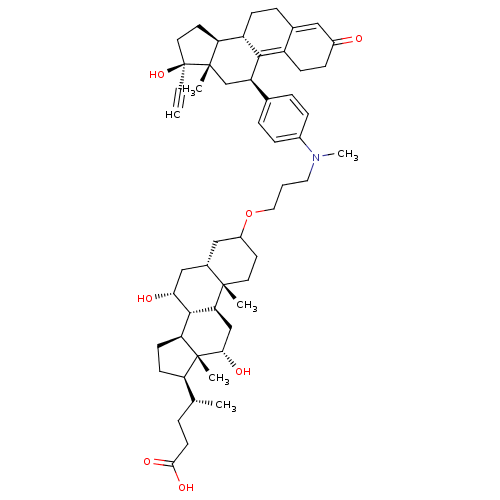
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[3-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:60,67| Show InChI InChI=1S/C54H75NO7/c1-7-54(61)24-22-43-40-16-12-34-27-37(56)15-17-39(34)49(40)41(31-52(43,54)4)33-10-13-36(14-11-33)55(6)25-8-26-62-38-21-23-51(3)35(28-38)29-46(57)50-44-19-18-42(32(2)9-20-48(59)60)53(44,5)47(58)30-45(50)51/h1,10-11,13-14,27,32,35,38,40-47,50,57-58,61H,8-9,12,15-26,28-31H2,2-6H3,(H,59,60)/t32-,35+,38?,40+,41-,42-,43+,44+,45+,46-,47+,50+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271325
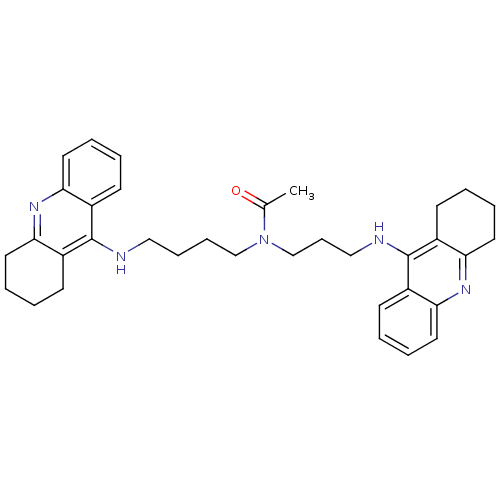
(CHEMBL451277 | N-{4-[(1,2,3,4-Tetrahydroacridin-9-...)Show SMILES CC(=O)N(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N5O/c1-25(41)40(24-12-22-37-35-28-15-4-8-19-32(28)39-33-20-9-5-16-29(33)35)23-11-10-21-36-34-26-13-2-6-17-30(26)38-31-18-7-3-14-27(31)34/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8975
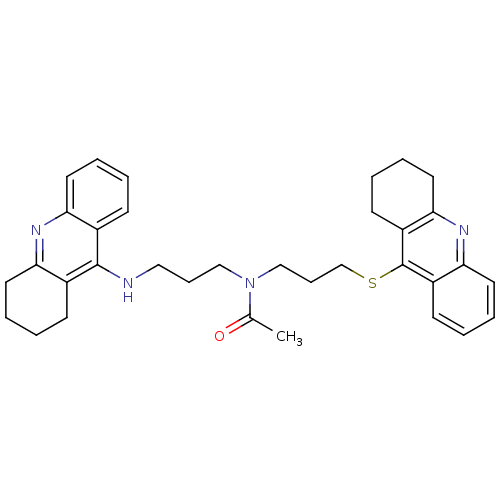
(CHEMBL179192 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...)Show SMILES CC(=O)N(CCCNc1c2CCCCc2nc2ccccc12)CCCSc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H40N4OS/c1-24(39)38(21-10-20-35-33-25-12-2-6-16-29(25)36-30-17-7-3-13-26(30)33)22-11-23-40-34-27-14-4-8-18-31(27)37-32-19-9-5-15-28(32)34/h2,4,6,8,12,14,16,18H,3,5,7,9-11,13,15,17,19-23H2,1H3,(H,35,36) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena
| Assay Description
Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... |
J Med Chem 48: 1919-29 (2005)
Article DOI: 10.1021/jm049510k
BindingDB Entry DOI: 10.7270/Q27P8WMJ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50152456
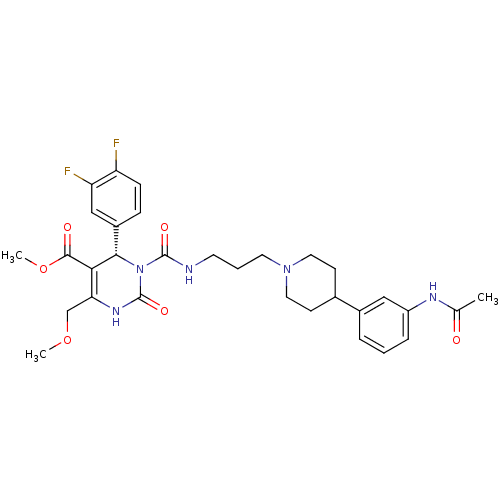
((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...)Show SMILES COCC1=C([C@@H](N(C(=O)NCCCN2CCC(CC2)c2cccc(NC(C)=O)c2)C(=O)N1)c1ccc(F)c(F)c1)C(=O)OC |t:3| Show InChI InChI=1S/C31H37F2N5O6/c1-19(39)35-23-7-4-6-21(16-23)20-10-14-37(15-11-20)13-5-12-34-30(41)38-28(22-8-9-24(32)25(33)17-22)27(29(40)44-3)26(18-43-2)36-31(38)42/h4,6-9,16-17,20,28H,5,10-15,18H2,1-3H3,(H,34,41)(H,35,39)(H,36,42)/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor in bovine cortical membranes using [3H]-CHA as radioligand |
J Med Chem 47: 3019-31 (2004)
Article DOI: 10.1021/jm030977p
BindingDB Entry DOI: 10.7270/Q2BC3Z07 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50152456

((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...)Show SMILES COCC1=C([C@@H](N(C(=O)NCCCN2CCC(CC2)c2cccc(NC(C)=O)c2)C(=O)N1)c1ccc(F)c(F)c1)C(=O)OC |t:3| Show InChI InChI=1S/C31H37F2N5O6/c1-19(39)35-23-7-4-6-21(16-23)20-10-14-37(15-11-20)13-5-12-34-30(41)38-28(22-8-9-24(32)25(33)17-22)27(29(40)44-3)26(18-43-2)36-31(38)42/h4,6-9,16-17,20,28H,5,10-15,18H2,1-3H3,(H,34,41)(H,35,39)(H,36,42)/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151067
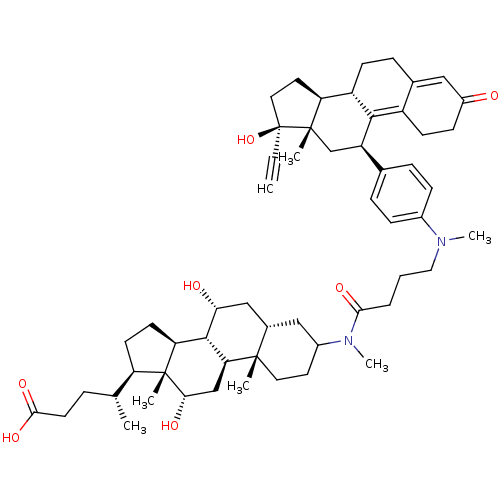
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-5-[4-({4...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)C(=O)CCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C56H78N2O7/c1-8-56(65)26-24-44-41-18-14-35-28-39(59)17-19-40(35)51(41)42(32-54(44,56)4)34-12-15-37(16-13-34)57(6)27-9-10-49(62)58(7)38-23-25-53(3)36(29-38)30-47(60)52-45-21-20-43(33(2)11-22-50(63)64)55(45,5)48(61)31-46(52)53/h1,12-13,15-16,28,33,36,38,41-48,52,60-61,65H,9-11,14,17-27,29-32H2,2-7H3,(H,63,64)/t33-,36+,38?,41+,42-,43-,44+,45+,46+,47-,48+,52+,53+,54+,55-,56+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271323
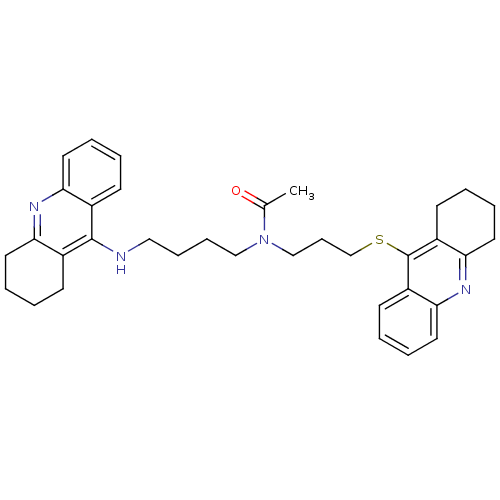
(CHEMBL501587 | N-{4-[(1,2,3, 4-Tetrahydroacridin-9...)Show SMILES CC(=O)N(CCCCNc1c2CCCCc2nc2ccccc12)CCCSc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H42N4OS/c1-25(40)39(23-12-24-41-35-28-15-4-8-19-32(28)38-33-20-9-5-16-29(33)35)22-11-10-21-36-34-26-13-2-6-17-30(26)37-31-18-7-3-14-27(31)34/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151059
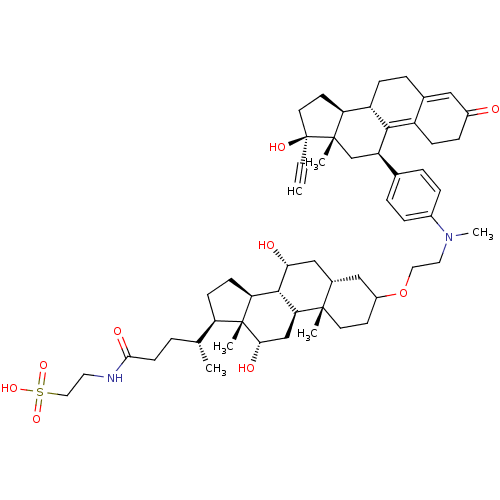
(2-[(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[2-...)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:65,72| Show InChI InChI=1S/C55H78N2O9S/c1-7-55(62)23-21-44-41-15-11-35-28-38(58)14-16-40(35)50(41)42(32-53(44,55)4)34-9-12-37(13-10-34)57(6)25-26-66-39-20-22-52(3)36(29-39)30-47(59)51-45-18-17-43(54(45,5)48(60)31-46(51)52)33(2)8-19-49(61)56-24-27-67(63,64)65/h1,9-10,12-13,28,33,36,39,41-48,51,59-60,62H,8,11,14-27,29-32H2,2-6H3,(H,56,61)(H,63,64,65)/t33-,36+,39?,41+,42-,43-,44+,45+,46+,47-,48+,51+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50353148
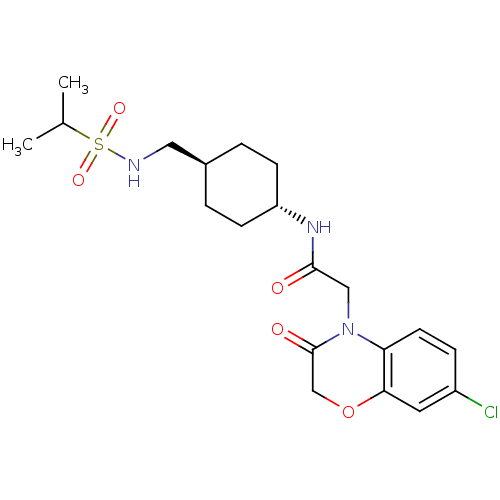
(CHEMBL1829320)Show SMILES CC(C)S(=O)(=O)NC[C@H]1CC[C@@H](CC1)NC(=O)CN1C(=O)COc2cc(Cl)ccc12 |r,wU:11.14,wD:8.7,(10,-2.04,;8.67,-1.27,;8.67,.27,;7.34,-2.03,;6.55,-3.36,;8.09,-3.36,;6.01,-1.26,;4.67,-2.02,;3.34,-1.25,;3.34,.29,;2,1.07,;.67,.29,;.67,-1.25,;2,-2.02,;-.66,1.06,;-1.99,.28,;-1.99,-1.26,;-3.33,1.06,;-4.66,.28,;-4.66,-1.26,;-3.33,-2.03,;-6,-2.03,;-7.33,-1.26,;-7.33,.27,;-8.67,1.04,;-8.67,2.59,;-10,3.35,;-7.34,3.36,;-6,2.6,;-5.99,1.05,)| Show InChI InChI=1S/C20H28ClN3O5S/c1-13(2)30(27,28)22-10-14-3-6-16(7-4-14)23-19(25)11-24-17-8-5-15(21)9-18(17)29-12-20(24)26/h5,8-9,13-14,16,22H,3-4,6-7,10-12H2,1-2H3,(H,23,25)/t14-,16- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptide YY from human NPY5 receptor |
Bioorg Med Chem Lett 22: 2167-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.117
BindingDB Entry DOI: 10.7270/Q2BV7HNQ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151060
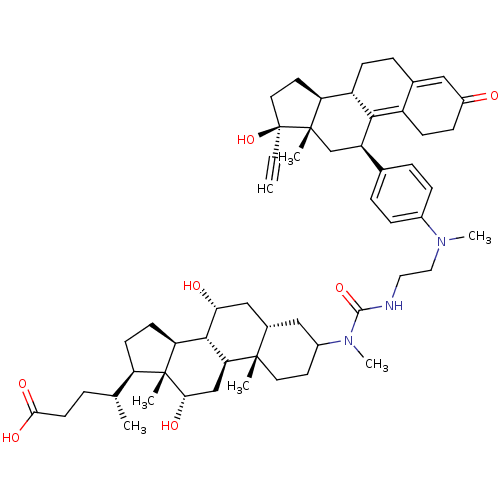
((4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)N(C)C(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:63,70| Show InChI InChI=1S/C55H77N3O7/c1-8-55(65)24-22-43-40-16-12-34-27-38(59)15-17-39(34)49(40)41(31-53(43,55)4)33-10-13-36(14-11-33)57(6)26-25-56-51(64)58(7)37-21-23-52(3)35(28-37)29-46(60)50-44-19-18-42(32(2)9-20-48(62)63)54(44,5)47(61)30-45(50)52/h1,10-11,13-14,27,32,35,37,40-47,50,60-61,65H,9,12,15-26,28-31H2,2-7H3,(H,56,64)(H,62,63)/t32-,35+,37?,40+,41-,42-,43+,44+,45+,46-,47+,50+,52+,53+,54-,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50379608

(CHEMBL2013020)Show SMILES CC(C)S(=O)(=O)NC[C@H]1CC[C@@H](CC1)NC(=O)Cn1ccc2cc(Cl)ccc12 |r,wU:8.7,wD:11.14,(12.87,-43.9,;11.53,-43.13,;11.53,-41.59,;10.2,-43.89,;10.96,-45.22,;9.43,-45.22,;8.86,-43.13,;7.53,-43.9,;6.2,-43.12,;4.87,-43.89,;3.54,-43.12,;3.54,-41.58,;4.87,-40.8,;6.2,-41.58,;2.2,-40.82,;.87,-41.59,;.88,-43.13,;-.46,-40.82,;-1.8,-41.6,;-1.96,-43.13,;-3.47,-43.45,;-4.24,-42.12,;-5.74,-41.81,;-6.22,-40.35,;-7.73,-40.04,;-5.19,-39.2,;-3.68,-39.52,;-3.21,-40.98,)| Show InChI InChI=1S/C20H28ClN3O3S/c1-14(2)28(26,27)22-12-15-3-6-18(7-4-15)23-20(25)13-24-10-9-16-11-17(21)5-8-19(16)24/h5,8-11,14-15,18,22H,3-4,6-7,12-13H2,1-2H3,(H,23,25)/t15-,18- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA
Curated by ChEMBL
| Assay Description
Displacement of [125I]peptide YY from human NPY5 receptor |
Bioorg Med Chem Lett 22: 2167-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.117
BindingDB Entry DOI: 10.7270/Q2BV7HNQ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50400513
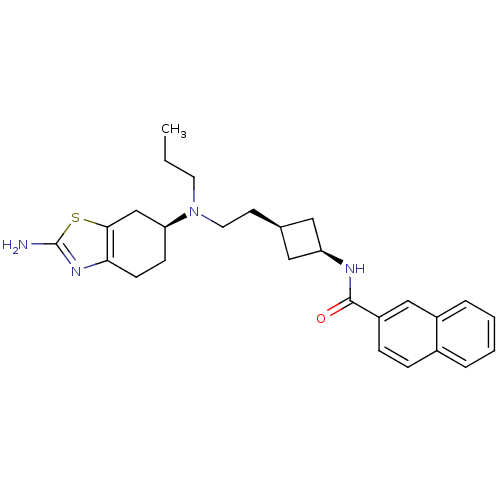
(CHEMBL2203404)Show SMILES CCCN(CC[C@H]1C[C@H](C1)NC(=O)c1ccc2ccccc2c1)[C@H]1CCc2nc(N)sc2C1 |r,wU:6.5,8.10,wD:23.25,(9.6,-44.79,;9.61,-43.25,;8.27,-42.48,;8.28,-40.94,;9.61,-40.17,;10.94,-40.94,;12.28,-40.18,;12.68,-38.7,;14.16,-39.1,;13.76,-40.58,;15.5,-38.33,;16.83,-39.11,;16.82,-40.65,;18.2,-38.37,;18.19,-36.83,;19.52,-36.07,;20.84,-36.83,;22.17,-36.06,;23.51,-36.84,;23.5,-38.38,;22.16,-39.14,;20.84,-38.36,;19.52,-39.12,;6.94,-40.17,;6.94,-38.63,;5.61,-37.85,;4.28,-38.62,;2.81,-38.14,;1.9,-39.39,;.36,-39.39,;2.81,-40.65,;4.28,-40.17,;5.61,-40.93,)| Show InChI InChI=1S/C27H34N4OS/c1-2-12-31(23-9-10-24-25(17-23)33-27(28)30-24)13-11-18-14-22(15-18)29-26(32)21-8-7-19-5-3-4-6-20(19)16-21/h3-8,16,18,22-23H,2,9-15,17H2,1H3,(H2,28,30)(H,29,32)/t18-,22+,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]R(+)-7-OH-DPAT from Sprague-Dawley rat dopamine D3 receptor after 90 mins |
ACS Med Chem Lett 2: 620-625 (2011)
Article DOI: 10.1021/ml200100t
BindingDB Entry DOI: 10.7270/Q2028SQ8 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151076

((4R)-4-[(1S,2S,7R,10R,11S,14R,15R,16S)-5-[2-({4-[(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:58,65| Show InChI InChI=1S/C53H73NO6/c1-7-53(59)25-23-44-41-16-11-34-28-37(55)15-18-39(34)49(41)42(31-51(44,53)4)33-9-13-36(14-10-33)54(6)26-27-60-38-22-24-50(3)35(29-38)12-17-40-45-20-19-43(32(2)8-21-48(57)58)52(45,5)47(56)30-46(40)50/h1,9-10,13-14,28,32,35,38,40-47,56,59H,8,11-12,15-27,29-31H2,2-6H3,(H,57,58)/t32-,35-,38?,40+,41+,42-,43-,44+,45+,46+,47+,50+,51+,52-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor dependent alkaline phosphatase activity |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50151069
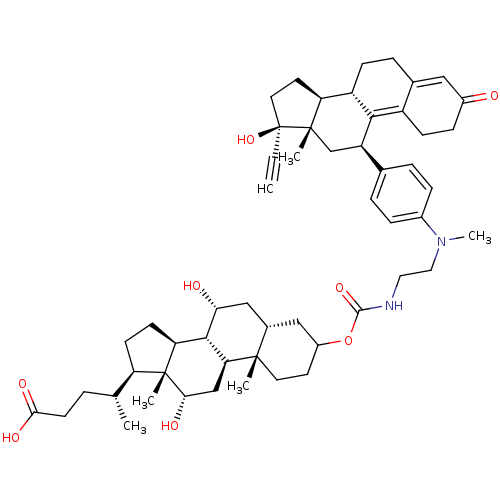
((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C54H74N2O8/c1-7-54(63)23-21-42-39-15-11-33-26-36(57)14-16-38(33)48(39)40(30-52(42,54)4)32-9-12-35(13-10-32)56(6)25-24-55-50(62)64-37-20-22-51(3)34(27-37)28-45(58)49-43-18-17-41(31(2)8-19-47(60)61)53(43,5)46(59)29-44(49)51/h1,9-10,12-13,26,31,34,37,39-46,49,58-59,63H,8,11,14-25,27-30H2,2-6H3,(H,55,62)(H,60,61)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151069

((4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-({[2-(...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OC(=O)NCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:62,69| Show InChI InChI=1S/C54H74N2O8/c1-7-54(63)23-21-42-39-15-11-33-26-36(57)14-16-38(33)48(39)40(30-52(42,54)4)32-9-12-35(13-10-32)56(6)25-24-55-50(62)64-37-20-22-51(3)34(27-37)28-45(58)49-43-18-17-41(31(2)8-19-47(60)61)53(43,5)46(59)29-44(49)51/h1,9-10,12-13,26,31,34,37,39-46,49,58-59,63H,8,11,14-25,27-30H2,2-6H3,(H,55,62)(H,60,61)/t31-,34+,37?,39+,40-,41-,42+,43+,44+,45-,46+,49+,51+,52+,53-,54+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50151074
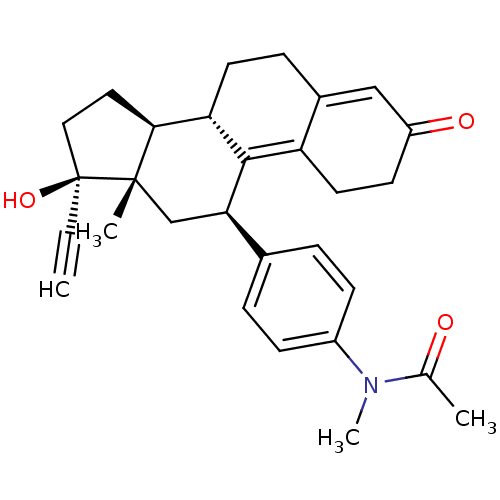
(CHEMBL364368 | N-[4-((2R,8S,13S,14S,17R)-17-Ethyny...)Show SMILES CN(C(C)=O)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:27,34| Show InChI InChI=1S/C29H33NO3/c1-5-29(33)15-14-26-24-12-8-20-16-22(32)11-13-23(20)27(24)25(17-28(26,29)3)19-6-9-21(10-7-19)30(4)18(2)31/h1,6-7,9-10,16,24-26,33H,8,11-15,17H2,2-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
J Med Chem 47: 4213-30 (2004)
Article DOI: 10.1021/jm0400045
BindingDB Entry DOI: 10.7270/Q22N532T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data