

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052442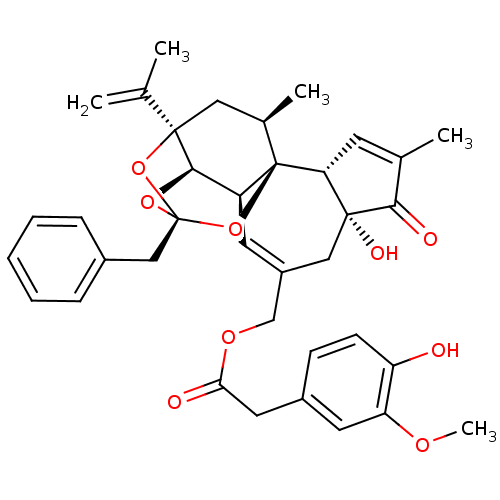 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsacin | J Med Chem 46: 3116-26 (2003) Article DOI: 10.1021/jm030089u BindingDB Entry DOI: 10.7270/Q2SB4551 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.220 | -56.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242266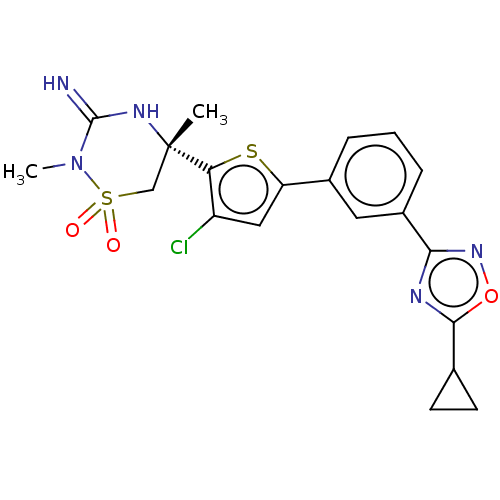 (US9416129, 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.240 | -55.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242250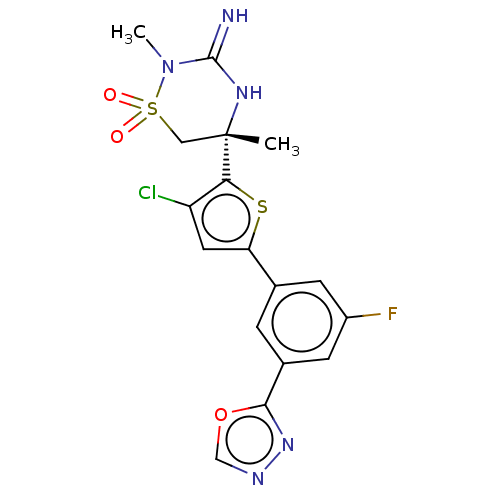 (US9416129, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.280 | -55.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242231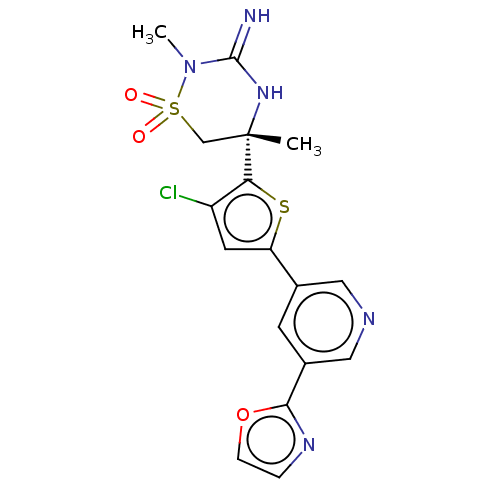 (US9416129, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.310 | -55.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50531563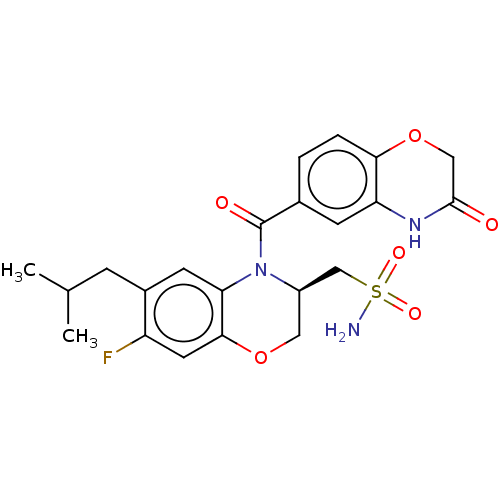 (CHEMBL4463240) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay | J Med Chem 62: 1385-1406 (2019) Article DOI: 10.1021/acs.jmedchem.8b01523 BindingDB Entry DOI: 10.7270/Q2GT5RP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50531598 (CHEMBL4572476) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay | J Med Chem 62: 1385-1406 (2019) Article DOI: 10.1021/acs.jmedchem.8b01523 BindingDB Entry DOI: 10.7270/Q2GT5RP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242269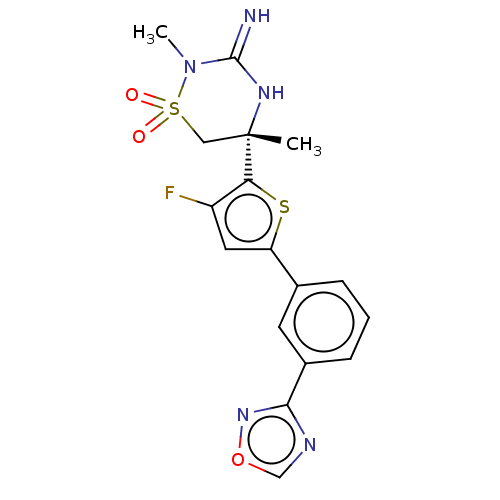 (US9416129, 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | -54.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242226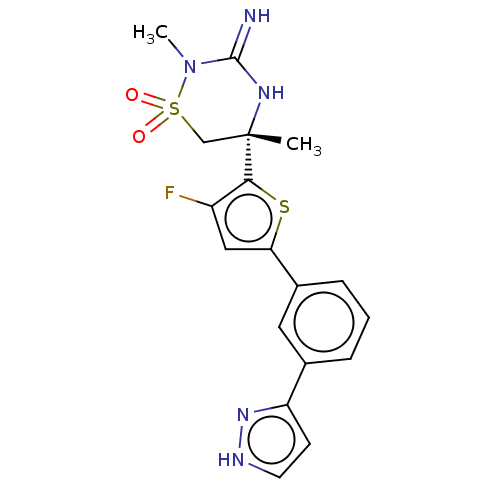 (US9416129, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | -54.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242263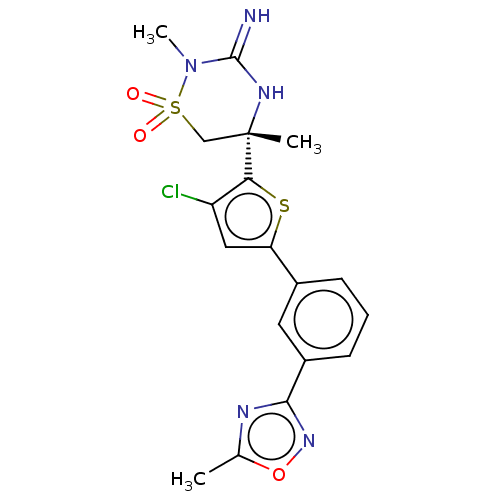 (US9416129, 41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | -54.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353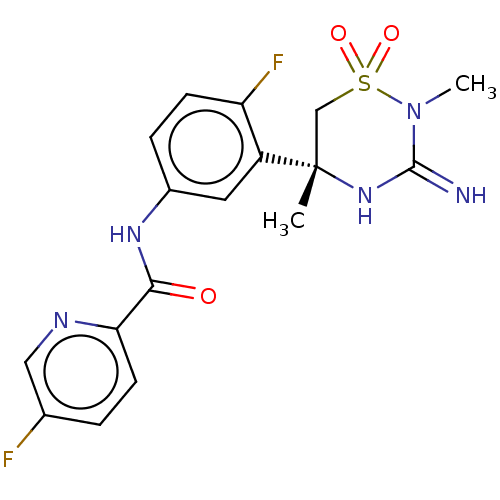 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.370 | -54.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143227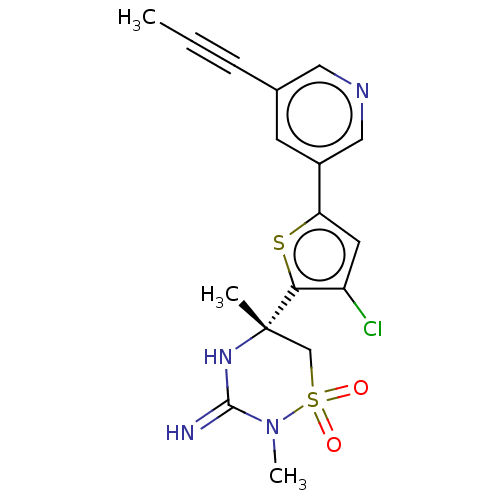 (US8940748, 52 | US9029362, 52 | US9687494, 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 0.370 | -54.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242268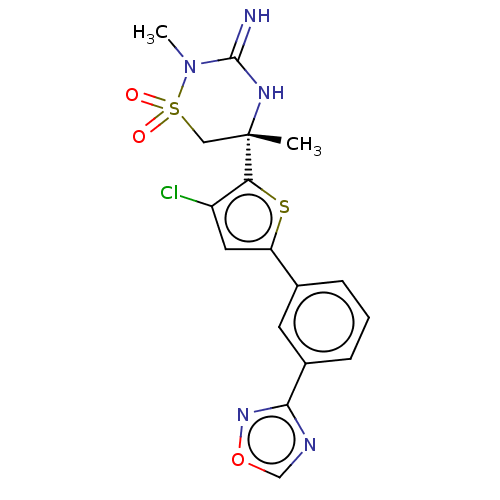 (US9416129, 46) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.410 | -54.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143223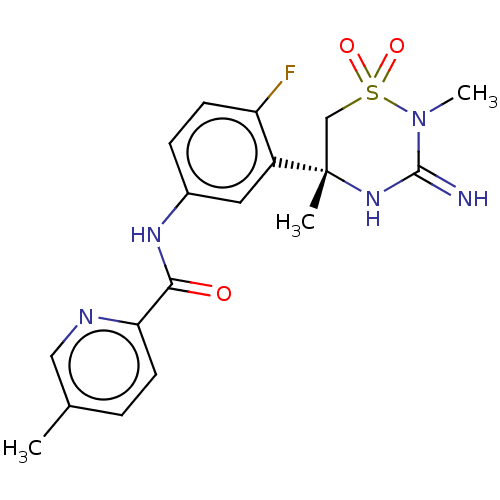 (US8940748, 35 | US9029362, 35 | US9687494, 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.450 | -54.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143224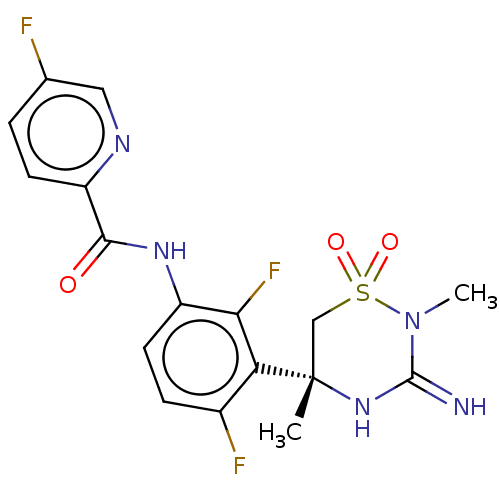 (US8940748, 173 | US9687494, 173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | -54.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242250 (US9416129, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50531587 (CHEMBL4471322) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay | J Med Chem 62: 1385-1406 (2019) Article DOI: 10.1021/acs.jmedchem.8b01523 BindingDB Entry DOI: 10.7270/Q2GT5RP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065428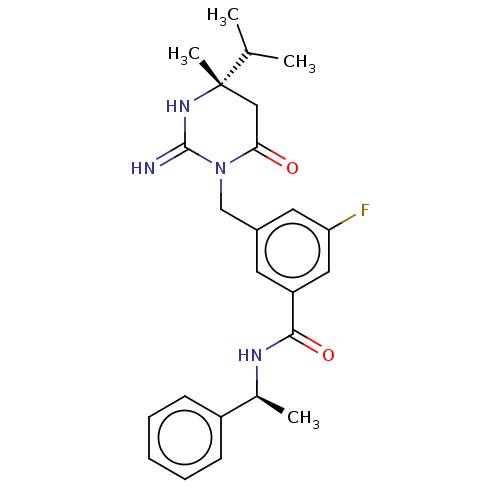 (CHEMBL3401350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242270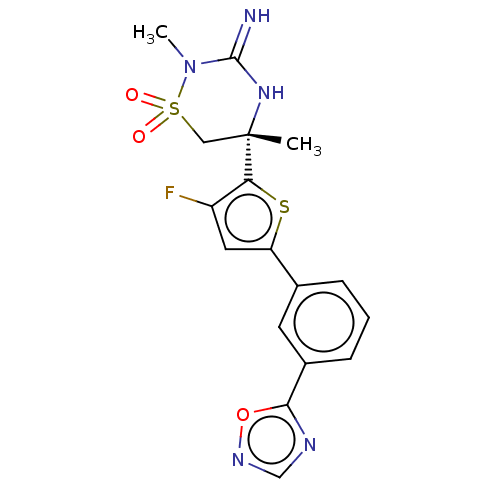 (US9416129, 48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | -53.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50531593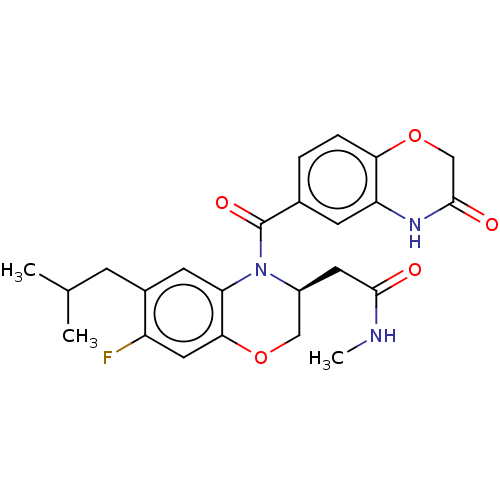 (CHEMBL4555842) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay | J Med Chem 62: 1385-1406 (2019) Article DOI: 10.1021/acs.jmedchem.8b01523 BindingDB Entry DOI: 10.7270/Q2GT5RP6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242938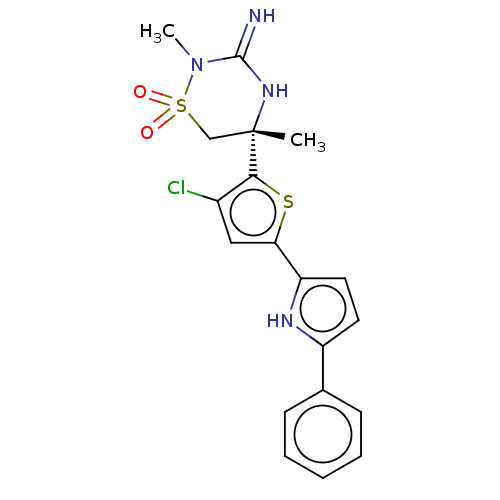 (US9422277, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.690 | -53.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9422277 (2016) BindingDB Entry DOI: 10.7270/Q2VX0FFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242229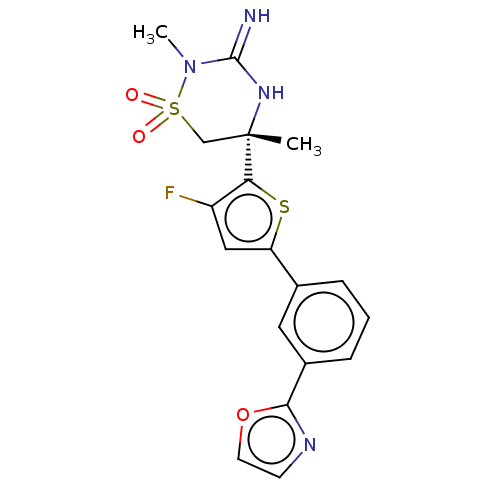 (US9416129, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.730 | -53.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50531569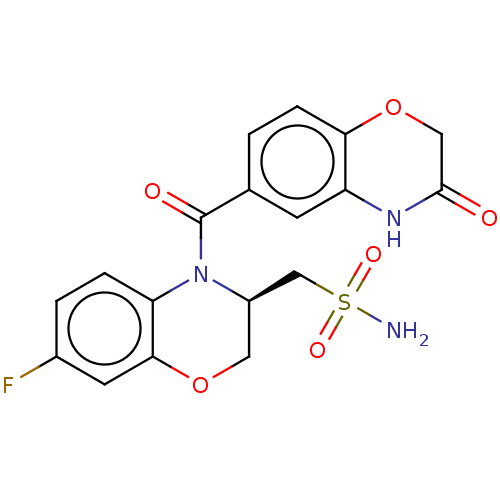 (CHEMBL4532472) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone to human mineralocorticoid receptor LBD by radiometric binding assay | J Med Chem 62: 1385-1406 (2019) Article DOI: 10.1021/acs.jmedchem.8b01523 BindingDB Entry DOI: 10.7270/Q2GT5RP6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242270 (US9416129, 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.870 | -52.6 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Curated by ChEMBL | Assay Description High inhibition constant against [3H]-spiperone binding to human Dopamine receptor D3 expressed in CHO cells | J Med Chem 48: 2493-508 (2005) Article DOI: 10.1021/jm049269+ BindingDB Entry DOI: 10.7270/Q2TT4QGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.949 | n/a | n/a | n/a | n/a | n/a | n/a | 5.01 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The protocol that was used to determine the recited values isdescribed as follows.BACE1 HTRF FRET AssayReagentsNa+-Acetate pH 5.01% Brij-35GlycerolDi... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256742 (US9489013, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242232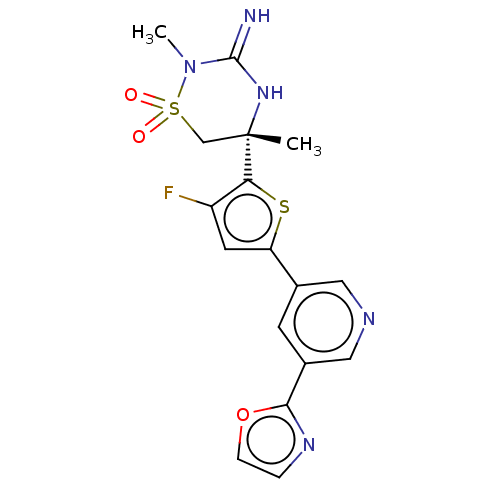 (US9416129, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256765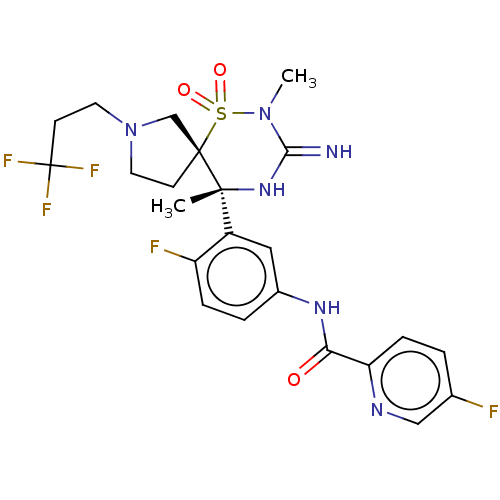 (US9489013, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM256742 (US9489013, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50's at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM256761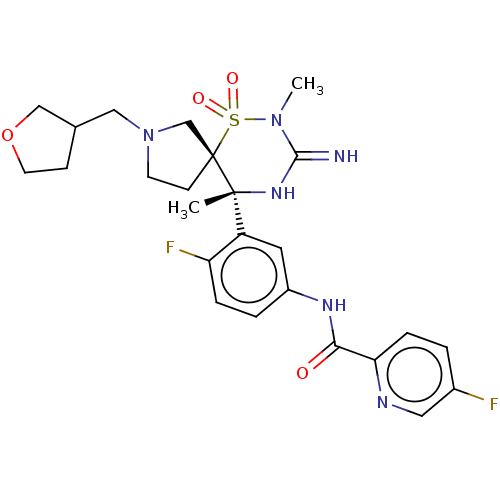 (US9489013, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50's at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM256765 (US9489013, 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50's at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256738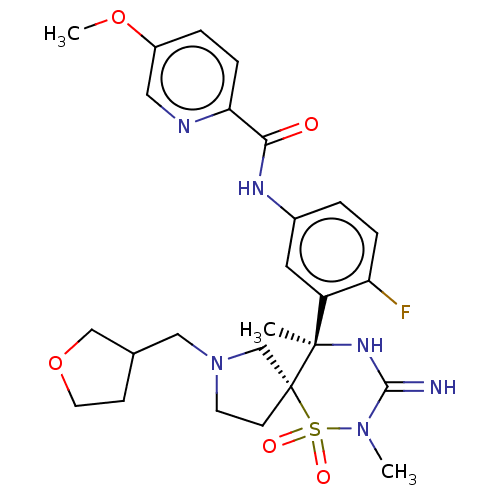 (US9489013, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256786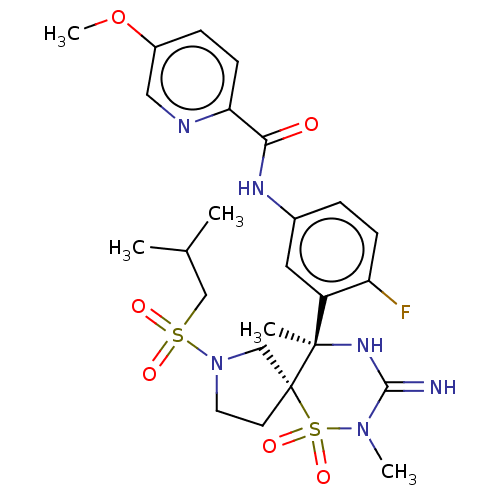 (US9489013, 57) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256789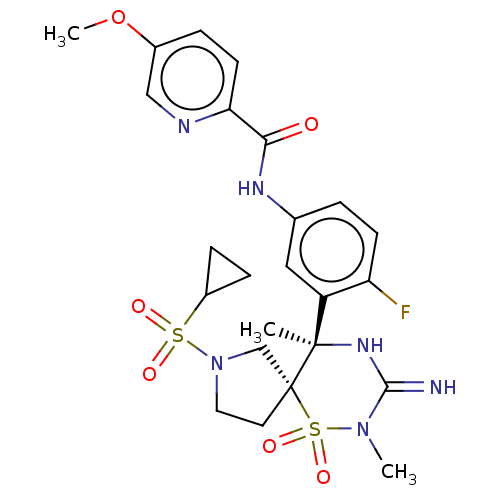 (US9489013, 60) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256808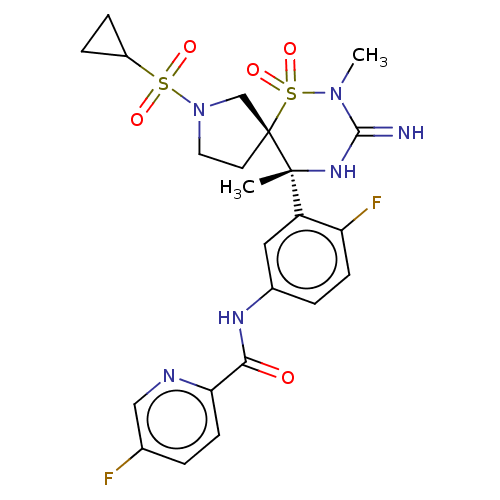 (US9489013, 79) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256785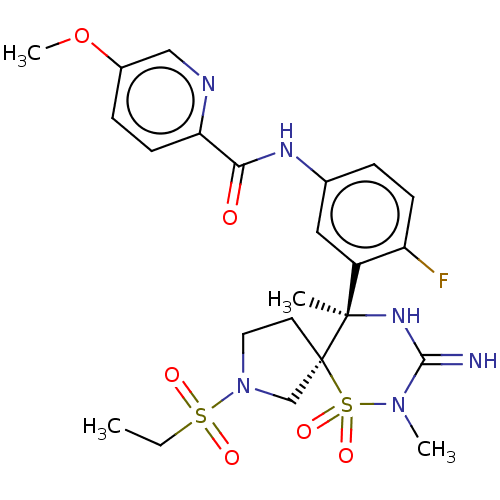 (US9489013, 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256761 (US9489013, 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143222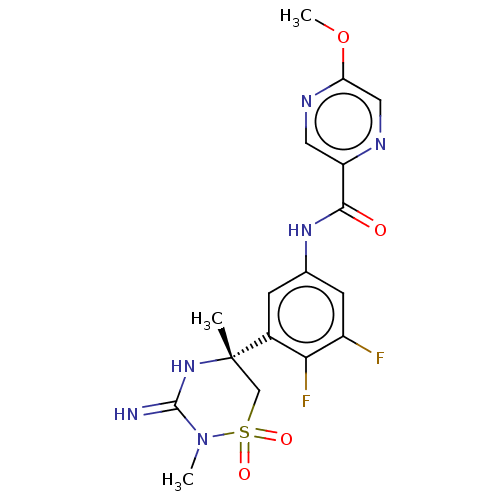 (US8940748, 40di | US9029362, 40di | US9687494, 40d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | 5.01 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The protocol that was used to determine the recited values isdescribed as follows.BACE1 HTRF FRET AssayReagentsNa+-Acetate pH 5.01% Brij-35GlycerolDi... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242254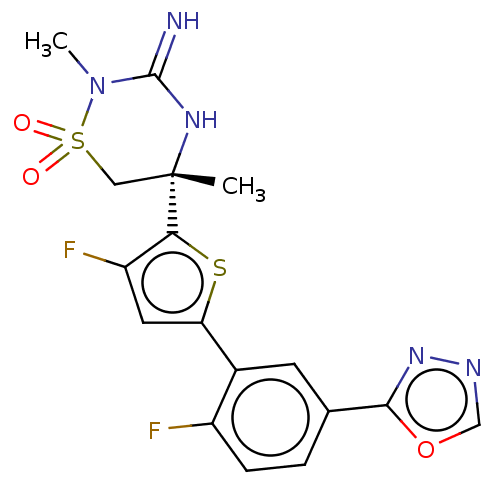 (US9416129, 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242264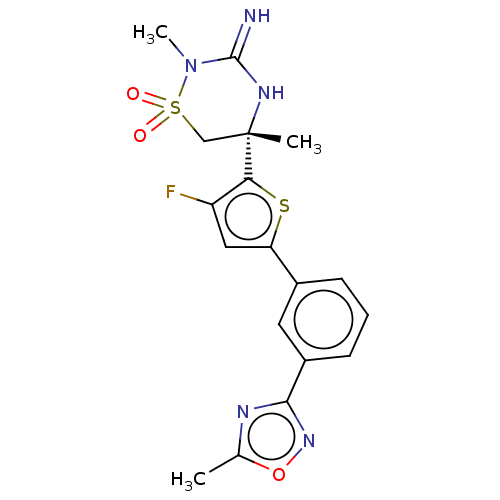 (US9416129, 42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242271 (US9416129, 49) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242251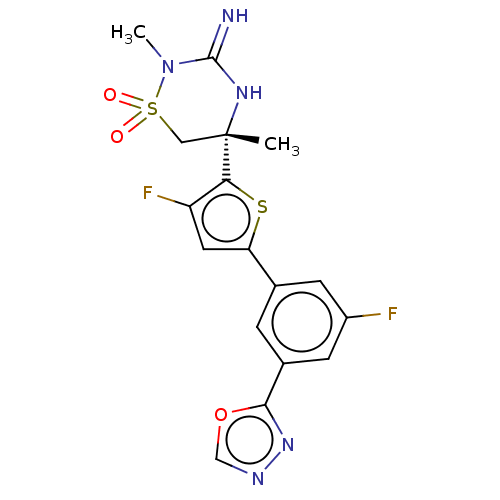 (US9416129, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | -51.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143219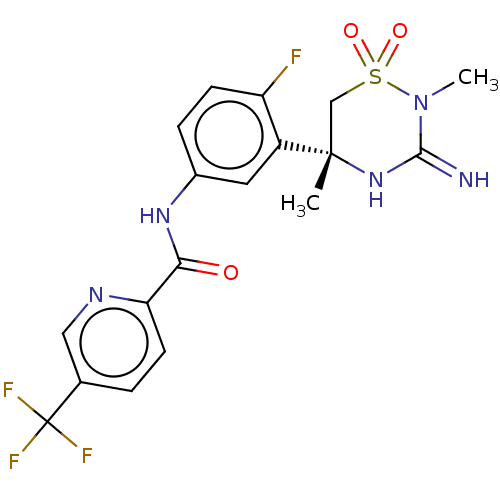 (US8940748, 26 | US9029362, 26 | US9687494, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | 5.01 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The protocol that was used to determine the recited values isdescribed as follows.BACE1 HTRF FRET AssayReagentsNa+-Acetate pH 5.01% Brij-35GlycerolDi... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242251 (US9416129, 29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | -51.6 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50531587 (CHEMBL4471322) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to recombinant human GR LBD by fluormone GS red-fluorescence polarization assay | J Med Chem 62: 1385-1406 (2019) Article DOI: 10.1021/acs.jmedchem.8b01523 BindingDB Entry DOI: 10.7270/Q2GT5RP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242228 (US9416129, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | -51.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242223 (US9416129, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | -51.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242282 (US9416129, 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | -51.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1956 total ) | Next | Last >> |