

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human thrombin | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 40 | -43.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 50.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human trypsin | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 57 | -43.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | 67 | -42.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 102 | -41.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | 110 | -41.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 140 | -40.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 780 | -36.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human plasmin | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human Coagulation factor Xa (fXa) | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 4.10E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.50E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 6.80E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 9.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 9.20E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+4 | -29.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1.60E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human Activated protein C | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 4.40E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory constant (Ki) was determined against human Tissue plasminogen activator (tissue plasminogen activator) | J Med Chem 45: 1757-66 (2002) BindingDB Entry DOI: 10.7270/Q2GX49W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17295 (BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | >5.00E+4 | >-25.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17297 (4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | >5.00E+4 | >-25.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211918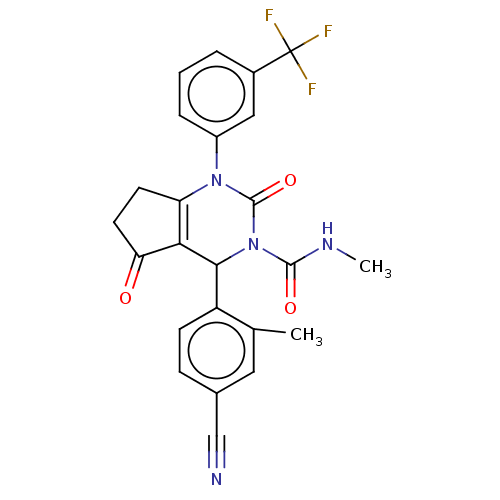 (US9290459, 50.2 | US9670166, 50.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211921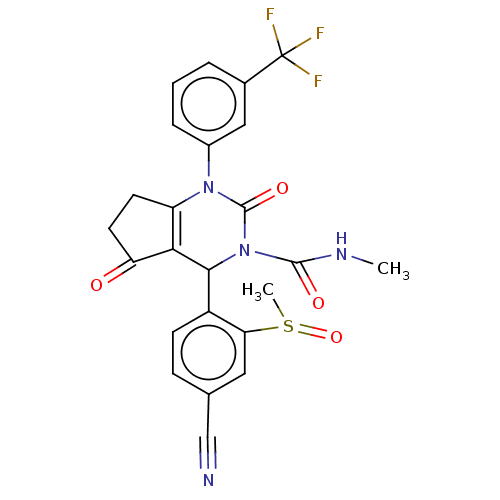 (US9290459, 50.5 | US9670166, 50.5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211923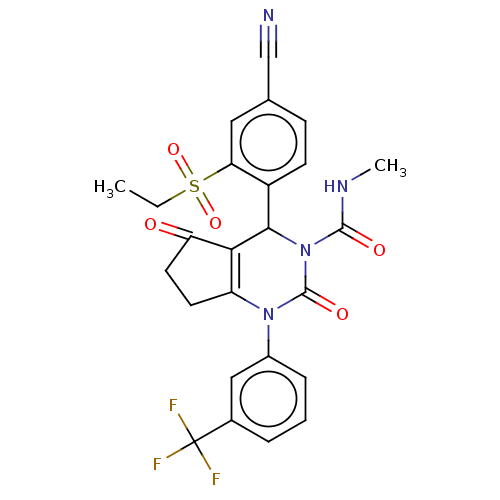 (US9290459, 50.7 | US9670166, 50.7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189407 (US9670166, 51.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211925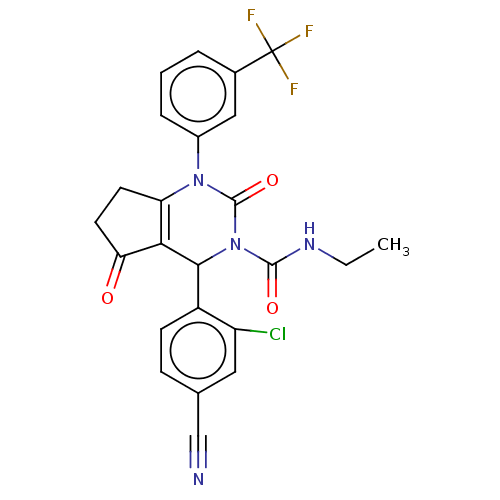 (US9290459, 51.2 | US9670166, 51.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211926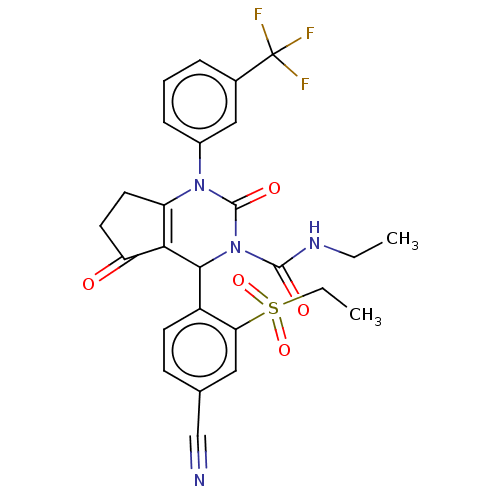 (US9290459, 51.3 | US9670166, 51.3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211927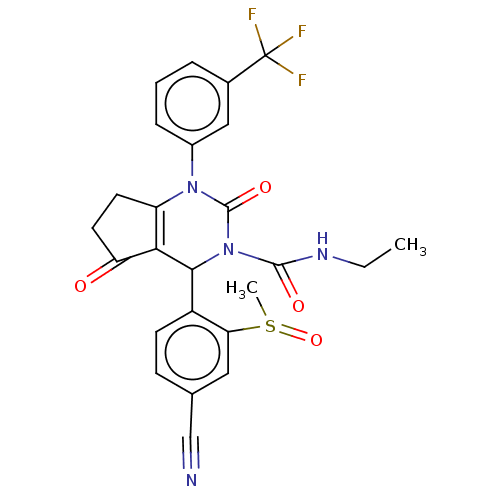 (US9290459, 51.4 | US9475779, C.3 | US9670166, 51.4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211928 (US9290459, 52 | US9670166, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211929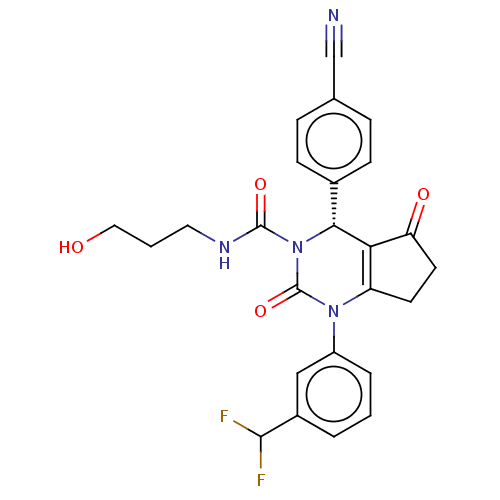 (US9290459, 52A | US9670166, 52A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211932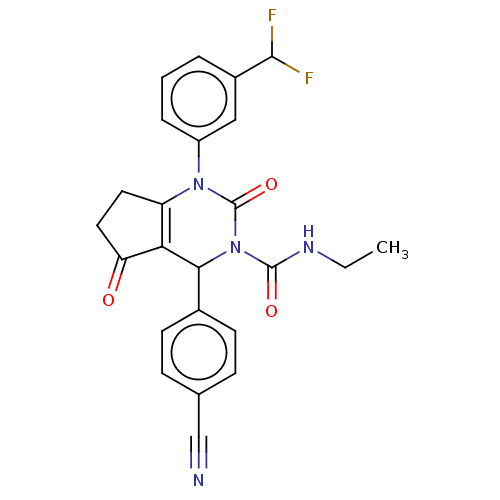 (US9290459, 52.2 | US9670166, 52.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211933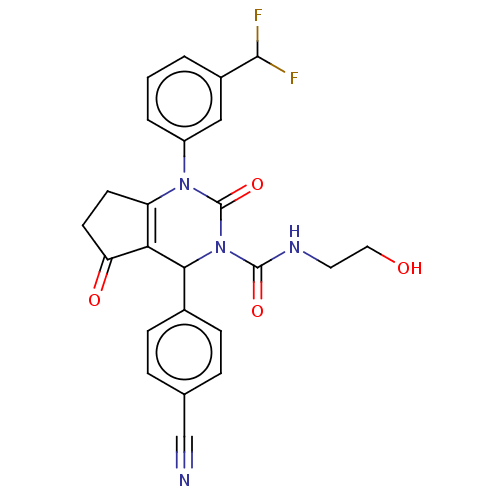 (US9290459, 52.3 | US9670166, 52.3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211934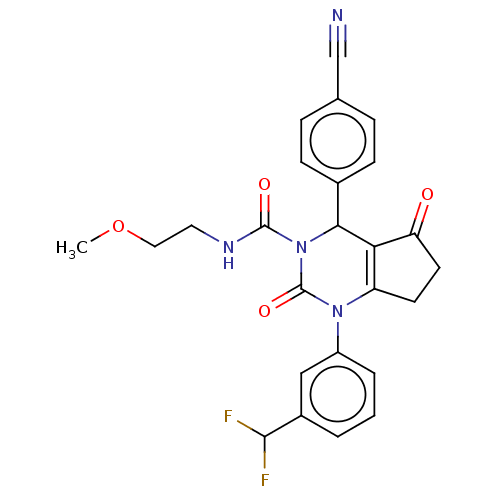 (US9290459, 52.4 | US9670166, 52.4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211935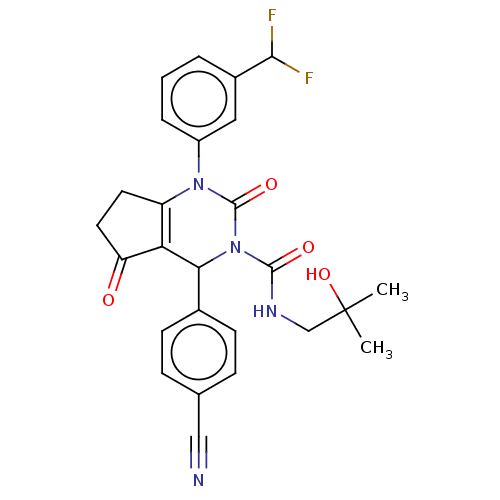 (US9290459, 52.5 | US9670166, 52.5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211936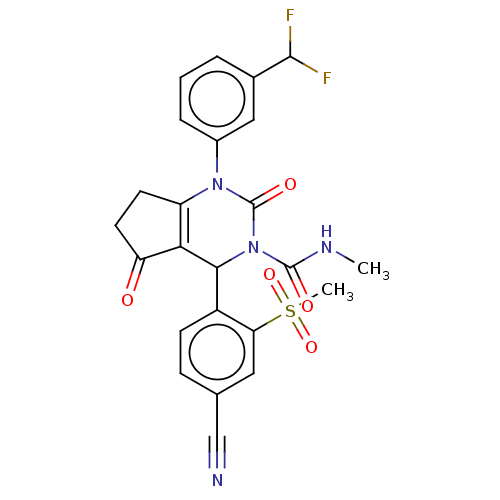 (US9290459, 53.1 | US9670166, 53.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211937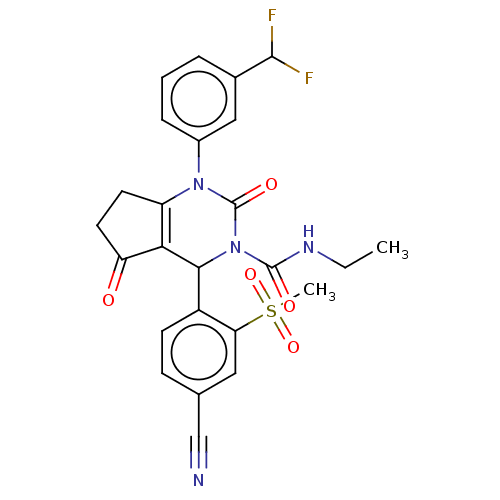 (US9290459, 53.2 | US9670166, 53.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211938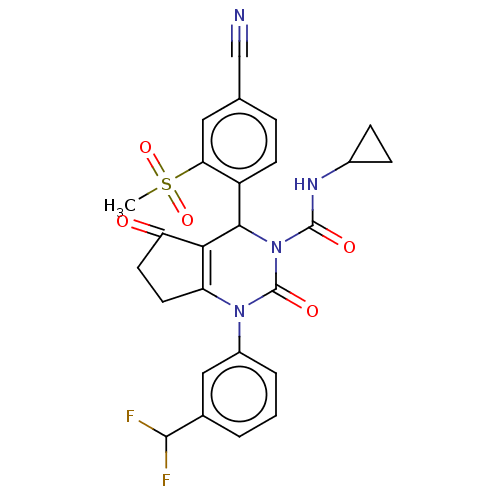 (US9290459, 53.3 | US9670166, 53.3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211939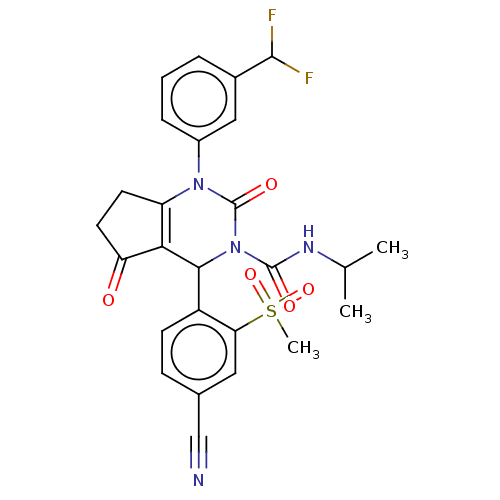 (US9290459, 53.4 | US9670166, 53.4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211940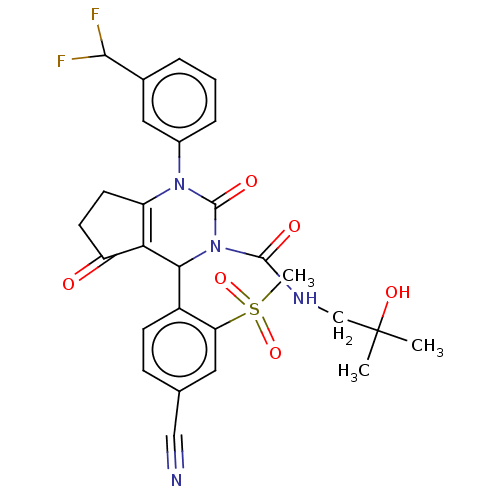 (US9290459, 53.5 | US9670166, 53.5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211946 (US9290459, 56.1 | US9670166, 56.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211947 (US9290459, 56.2 | US9475779, D.4.10 | US9670166, 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1163 total ) | Next | Last >> |