 Found 1681 hits with Last Name = 'palombella' and Initial = 'vj'
Found 1681 hits with Last Name = 'palombella' and Initial = 'vj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50192880
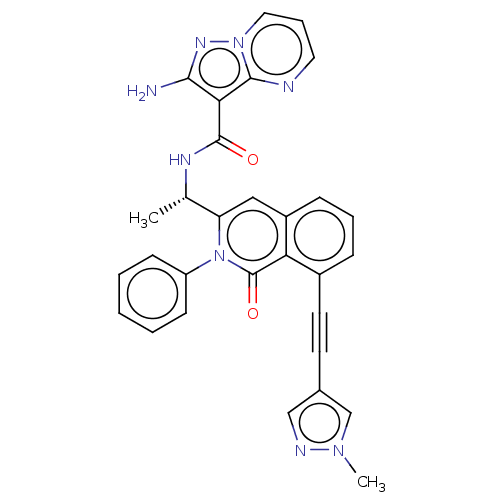
(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in C5a-stimulated mouse RAW264.7 cells assessed as reduction in AKT phosphorylation at S473 incubated for 30 mins followed by... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50293788
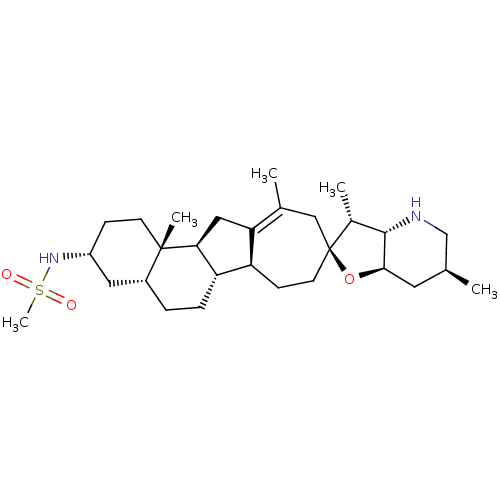
(CHEMBL538867 | N-((2S,3R,3aS,3'R,4a'R,6S,6a'R,6b'S...)Show SMILES C[C@@H]1[C@@H]2NC[C@@H](C)C[C@H]2O[C@]11CC[C@H]2[C@@H]3CC[C@@H]4C[C@@H](CC[C@]4(C)[C@H]3CC2=C(C)C1)NS(C)(=O)=O |r,t:31| Show InChI InChI=1S/C29H48N2O3S/c1-17-12-26-27(30-16-17)19(3)29(34-26)11-9-22-23-7-6-20-13-21(31-35(5,32)33)8-10-28(20,4)25(23)14-24(22)18(2)15-29/h17,19-23,25-27,30-31H,6-16H2,1-5H3/t17-,19+,20+,21+,22-,23-,25-,26+,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant SMO expressed in mouse C3H10T1/2 cells assessed as inhibition of association of BODIPY-cyclopamine |
J Med Chem 52: 4400-18 (2009)
Article DOI: 10.1021/jm900305z
BindingDB Entry DOI: 10.7270/Q2CC10QM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192889
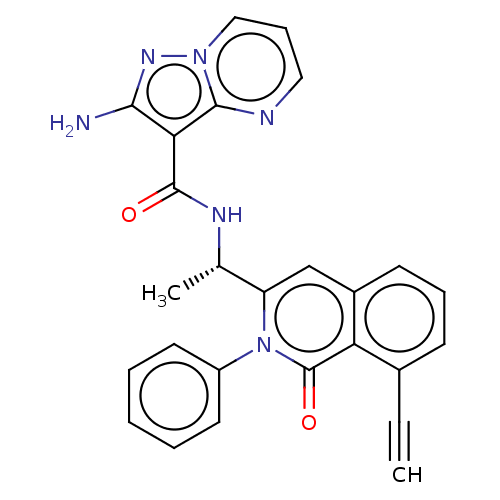
(CHEMBL3975359)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H20N6O2/c1-3-17-9-7-10-18-15-20(32(26(34)21(17)18)19-11-5-4-6-12-19)16(2)29-25(33)22-23(27)30-31-14-8-13-28-24(22)31/h1,4-16H,2H3,(H2,27,30)(H,29,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192880

(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192882
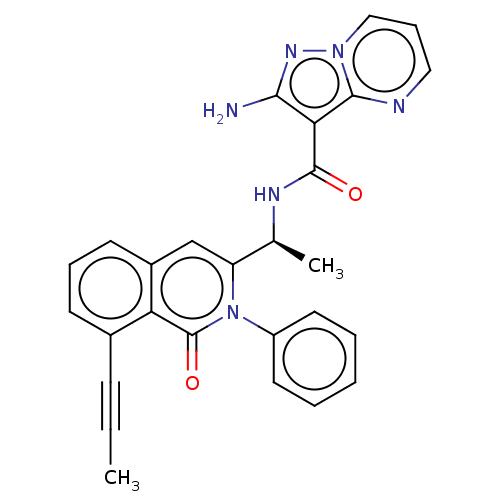
(CHEMBL3937119)Show SMILES CC#Cc1cccc2cc([C@H](C)NC(=O)c3c(N)nn4cccnc34)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C27H22N6O2/c1-3-9-18-10-7-11-19-16-21(33(27(35)22(18)19)20-12-5-4-6-13-20)17(2)30-26(34)23-24(28)31-32-15-8-14-29-25(23)32/h4-8,10-17H,1-2H3,(H2,28,31)(H,30,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192899
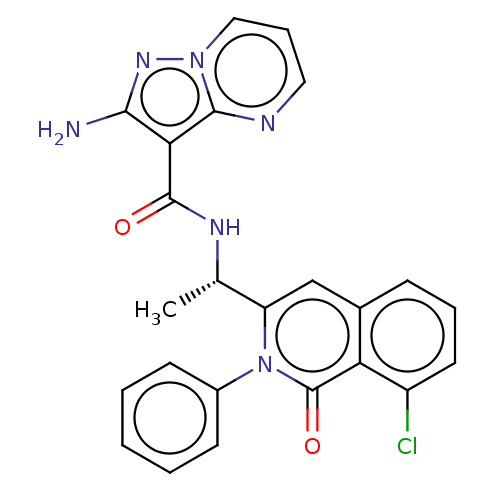
(CHEMBL3963736)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H19ClN6O2/c1-14(28-23(32)20-21(26)29-30-12-6-11-27-22(20)30)18-13-15-7-5-10-17(25)19(15)24(33)31(18)16-8-3-2-4-9-16/h2-14H,1H3,(H2,26,29)(H,28,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192883
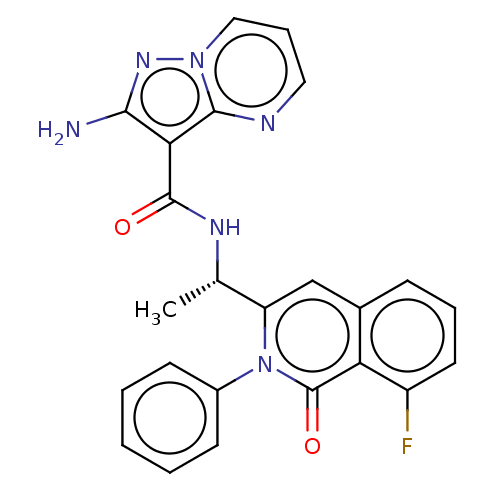
(CHEMBL3976330)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H19FN6O2/c1-14(28-23(32)20-21(26)29-30-12-6-11-27-22(20)30)18-13-15-7-5-10-17(25)19(15)24(33)31(18)16-8-3-2-4-9-16/h2-14H,1H3,(H2,26,29)(H,28,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192901
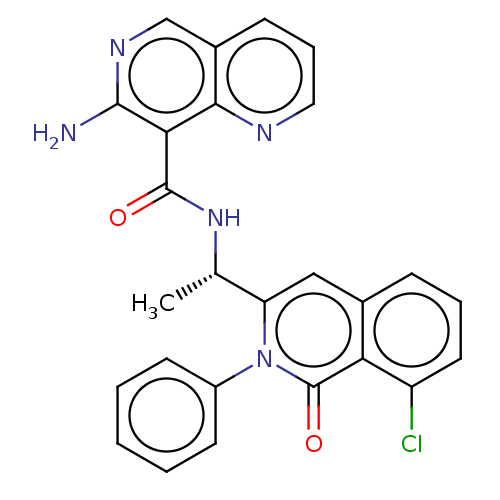
(CHEMBL3911278)Show SMILES C[C@H](NC(=O)c1c(N)ncc2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H20ClN5O2/c1-15(31-25(33)22-23-17(8-6-12-29-23)14-30-24(22)28)20-13-16-7-5-11-19(27)21(16)26(34)32(20)18-9-3-2-4-10-18/h2-15H,1H3,(H2,28,30)(H,31,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192885
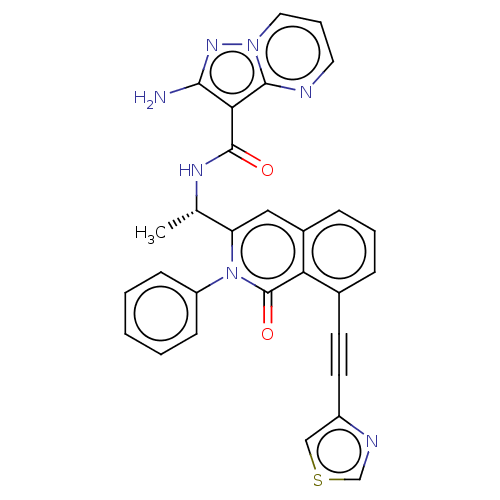
(CHEMBL3923629 | US10329299, Compound 54 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cscn3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C29H21N7O2S/c1-18(33-28(37)25-26(30)34-35-14-6-13-31-27(25)35)23-15-20-8-5-7-19(11-12-21-16-39-17-32-21)24(20)29(38)36(23)22-9-3-2-4-10-22/h2-10,13-18H,1H3,(H2,30,34)(H,33,37)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192881
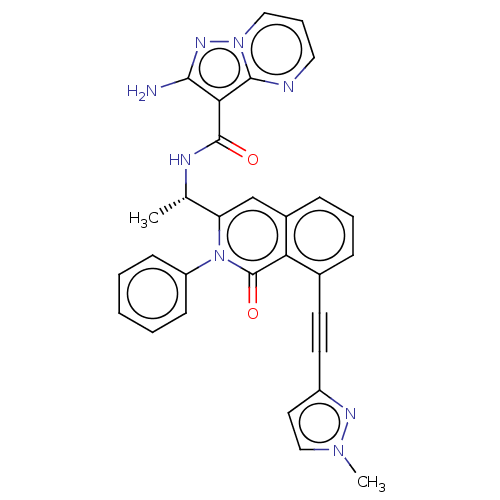
(CHEMBL3914602 | US10329299, Compound 30 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3ccn(C)n3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(33-29(39)26-27(31)35-37-16-7-15-32-28(26)37)24-18-21-9-6-8-20(12-13-22-14-17-36(2)34-22)25(21)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,33,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192888
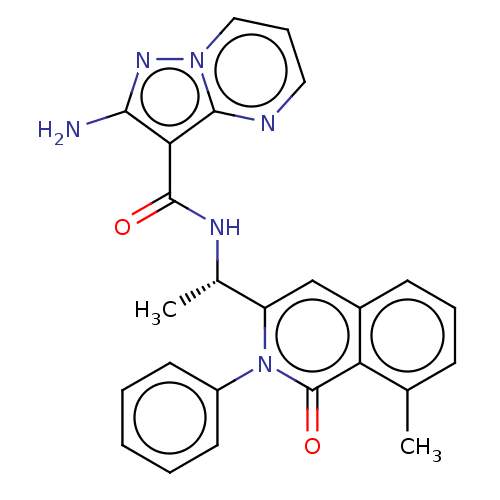
(CHEMBL3947903)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H22N6O2/c1-15-8-6-9-17-14-19(31(25(33)20(15)17)18-10-4-3-5-11-18)16(2)28-24(32)21-22(26)29-30-13-7-12-27-23(21)30/h3-14,16H,1-2H3,(H2,26,29)(H,28,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192909
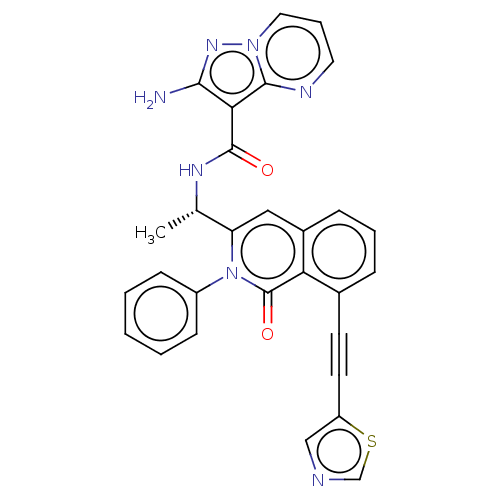
(CHEMBL3921445 | US10329299, Compound 41 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cncs3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C29H21N7O2S/c1-18(33-28(37)25-26(30)34-35-14-6-13-32-27(25)35)23-15-20-8-5-7-19(11-12-22-16-31-17-39-22)24(20)29(38)36(23)21-9-3-2-4-10-21/h2-10,13-18H,1H3,(H2,30,34)(H,33,37)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192895
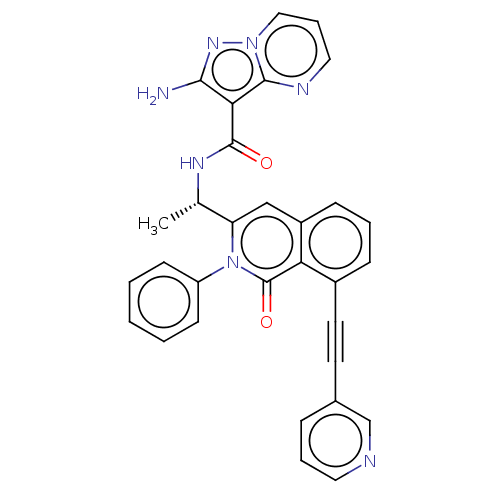
(CHEMBL3928146 | US10329299, Compound 17 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cccnc3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C31H23N7O2/c1-20(35-30(39)27-28(32)36-37-17-7-16-34-29(27)37)25-18-23-10-5-9-22(14-13-21-8-6-15-33-19-21)26(23)31(40)38(25)24-11-3-2-4-12-24/h2-12,15-20H,1H3,(H2,32,36)(H,35,39)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192892
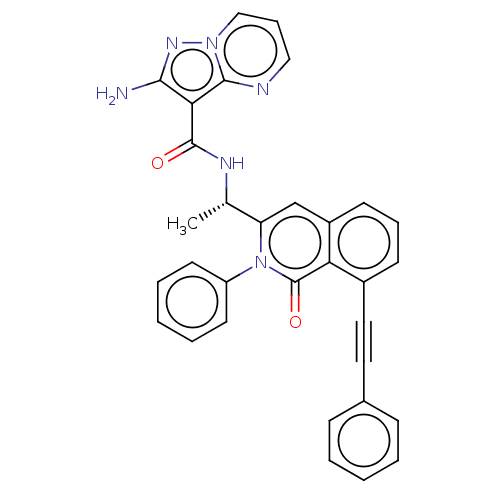
(CHEMBL3966879 | US10329299, Compound 5 | US1067528...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3ccccc3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C32H24N6O2/c1-21(35-31(39)28-29(33)36-37-19-9-18-34-30(28)37)26-20-24-13-8-12-23(17-16-22-10-4-2-5-11-22)27(24)32(40)38(26)25-14-6-3-7-15-25/h2-15,18-21H,1H3,(H2,33,36)(H,35,39)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192891
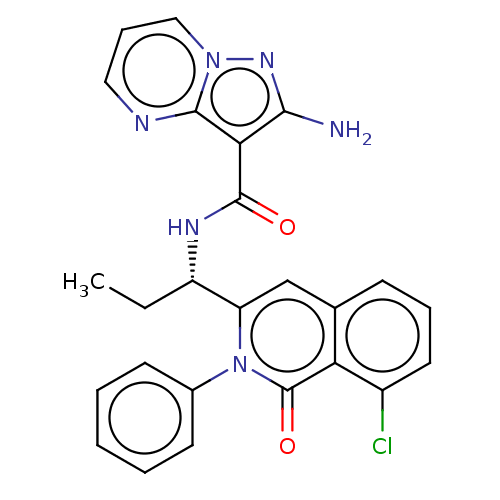
(CHEMBL3959293)Show SMILES CC[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H21ClN6O2/c1-2-18(29-24(33)21-22(27)30-31-13-7-12-28-23(21)31)19-14-15-8-6-11-17(26)20(15)25(34)32(19)16-9-4-3-5-10-16/h3-14,18H,2H2,1H3,(H2,27,30)(H,29,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192894
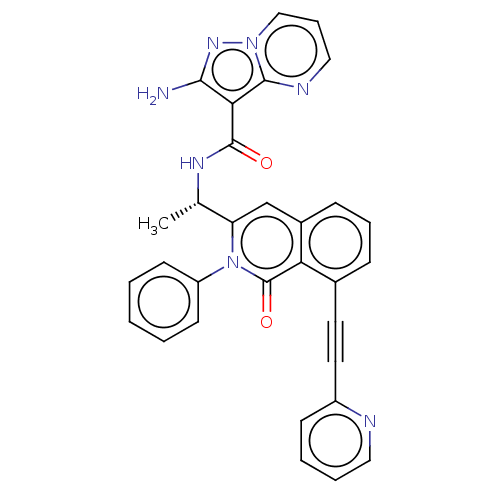
(CHEMBL3893430 | US10329299, Compound 32 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3ccccn3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C31H23N7O2/c1-20(35-30(39)27-28(32)36-37-18-8-17-34-29(27)37)25-19-22-10-7-9-21(14-15-23-11-5-6-16-33-23)26(22)31(40)38(25)24-12-3-2-4-13-24/h2-13,16-20H,1H3,(H2,32,36)(H,35,39)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192880

(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in IgM-stimulated human Raji cells assessed as reduction in AKT phosphorylation at S473 incubated for 30 mins followed by sti... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192897
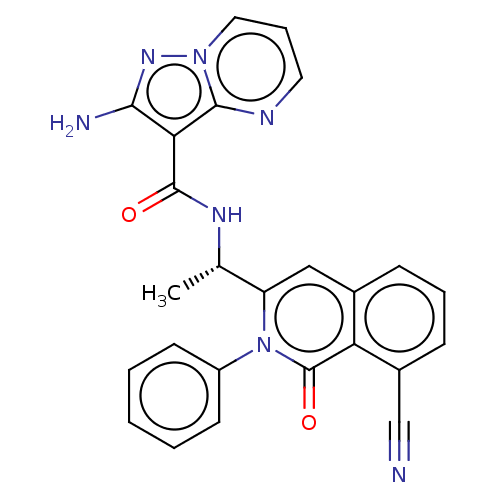
(CHEMBL3944056)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#N)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H19N7O2/c1-15(29-24(33)21-22(27)30-31-12-6-11-28-23(21)31)19-13-16-7-5-8-17(14-26)20(16)25(34)32(19)18-9-3-2-4-10-18/h2-13,15H,1H3,(H2,27,30)(H,29,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50192880

(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human 786-O cells assessed as reduction in AKT phosphorylation at S473 after 30 mins by ELISA |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50192880

(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human SKOV-3 cells assessed as reduction in AKT phosphorylation at S473 after 30 mins by ELISA |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192892

(CHEMBL3966879 | US10329299, Compound 5 | US1067528...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3ccccc3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C32H24N6O2/c1-21(35-31(39)28-29(33)36-37-19-9-18-34-30(28)37)26-20-24-13-8-12-23(17-16-22-10-4-2-5-11-22)27(24)32(40)38(26)25-14-6-3-7-15-25/h2-15,18-21H,1H3,(H2,33,36)(H,35,39)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192900
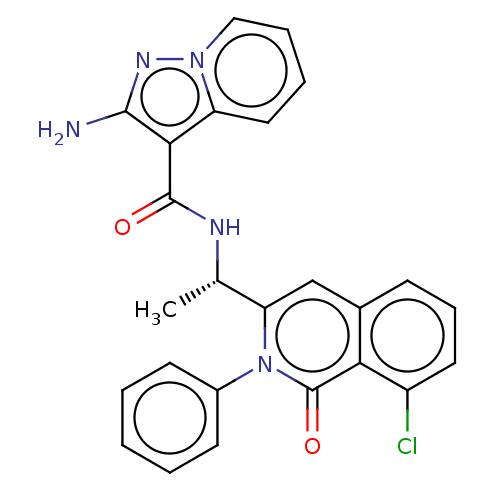
(CHEMBL3917188 | US10919914, Compound 31)Show SMILES C[C@H](NC(=O)c1c(N)nn2ccccc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H20ClN5O2/c1-15(28-24(32)22-19-12-5-6-13-30(19)29-23(22)27)20-14-16-8-7-11-18(26)21(16)25(33)31(20)17-9-3-2-4-10-17/h2-15H,1H3,(H2,27,29)(H,28,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192901

(CHEMBL3911278)Show SMILES C[C@H](NC(=O)c1c(N)ncc2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H20ClN5O2/c1-15(31-25(33)22-23-17(8-6-12-29-23)14-30-24(22)28)20-13-16-7-5-11-19(27)21(16)26(34)32(20)18-9-3-2-4-10-18/h2-15H,1H3,(H2,28,30)(H,31,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192886
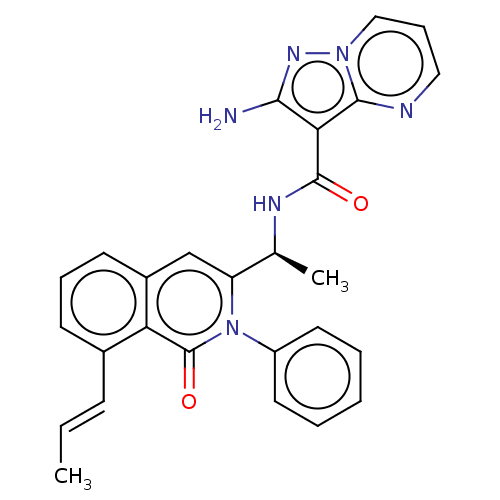
(CHEMBL3946030 | US10329299, Compound 70 | US106752...)Show SMILES C\C=C\c1cccc2cc([C@H](C)NC(=O)c3c(N)nn4cccnc34)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C27H24N6O2/c1-3-9-18-10-7-11-19-16-21(33(27(35)22(18)19)20-12-5-4-6-13-20)17(2)30-26(34)23-24(28)31-32-15-8-14-29-25(23)32/h3-17H,1-2H3,(H2,28,31)(H,30,34)/b9-3+/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192911
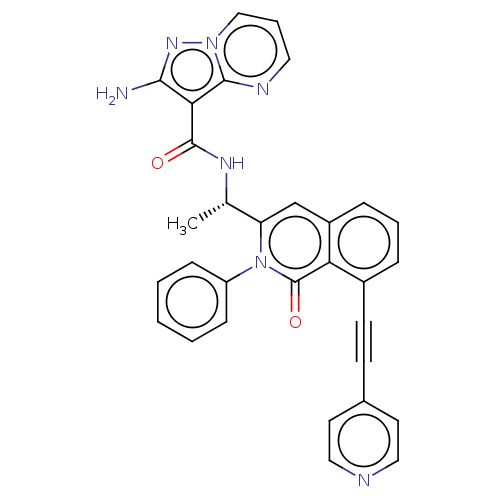
(CHEMBL3955769 | US10329299, Compound 26 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3ccncc3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C31H23N7O2/c1-20(35-30(39)27-28(32)36-37-18-6-15-34-29(27)37)25-19-23-8-5-7-22(12-11-21-13-16-33-17-14-21)26(23)31(40)38(25)24-9-3-2-4-10-24/h2-10,13-20H,1H3,(H2,32,36)(H,35,39)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192908
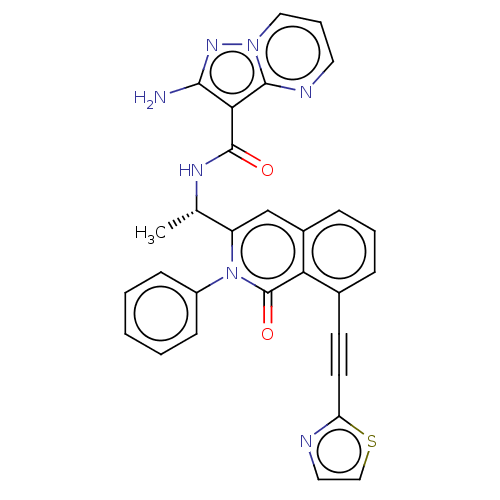
(CHEMBL3895629 | US10329299, Compound 59 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3nccs3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C29H21N7O2S/c1-18(33-28(37)25-26(30)34-35-15-6-13-32-27(25)35)22-17-20-8-5-7-19(11-12-23-31-14-16-39-23)24(20)29(38)36(22)21-9-3-2-4-10-21/h2-10,13-18H,1H3,(H2,30,34)(H,33,37)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192890
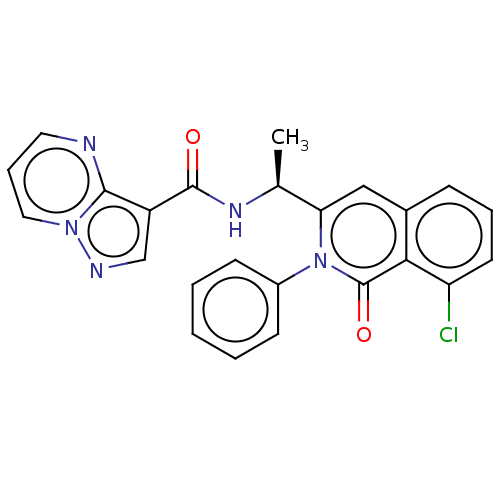
(CHEMBL3908224)Show SMILES C[C@H](NC(=O)c1cnn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H18ClN5O2/c1-15(28-23(31)18-14-27-29-12-6-11-26-22(18)29)20-13-16-7-5-10-19(25)21(16)24(32)30(20)17-8-3-2-4-9-17/h2-15H,1H3,(H,28,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192899

(CHEMBL3963736)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H19ClN6O2/c1-14(28-23(32)20-21(26)29-30-12-6-11-27-22(20)30)18-13-15-7-5-10-17(25)19(15)24(33)31(18)16-8-3-2-4-9-16/h2-14H,1H3,(H2,26,29)(H,28,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192883

(CHEMBL3976330)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H19FN6O2/c1-14(28-23(32)20-21(26)29-30-12-6-11-27-22(20)30)18-13-15-7-5-10-17(25)19(15)24(33)31(18)16-8-3-2-4-9-16/h2-14H,1H3,(H2,26,29)(H,28,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192891

(CHEMBL3959293)Show SMILES CC[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H21ClN6O2/c1-2-18(29-24(33)21-22(27)30-31-13-7-12-28-23(21)31)19-14-15-8-6-11-17(26)20(15)25(34)32(19)16-9-4-3-5-10-16/h3-14,18H,2H2,1H3,(H2,27,30)(H,29,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192889

(CHEMBL3975359)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H20N6O2/c1-3-17-9-7-10-18-15-20(32(26(34)21(17)18)19-11-5-4-6-12-19)16(2)29-25(33)22-23(27)30-31-14-8-13-28-24(22)31/h1,4-16H,2H3,(H2,27,30)(H,29,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192890

(CHEMBL3908224)Show SMILES C[C@H](NC(=O)c1cnn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H18ClN5O2/c1-15(28-23(31)18-14-27-29-12-6-11-26-22(18)29)20-13-16-7-5-10-19(25)21(16)24(32)30(20)17-8-3-2-4-9-17/h2-15H,1H3,(H,28,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
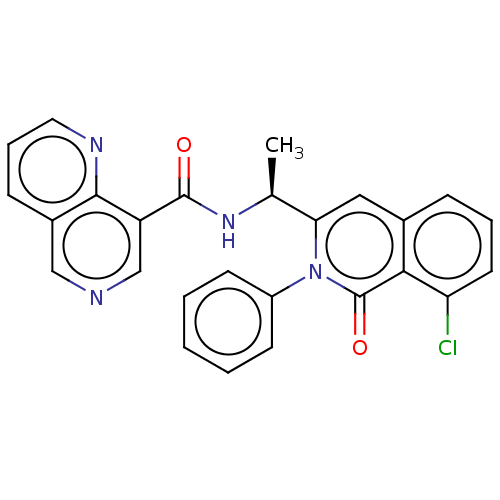
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192884
(CHEMBL3978650)Show SMILES C[C@H](NC(=O)c1cncc2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H19ClN4O2/c1-16(30-25(32)20-15-28-14-18-8-6-12-29-24(18)20)22-13-17-7-5-11-21(27)23(17)26(33)31(22)19-9-3-2-4-10-19/h2-16H,1H3,(H,30,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50192882

(CHEMBL3937119)Show SMILES CC#Cc1cccc2cc([C@H](C)NC(=O)c3c(N)nn4cccnc34)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C27H22N6O2/c1-3-9-18-10-7-11-19-16-21(33(27(35)22(18)19)20-12-5-4-6-13-20)17(2)30-26(34)23-24(28)31-32-15-8-14-29-25(23)32/h4-8,10-17H,1-2H3,(H2,28,31)(H,30,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged PI3K p110delta/p85alpha coexpressed in baculovirus infected Sf9 insect cells using ... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192896
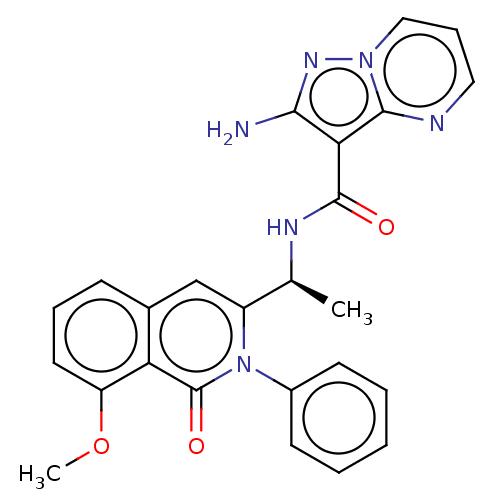
(CHEMBL3916095)Show SMILES COc1cccc2cc([C@H](C)NC(=O)c3c(N)nn4cccnc34)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C25H22N6O3/c1-15(28-24(32)21-22(26)29-30-13-7-12-27-23(21)30)18-14-16-8-6-11-19(34-2)20(16)25(33)31(18)17-9-4-3-5-10-17/h3-15H,1-2H3,(H2,26,29)(H,28,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192884

(CHEMBL3978650)Show SMILES C[C@H](NC(=O)c1cncc2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H19ClN4O2/c1-16(30-25(32)20-15-28-14-18-8-6-12-29-24(18)20)22-13-17-7-5-11-21(27)23(17)26(33)31(22)19-9-3-2-4-10-19/h2-16H,1H3,(H,30,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192910
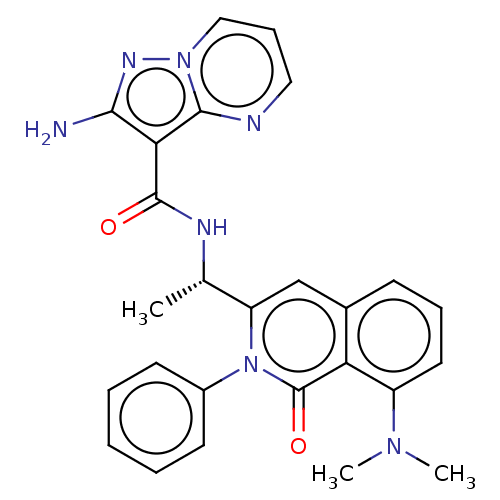
(CHEMBL3983349)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(N(C)C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H25N7O2/c1-16(29-25(34)22-23(27)30-32-14-8-13-28-24(22)32)20-15-17-9-7-12-19(31(2)3)21(17)26(35)33(20)18-10-5-4-6-11-18/h4-16H,1-3H3,(H2,27,30)(H,29,34)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192893
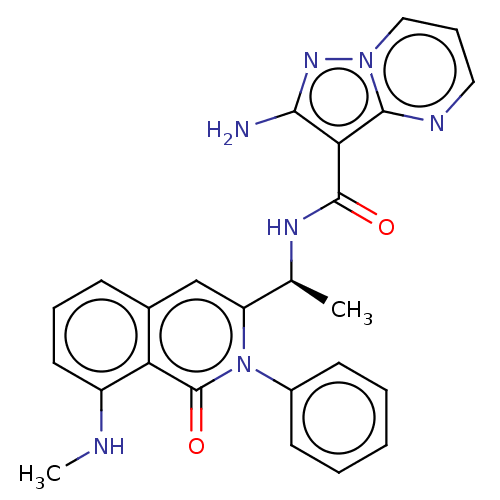
(CHEMBL3954718)Show SMILES CNc1cccc2cc([C@H](C)NC(=O)c3c(N)nn4cccnc34)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C25H23N7O2/c1-15(29-24(33)21-22(26)30-31-13-7-12-28-23(21)31)19-14-16-8-6-11-18(27-2)20(16)25(34)32(19)17-9-4-3-5-10-17/h3-15,27H,1-2H3,(H2,26,30)(H,29,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM342550
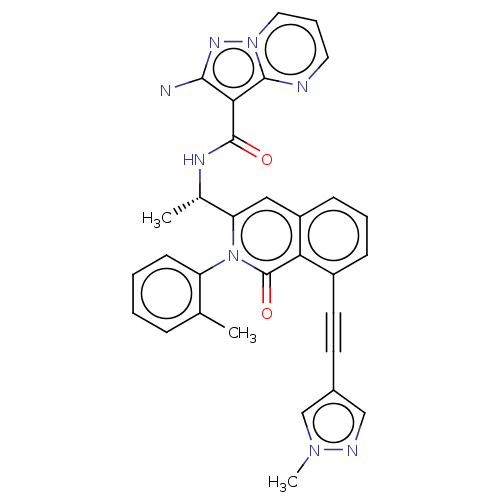
(US10329299, Compound 101 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1C |r,wD:1.0,$;;;;;;;N;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;$,(-3.79,1.15,;-2.29,1.47,;-1.81,2.94,;-.3,3.26,;.73,2.11,;.17,4.72,;-.73,5.97,;-2.27,5.97,;.17,7.21,;1.64,6.74,;2.97,7.51,;4.3,6.74,;4.3,5.2,;2.97,4.43,;1.64,5.2,;-1.26,.33,;.25,.65,;1.28,-.5,;2.79,-.17,;3.82,-1.32,;3.34,-2.78,;1.84,-3.1,;1.36,-4.57,;.88,-6.03,;.41,-7.5,;-1.06,-7.97,;-1.06,-9.51,;.41,-9.99,;.88,-11.45,;1.31,-8.74,;.81,-1.96,;-.7,-2.28,;-1.18,-3.74,;-1.73,-1.14,;-3.24,-1.46,;-4.27,-.31,;-5.77,-.63,;-6.25,-2.1,;-5.22,-3.24,;-3.71,-2.92,;-2.68,-4.06,)| Show InChI InChI=1S/C31H24N8O2/c1-19-8-4-5-11-24(19)39-25(20(2)35-30(40)27-28(32)36-38-15-7-14-33-29(27)38)16-23-10-6-9-22(26(23)31(39)41)13-12-21-17-34-37(3)18-21/h4-11,14-18,20H,1-3H3,(H,35,40)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM342550

(US10329299, Compound 101 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1C |r,wD:1.0,$;;;;;;;N;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;$,(-3.79,1.15,;-2.29,1.47,;-1.81,2.94,;-.3,3.26,;.73,2.11,;.17,4.72,;-.73,5.97,;-2.27,5.97,;.17,7.21,;1.64,6.74,;2.97,7.51,;4.3,6.74,;4.3,5.2,;2.97,4.43,;1.64,5.2,;-1.26,.33,;.25,.65,;1.28,-.5,;2.79,-.17,;3.82,-1.32,;3.34,-2.78,;1.84,-3.1,;1.36,-4.57,;.88,-6.03,;.41,-7.5,;-1.06,-7.97,;-1.06,-9.51,;.41,-9.99,;.88,-11.45,;1.31,-8.74,;.81,-1.96,;-.7,-2.28,;-1.18,-3.74,;-1.73,-1.14,;-3.24,-1.46,;-4.27,-.31,;-5.77,-.63,;-6.25,-2.1,;-5.22,-3.24,;-3.71,-2.92,;-2.68,-4.06,)| Show InChI InChI=1S/C31H24N8O2/c1-19-8-4-5-11-24(19)39-25(20(2)35-30(40)27-28(32)36-38-15-7-14-33-29(27)38)16-23-10-6-9-22(26(23)31(39)41)13-12-21-17-34-37(3)18-21/h4-11,14-18,20H,1-3H3,(H,35,40)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM342550

(US10329299, Compound 101 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1C |r,wD:1.0,$;;;;;;;N;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;$,(-3.79,1.15,;-2.29,1.47,;-1.81,2.94,;-.3,3.26,;.73,2.11,;.17,4.72,;-.73,5.97,;-2.27,5.97,;.17,7.21,;1.64,6.74,;2.97,7.51,;4.3,6.74,;4.3,5.2,;2.97,4.43,;1.64,5.2,;-1.26,.33,;.25,.65,;1.28,-.5,;2.79,-.17,;3.82,-1.32,;3.34,-2.78,;1.84,-3.1,;1.36,-4.57,;.88,-6.03,;.41,-7.5,;-1.06,-7.97,;-1.06,-9.51,;.41,-9.99,;.88,-11.45,;1.31,-8.74,;.81,-1.96,;-.7,-2.28,;-1.18,-3.74,;-1.73,-1.14,;-3.24,-1.46,;-4.27,-.31,;-5.77,-.63,;-6.25,-2.1,;-5.22,-3.24,;-3.71,-2.92,;-2.68,-4.06,)| Show InChI InChI=1S/C31H24N8O2/c1-19-8-4-5-11-24(19)39-25(20(2)35-30(40)27-28(32)36-38-15-7-14-33-29(27)38)16-23-10-6-9-22(26(23)31(39)41)13-12-21-17-34-37(3)18-21/h4-11,14-18,20H,1-3H3,(H,35,40)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM342551
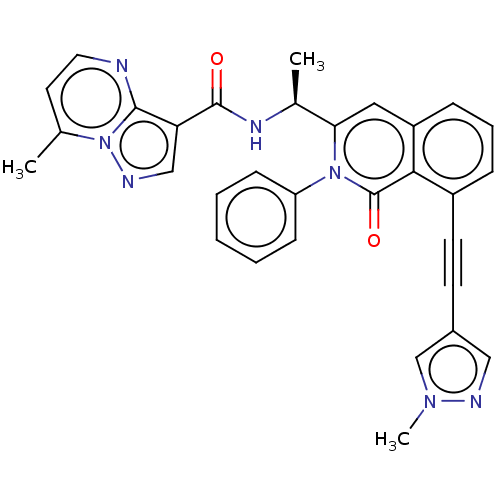
(US10329299, Compound 102 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1cnn2c(C)ccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r,$;;;;O;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;$| Show InChI InChI=1S/C31H25N7O2/c1-20-14-15-32-29-26(18-34-38(20)29)30(39)35-21(2)27-16-24-9-7-8-23(13-12-22-17-33-36(3)19-22)28(24)31(40)37(27)25-10-5-4-6-11-25/h4-11,14-19,21H,1-3H3,(H,35,39)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM342551

(US10329299, Compound 102 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1cnn2c(C)ccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r,$;;;;O;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;$| Show InChI InChI=1S/C31H25N7O2/c1-20-14-15-32-29-26(18-34-38(20)29)30(39)35-21(2)27-16-24-9-7-8-23(13-12-22-17-33-36(3)19-22)28(24)31(40)37(27)25-10-5-4-6-11-25/h4-11,14-19,21H,1-3H3,(H,35,39)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM342551

(US10329299, Compound 102 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1cnn2c(C)ccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r,$;;;;O;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;$| Show InChI InChI=1S/C31H25N7O2/c1-20-14-15-32-29-26(18-34-38(20)29)30(39)35-21(2)27-16-24-9-7-8-23(13-12-22-17-33-36(3)19-22)28(24)31(40)37(27)25-10-5-4-6-11-25/h4-11,14-19,21H,1-3H3,(H,35,39)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
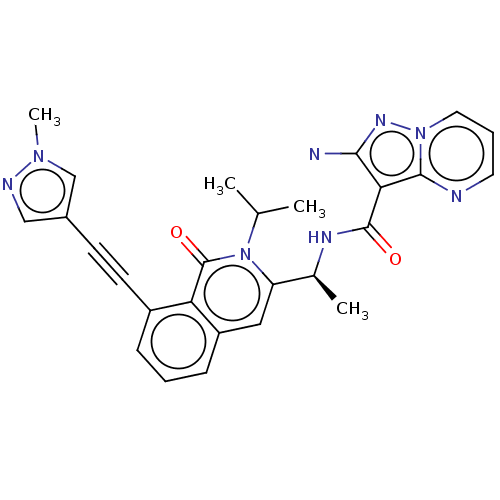
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM342552
(US10329299, Compound 103 | US10675286, Compound 10...)Show SMILES CC(C)n1c(cc2cccc(C#Cc3cnn(C)c3)c2c1=O)[C@H](C)NC(=O)c1c(N)nn2cccnc12 |r,$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;N;;;;;;;$| Show InChI InChI=1S/C27H24N8O2/c1-16(2)35-21(17(3)31-26(36)23-24(28)32-34-12-6-11-29-25(23)34)13-20-8-5-7-19(22(20)27(35)37)10-9-18-14-30-33(4)15-18/h5-8,11-17H,1-4H3,(H,31,36)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM342552

(US10329299, Compound 103 | US10675286, Compound 10...)Show SMILES CC(C)n1c(cc2cccc(C#Cc3cnn(C)c3)c2c1=O)[C@H](C)NC(=O)c1c(N)nn2cccnc12 |r,$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;N;;;;;;;$| Show InChI InChI=1S/C27H24N8O2/c1-16(2)35-21(17(3)31-26(36)23-24(28)32-34-12-6-11-29-25(23)34)13-20-8-5-7-19(22(20)27(35)37)10-9-18-14-30-33(4)15-18/h5-8,11-17H,1-4H3,(H,31,36)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM342552

(US10329299, Compound 103 | US10675286, Compound 10...)Show SMILES CC(C)n1c(cc2cccc(C#Cc3cnn(C)c3)c2c1=O)[C@H](C)NC(=O)c1c(N)nn2cccnc12 |r,$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;N;;;;;;;$| Show InChI InChI=1S/C27H24N8O2/c1-16(2)35-21(17(3)31-26(36)23-24(28)32-34-12-6-11-29-25(23)34)13-20-8-5-7-19(22(20)27(35)37)10-9-18-14-30-33(4)15-18/h5-8,11-17H,1-4H3,(H,31,36)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM342553
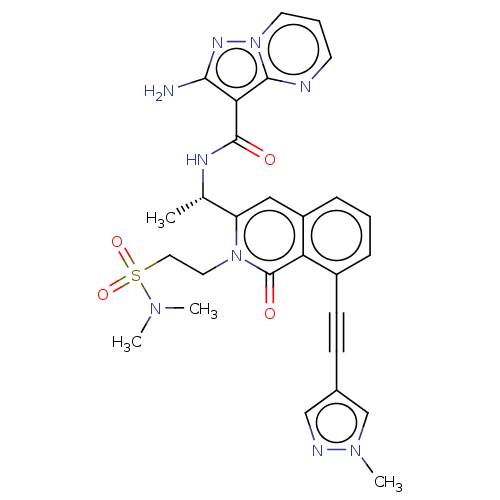
(US10329299, Compound 104 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1CCS(=O)(=O)N(C)C |r| Show InChI InChI=1S/C28H29N9O4S/c1-18(32-27(38)24-25(29)33-37-12-6-11-30-26(24)37)22-15-21-8-5-7-20(10-9-19-16-31-35(4)17-19)23(21)28(39)36(22)13-14-42(40,41)34(2)3/h5-8,11-12,15-18H,13-14H2,1-4H3,(H2,29,33)(H,32,38)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM342553

(US10329299, Compound 104 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1CCS(=O)(=O)N(C)C |r| Show InChI InChI=1S/C28H29N9O4S/c1-18(32-27(38)24-25(29)33-37-12-6-11-30-26(24)37)22-15-21-8-5-7-20(10-9-19-16-31-35(4)17-19)23(21)28(39)36(22)13-14-42(40,41)34(2)3/h5-8,11-12,15-18H,13-14H2,1-4H3,(H2,29,33)(H,32,38)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM342553

(US10329299, Compound 104 | US10675286, Compound 10...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1CCS(=O)(=O)N(C)C |r| Show InChI InChI=1S/C28H29N9O4S/c1-18(32-27(38)24-25(29)33-37-12-6-11-30-26(24)37)22-15-21-8-5-7-20(10-9-19-16-31-35(4)17-19)23(21)28(39)36(22)13-14-42(40,41)34(2)3/h5-8,11-12,15-18H,13-14H2,1-4H3,(H2,29,33)(H,32,38)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9775844 (2017)
BindingDB Entry DOI: 10.7270/Q2GQ70WH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data