 Found 289 hits with Last Name = 'doppalapudi' and Initial = 'vr'
Found 289 hits with Last Name = 'doppalapudi' and Initial = 'vr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Placenta growth factor
(Mus musculus) | BDBM50339214
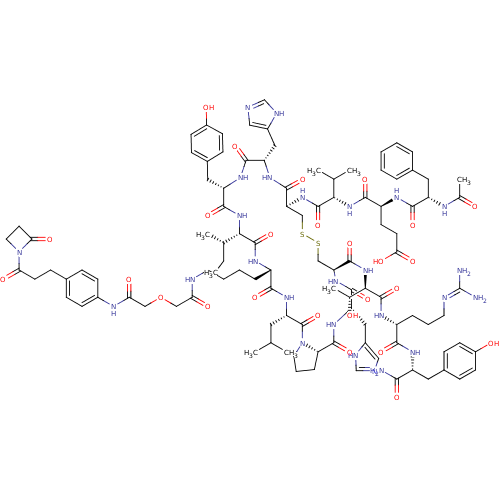
(CHEMBL1689480)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCNC(=O)COCC(=O)Nc2ccc(CCC(=O)N3CCC3=O)cc2)NC1=O)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(C)C |r,wU:39.41,4.3,64.68,49.56,2.2,145.161,106.112,132.140,20.21,8.8,wD:56.60,100.108,117.123,99.105,136.149,35.102,30.136,(28.71,-31.85,;27.38,-32.62,;26.04,-31.85,;26.04,-30.32,;24.71,-32.63,;20.77,-34.9,;19.44,-34.12,;18.1,-34.87,;19.46,-32.59,;20.78,-31.84,;20.81,-30.3,;22.14,-29.56,;22.16,-28.02,;20.84,-27.24,;20.86,-25.7,;19.5,-27.99,;19.49,-29.52,;18.13,-31.79,;16.8,-32.55,;16.78,-34.09,;15.46,-31.76,;15.48,-30.23,;14.16,-29.44,;14.02,-27.92,;12.52,-27.58,;11.73,-28.9,;12.74,-30.05,;14.12,-32.54,;14.18,-34.02,;15.53,-34.76,;12.81,-34.79,;12.81,-36.34,;14.15,-37.14,;14.14,-38.71,;15.45,-39.53,;15.45,-41.06,;16.77,-41.84,;16.77,-43.38,;15.44,-44.14,;18.09,-44.15,;18.09,-45.7,;16.74,-46.46,;15.34,-45.83,;14.31,-46.97,;15.08,-48.31,;16.59,-47.99,;19.43,-43.4,;20.74,-44.16,;20.74,-45.7,;22.09,-43.4,;23.55,-43.87,;24.45,-42.64,;23.55,-41.4,;22.1,-41.87,;20.75,-41.12,;19.44,-41.88,;20.75,-39.58,;19.42,-38.82,;18.09,-39.59,;16.75,-38.83,;18.09,-41.13,;22.09,-38.79,;22.07,-37.26,;20.73,-36.51,;23.4,-36.48,;24.73,-37.25,;24.72,-38.79,;26.05,-39.57,;26.04,-41.12,;27.39,-41.91,;28.74,-41.13,;28.75,-39.57,;30.09,-41.91,;31.44,-41.14,;32.78,-41.92,;34.14,-41.14,;34.14,-39.59,;35.48,-41.93,;36.83,-41.15,;38.17,-41.94,;39.52,-41.16,;39.53,-39.61,;40.88,-38.83,;42.22,-39.61,;43.57,-38.83,;43.57,-37.28,;44.92,-39.61,;45.32,-41.11,;46.82,-40.71,;46.42,-39.2,;47.2,-37.86,;38.17,-38.82,;36.83,-39.6,;23.43,-34.93,;24.73,-34.18,;26.05,-34.93,;14.11,-41.84,;14.11,-43.36,;12.79,-41.07,;11.45,-41.84,;11.45,-43.37,;10.13,-44.15,;12.79,-44.14,;10.13,-41.06,;10.13,-39.52,;8.79,-41.84,;7.45,-41.06,;7.45,-39.52,;6.11,-38.75,;4.79,-39.53,;3.46,-38.76,;2.12,-39.53,;.8,-38.77,;2.13,-41.07,;6.11,-41.84,;4.78,-41.07,;6.12,-43.37,;4.8,-44.14,;4.81,-45.7,;3.46,-46.48,;2.11,-45.71,;.77,-46.49,;.78,-48.05,;-.57,-48.83,;2.13,-48.82,;3.47,-48.03,;3.46,-43.38,;2.13,-44.16,;3.46,-41.84,;11.49,-34.02,;10.15,-34.77,;10.15,-36.31,;8.81,-34.01,;7.47,-34.78,;6.16,-34.02,;6.15,-32.49,;4.8,-34.8,;4.82,-36.33,;3.5,-37.12,;2.16,-36.36,;.85,-37.15,;2.14,-34.83,;3.5,-34.03,;3.5,-32.5,;4.84,-31.73,;2.17,-31.72,;2.19,-30.19,;3.52,-29.43,;4.84,-30.2,;6.18,-29.44,;6.19,-27.9,;4.85,-27.13,;3.52,-27.89,;.84,-32.48,;-.49,-31.71,;-1.83,-32.48,;-.49,-30.16,;8.81,-32.48,;7.47,-31.71,;10.13,-31.7,)| Show InChI InChI=1S/C106H146N26O26S2/c1-9-59(6)89-103(155)119-71(19-13-14-39-112-83(137)51-158-52-84(138)117-66-29-22-62(23-30-66)28-36-85(139)132-42-38-86(132)140)93(145)125-79(43-57(2)3)105(157)131-41-16-21-82(131)101(153)124-78(48-68-50-111-56-115-68)97(149)126-81(100(152)130-90(60(7)133)104(156)120-72(20-15-40-113-106(108)109)92(144)121-74(91(107)143)44-64-24-31-69(135)32-25-64)54-160-159-53-80(99(151)123-77(47-67-49-110-55-114-67)96(148)122-76(98(150)129-89)46-65-26-33-70(136)34-27-65)127-102(154)88(58(4)5)128-94(146)73(35-37-87(141)142)118-95(147)75(116-61(8)134)45-63-17-11-10-12-18-63/h10-12,17-18,22-27,29-34,49-50,55-60,71-82,88-90,133,135-136H,9,13-16,19-21,28,35-48,51-54H2,1-8H3,(H2,107,143)(H,110,114)(H,111,115)(H,112,137)(H,116,134)(H,117,138)(H,118,147)(H,119,155)(H,120,156)(H,121,144)(H,122,148)(H,123,151)(H,124,153)(H,125,145)(H,126,149)(H,127,154)(H,128,146)(H,129,150)(H,130,152)(H,141,142)(H4,108,109,113)/t59-,60+,71-,72+,73-,74+,75-,76-,77-,78-,79-,80-,81-,82-,88-,89-,90+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PlGF-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50339212
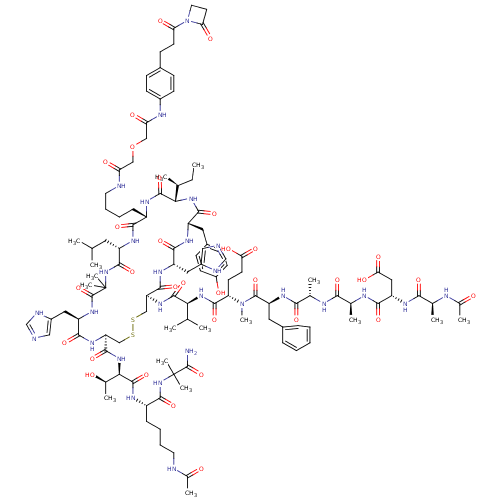
(CHEMBL1689483)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)C(C)(C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCNC(=O)COCC(=O)Nc2ccc(CCC(=O)N3CCC3=O)cc2)NC1=O)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCCNC(C)=O)C(=O)NC(C)(C)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(C)=O)C(C)C |r| Show InChI InChI=1S/C116H168N28O32S2/c1-18-62(6)94-110(171)128-76(28-23-25-44-121-87(149)54-176-55-88(150)127-72-35-30-69(31-36-72)34-40-89(151)144-45-42-90(144)152)99(160)131-78(46-60(2)3)107(168)142-116(15,16)114(175)137-81(50-74-53-119-59-123-74)102(163)135-85(105(166)140-95(66(10)145)111(172)129-77(29-22-24-43-120-67(11)146)106(167)141-115(13,14)113(117)174)57-178-177-56-84(104(165)133-80(49-73-52-118-58-122-73)101(162)132-79(103(164)139-94)47-71-32-37-75(148)38-33-71)136-109(170)93(61(4)5)138-108(169)86(39-41-91(153)154)143(17)112(173)83(48-70-26-20-19-21-27-70)134-98(159)65(9)125-96(157)64(8)126-100(161)82(51-92(155)156)130-97(158)63(7)124-68(12)147/h19-21,26-27,30-33,35-38,52-53,58-66,76-86,93-95,145,148H,18,22-25,28-29,34,39-51,54-57H2,1-17H3,(H2,117,174)(H,118,122)(H,119,123)(H,120,146)(H,121,149)(H,124,147)(H,125,157)(H,126,161)(H,127,150)(H,128,171)(H,129,172)(H,130,158)(H,131,160)(H,132,162)(H,133,165)(H,134,159)(H,135,163)(H,136,170)(H,137,175)(H,138,169)(H,139,164)(H,140,166)(H,141,167)(H,142,168)(H,153,154)(H,155,156)/t62-,63-,64-,65-,66+,76-,77-,78-,79-,80-,81?,82-,83-,84-,85-,86-,93-,94-,95+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PlGF-1-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Mus musculus) | BDBM50339209

(CHEMBL1689479)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCNC(=O)COCC(=O)Nc2ccc(CCC(=O)N3CCC3=O)cc2)NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(C)C |r,wU:4.3,64.68,49.56,2.2,145.161,117.123,99.105,132.140,20.21,8.8,39.41,wD:56.60,100.108,106.112,136.149,35.102,30.136,(16.25,-25.17,;14.91,-25.94,;13.58,-25.18,;13.58,-23.65,;12.25,-25.96,;8.31,-28.23,;6.97,-27.45,;5.63,-28.2,;6.99,-25.91,;8.32,-25.16,;8.34,-23.62,;9.68,-22.88,;9.7,-21.35,;8.38,-20.56,;8.4,-19.02,;7.04,-21.32,;7.02,-22.85,;5.67,-25.12,;4.34,-25.88,;4.32,-27.42,;2.99,-25.09,;3.01,-23.56,;1.69,-22.77,;1.55,-21.25,;.05,-20.91,;-.73,-22.23,;.28,-23.39,;1.66,-25.86,;1.72,-27.35,;3.06,-28.09,;.35,-28.11,;.34,-29.66,;1.68,-30.47,;1.67,-32.03,;2.98,-32.85,;2.98,-34.39,;4.31,-35.17,;4.31,-36.71,;2.97,-37.47,;5.63,-37.48,;5.62,-39.03,;4.28,-39.79,;2.88,-39.16,;1.85,-40.3,;2.61,-41.64,;4.12,-41.32,;6.97,-36.72,;8.28,-37.49,;8.28,-39.03,;9.63,-36.73,;11.08,-37.21,;11.99,-35.97,;11.09,-34.73,;9.63,-35.2,;8.29,-34.44,;6.97,-35.21,;8.29,-32.9,;6.95,-32.14,;5.63,-32.92,;4.29,-32.16,;5.63,-34.46,;9.63,-32.12,;9.61,-30.59,;8.27,-29.83,;10.94,-29.81,;12.27,-30.58,;12.26,-32.12,;13.59,-32.89,;13.58,-34.45,;14.93,-35.23,;16.28,-34.46,;16.28,-32.9,;17.62,-35.24,;18.97,-34.47,;20.32,-35.25,;21.67,-34.47,;21.67,-32.91,;23.02,-35.25,;24.37,-34.48,;25.71,-35.27,;27.06,-34.49,;27.07,-32.93,;28.41,-32.16,;29.76,-32.94,;31.11,-32.16,;31.11,-30.6,;32.46,-32.94,;32.86,-34.44,;34.36,-34.04,;33.96,-32.54,;34.74,-31.19,;25.71,-32.15,;24.37,-32.93,;10.97,-28.26,;12.26,-27.51,;13.59,-28.26,;1.65,-35.17,;1.65,-36.69,;.32,-34.4,;-1.01,-35.17,;-1.01,-36.7,;-2.34,-37.47,;.32,-37.47,;-2.34,-34.38,;-2.34,-32.85,;-3.68,-35.17,;-5.01,-34.38,;-5.02,-32.85,;-6.35,-32.08,;-7.68,-32.86,;-9.01,-32.09,;-10.34,-32.86,;-11.68,-32.09,;-10.34,-34.39,;-6.35,-35.17,;-7.68,-34.4,;-6.34,-36.7,;-7.67,-37.47,;-7.66,-39.03,;-9,-39.81,;-10.35,-39.03,;-11.7,-39.82,;-11.71,-41.38,;-13.04,-42.16,;-10.33,-42.15,;-8.99,-41.36,;-9.01,-36.71,;-10.34,-37.48,;-9.01,-35.17,;-.97,-27.34,;-2.31,-28.09,;-2.31,-29.63,;-3.65,-27.34,;-5,-28.1,;-6.31,-27.35,;-6.32,-25.82,;-7.67,-28.12,;-7.65,-29.66,;-8.97,-30.44,;-10.31,-29.69,;-11.64,-30.47,;-10.32,-28.16,;-8.97,-27.36,;-8.96,-25.83,;-7.63,-25.06,;-10.29,-25.05,;-10.28,-23.51,;-8.94,-22.75,;-7.62,-23.53,;-6.29,-22.76,;-6.28,-21.23,;-7.61,-20.46,;-8.94,-21.22,;-11.63,-25.81,;-12.98,-25.03,;-14.33,-25.81,;-12.98,-23.48,;-3.66,-25.8,;-4.99,-25.03,;-2.33,-25.03,)| Show InChI InChI=1S/C106H146N26O26S2/c1-9-59(6)89-103(155)119-71(19-13-14-39-112-83(137)51-158-52-84(138)117-66-29-22-62(23-30-66)28-36-85(139)132-42-38-86(132)140)93(145)125-79(43-57(2)3)105(157)131-41-16-21-82(131)101(153)124-78(48-68-50-111-56-115-68)97(149)126-81(100(152)130-90(60(7)133)104(156)120-72(20-15-40-113-106(108)109)92(144)121-74(91(107)143)44-64-24-31-69(135)32-25-64)54-160-159-53-80(99(151)123-77(47-67-49-110-55-114-67)96(148)122-76(98(150)129-89)46-65-26-33-70(136)34-27-65)127-102(154)88(58(4)5)128-94(146)73(35-37-87(141)142)118-95(147)75(116-61(8)134)45-63-17-11-10-12-18-63/h10-12,17-18,22-27,29-34,49-50,55-60,71-82,88-90,133,135-136H,9,13-16,19-21,28,35-48,51-54H2,1-8H3,(H2,107,143)(H,110,114)(H,111,115)(H,112,137)(H,116,134)(H,117,138)(H,118,147)(H,119,155)(H,120,156)(H,121,144)(H,122,148)(H,123,151)(H,124,153)(H,125,145)(H,126,149)(H,127,154)(H,128,146)(H,129,150)(H,130,152)(H,141,142)(H4,108,109,113)/t59-,60+,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,88-,89-,90-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PlGF-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Staphylococcus aureus) | BDBM50079694

(CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...)Show SMILES C[C@]1(COC(=O)Cc2ccccc2)[C@@H](N2C(\C(=C/c3ccccn3)C2=O)S1(=O)=O)C([O-])=O Show InChI InChI=1S/C22H20N2O7S/c1-22(13-31-17(25)11-14-7-3-2-4-8-14)18(21(27)28)24-19(26)16(20(24)32(22,29)30)12-15-9-5-6-10-23-15/h2-10,12,18,20H,11,13H2,1H3,(H,27,28)/p-1/b16-12-/t18-,20?,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus |
Bioorg Med Chem Lett 9: 1997-2002 (1999)
BindingDB Entry DOI: 10.7270/Q2TQ60RF |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50197001
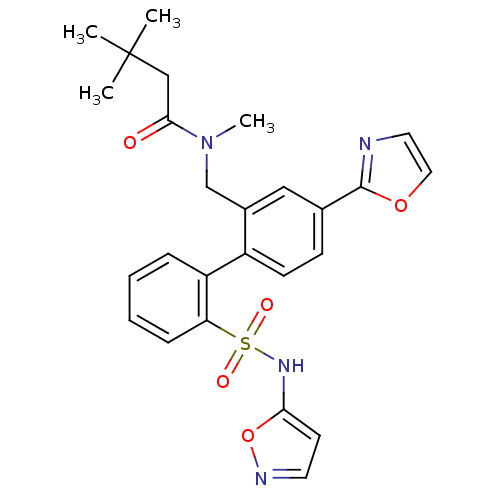
(CHEMBL238539 | N-[2'-(isoxazol-5-ylsulfamoyl)-4-ox...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1ccno1)-c1ncco1)C(=O)CC(C)(C)C Show InChI InChI=1S/C26H28N4O5S/c1-26(2,3)16-24(31)30(4)17-19-15-18(25-27-13-14-34-25)9-10-20(19)21-7-5-6-8-22(21)36(32,33)29-23-11-12-28-35-23/h5-15,29H,16-17H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
CovX Research LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET1 from human ETA receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 501-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.009
BindingDB Entry DOI: 10.7270/Q2DZ07ZF |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50339210
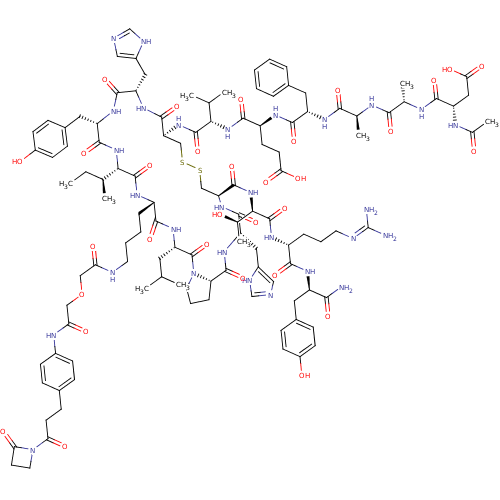
(CHEMBL1689481)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCNC(=O)COCC(=O)Nc2ccc(CCC(=O)N3CCC3=O)cc2)NC1=O)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(C)C |r,wU:39.41,4.3,64.68,49.56,2.2,145.161,161.170,106.112,132.140,20.21,8.8,wD:56.60,100.108,117.123,156.165,99.105,136.149,35.102,30.136,166.179,(33.81,-1.12,;32.48,-1.89,;31.15,-1.12,;31.14,.41,;29.82,-1.9,;25.88,-4.17,;24.55,-3.39,;23.21,-4.14,;24.57,-1.86,;25.89,-1.11,;25.92,.43,;27.25,1.17,;27.26,2.71,;25.95,3.49,;25.97,5.03,;24.61,2.74,;24.59,1.21,;23.24,-1.06,;21.91,-1.82,;21.89,-3.36,;20.57,-1.03,;20.59,.5,;19.27,1.29,;19.13,2.81,;17.63,3.15,;16.84,1.83,;17.85,.68,;19.23,-1.81,;19.29,-3.29,;20.64,-4.03,;17.93,-4.05,;17.92,-5.6,;19.26,-6.41,;19.25,-7.97,;20.56,-8.79,;20.56,-10.33,;21.88,-11.11,;21.88,-12.65,;20.55,-13.41,;23.2,-13.41,;23.19,-14.96,;21.85,-15.72,;20.45,-15.09,;19.42,-16.23,;20.19,-17.57,;21.7,-17.25,;24.54,-12.66,;25.85,-13.43,;25.84,-14.96,;27.2,-12.66,;28.65,-13.14,;29.56,-11.91,;28.66,-10.66,;27.2,-11.13,;25.86,-10.38,;24.54,-11.14,;25.86,-8.84,;24.53,-8.08,;23.2,-8.85,;21.86,-8.09,;23.2,-10.39,;27.19,-8.06,;27.18,-6.53,;25.84,-5.77,;28.51,-5.75,;29.84,-6.52,;29.83,-8.06,;31.15,-8.83,;31.15,-10.39,;32.49,-11.17,;33.85,-10.39,;33.85,-8.84,;35.19,-11.18,;36.54,-10.4,;37.88,-11.18,;39.24,-10.41,;39.24,-8.85,;40.58,-11.19,;41.93,-10.41,;43.27,-11.2,;44.62,-10.43,;44.63,-8.87,;45.98,-8.09,;47.32,-8.87,;48.67,-8.1,;48.67,-6.54,;50.02,-8.88,;50.41,-10.38,;51.92,-9.97,;51.52,-8.47,;52.3,-7.13,;43.27,-8.09,;41.93,-8.87,;28.54,-4.2,;29.83,-3.45,;31.16,-4.2,;19.22,-11.1,;19.22,-12.63,;17.9,-10.34,;16.57,-11.1,;16.57,-12.63,;15.24,-13.41,;17.9,-13.4,;15.24,-10.32,;15.24,-8.79,;13.9,-11.1,;12.57,-10.32,;12.56,-8.79,;11.22,-8.02,;9.9,-8.79,;8.57,-8.03,;7.24,-8.8,;5.92,-8.03,;7.24,-10.33,;11.22,-11.1,;9.9,-10.34,;11.23,-12.63,;9.92,-13.4,;9.92,-14.96,;8.58,-15.74,;7.23,-14.97,;5.89,-15.75,;5.89,-17.31,;4.55,-18.09,;7.25,-18.08,;8.59,-17.29,;8.58,-12.64,;7.24,-13.42,;8.58,-11.11,;16.61,-3.28,;15.27,-4.03,;15.27,-5.57,;13.93,-3.28,;12.58,-4.05,;11.27,-3.29,;11.27,-1.76,;9.92,-4.07,;9.94,-5.6,;8.61,-6.38,;7.27,-5.63,;5.97,-6.41,;7.26,-4.1,;8.62,-3.3,;8.62,-1.77,;9.95,-1,;7.29,-.99,;7.3,.54,;8.64,1.3,;9.96,.53,;11.29,1.29,;11.31,2.83,;9.97,3.6,;8.64,2.83,;5.96,-1.75,;4.63,-.98,;4.63,.57,;3.29,-1.75,;3.29,-3.3,;1.95,-.98,;.62,-1.75,;.62,-3.3,;-.72,-.98,;-.72,.57,;-2.05,-1.75,;-3.39,-.98,;-3.39,.57,;-4.73,-1.75,;-4.73,-3.3,;-3.39,-4.07,;-2.05,-3.3,;-3.39,-5.62,;-6.07,-.98,;-7.4,-1.76,;-8.73,-.99,;-7.4,-3.3,;13.92,-1.75,;12.58,-.97,;15.25,-.97,)| Show InChI InChI=1S/C116H161N29O31S2/c1-11-62(6)96-113(173)131-76(21-15-16-41-122-89(150)54-176-55-90(151)129-71-31-24-67(25-32-71)30-38-91(152)145-44-40-92(145)153)102(162)138-85(45-60(2)3)115(175)144-43-18-23-88(144)111(171)137-83(50-73-53-121-59-125-73)107(167)139-87(110(170)143-97(65(9)146)114(174)132-77(22-17-42-123-116(118)119)101(161)133-79(98(117)158)46-69-26-33-74(148)34-27-69)57-178-177-56-86(109(169)136-82(49-72-52-120-58-124-72)106(166)135-81(108(168)142-96)48-70-28-35-75(149)36-29-70)140-112(172)95(61(4)5)141-103(163)78(37-39-93(154)155)130-105(165)80(47-68-19-13-12-14-20-68)134-100(160)64(8)126-99(159)63(7)127-104(164)84(51-94(156)157)128-66(10)147/h12-14,19-20,24-29,31-36,52-53,58-65,76-88,95-97,146,148-149H,11,15-18,21-23,30,37-51,54-57H2,1-10H3,(H2,117,158)(H,120,124)(H,121,125)(H,122,150)(H,126,159)(H,127,164)(H,128,147)(H,129,151)(H,130,165)(H,131,173)(H,132,174)(H,133,161)(H,134,160)(H,135,166)(H,136,169)(H,137,171)(H,138,162)(H,139,167)(H,140,172)(H,141,163)(H,142,168)(H,143,170)(H,154,155)(H,156,157)(H4,118,119,123)/t62-,63-,64-,65+,76-,77+,78-,79+,80-,81-,82-,83-,84-,85-,86-,87-,88-,95-,96-,97+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PlGF-1-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50197003

(CHEMBL241460 | N-[4-(3,5-dioxo-hexyl)-phenyl]-3-(2...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1ccno1)-c1ncco1)C(=O)COCCOCCOCCOCCC(=O)Nc1ccc(CCC(=O)CC(C)=O)cc1 Show InChI InChI=1S/C43H49N5O12S/c1-31(49)27-36(50)13-9-32-7-11-35(12-8-32)46-40(51)16-19-55-21-22-56-23-24-57-25-26-58-30-42(52)48(2)29-34-28-33(43-44-18-20-59-43)10-14-37(34)38-5-3-4-6-39(38)61(53,54)47-41-15-17-45-60-41/h3-8,10-12,14-15,17-18,20,28,47H,9,13,16,19,21-27,29-30H2,1-2H3,(H,46,51) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CovX Research LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET1 from human ETA receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 501-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.009
BindingDB Entry DOI: 10.7270/Q2DZ07ZF |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50339191
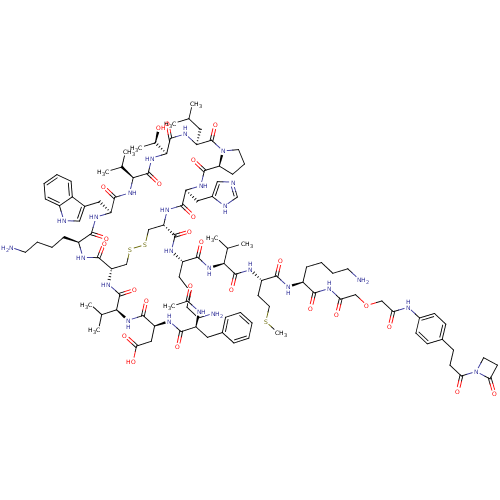
(CHEMBL1689460)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2ccccc2)NC(C)=O)C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N1)C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NC(=O)COCC(=O)Nc1ccc(CCC(=O)N2CCC2=O)cc1 |r| Show InChI InChI=1S/C107H157N25O25S3/c1-58(2)46-79-107(156)131-43-23-31-82(131)102(151)122-77(49-68-52-111-57-113-68)97(146)124-80(100(149)118-73(30-19-22-42-110)95(144)127-88(59(3)4)103(152)119-74(39-45-158-11)93(142)116-72(29-18-21-41-109)94(143)126-84(136)54-157-53-83(135)115-67-35-32-64(33-36-67)34-37-85(137)132-44-38-86(132)138)55-159-160-56-81(125-104(153)89(60(5)6)128-99(148)78(50-87(139)140)121-96(145)75(114-63(10)134)47-65-24-13-12-14-25-65)101(150)117-71(28-17-20-40-108)92(141)120-76(48-66-51-112-70-27-16-15-26-69(66)70)98(147)129-90(61(7)8)105(154)130-91(62(9)133)106(155)123-79/h12-16,24-27,32-33,35-36,51-52,57-62,71-82,88-91,112,133H,17-23,28-31,34,37-50,53-56,108-110H2,1-11H3,(H,111,113)(H,114,134)(H,115,135)(H,116,142)(H,117,150)(H,118,149)(H,119,152)(H,120,141)(H,121,145)(H,122,151)(H,123,155)(H,124,146)(H,125,153)(H,127,144)(H,128,148)(H,129,147)(H,130,154)(H,139,140)(H,126,136,143)/t62-,71+,72+,73+,74+,75+,76+,77+,78+,79+,80+,81+,82+,88+,89+,90+,91+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PlGF-1-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114512

(CHEMBL45136 | Sodium; (R)-3-bromo-5,5,8-trioxo-7-[...)Show SMILES [O-]C(=O)C1=C(Br)CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1 |c:3| Show InChI InChI=1S/C13H9BrN2O5S/c14-9-6-22(20,21)12-8(5-7-3-1-2-4-15-7)11(17)16(12)10(9)13(18)19/h1-5,12H,6H2,(H,18,19)/p-1/b8-5-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114505
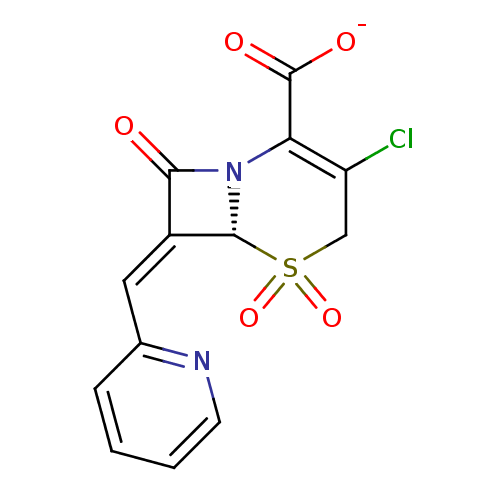
(CHEMBL295625 | Sodium; (R)-3-chloro-5,5,8-trioxo-7...)Show SMILES [O-]C(=O)C1=C(Cl)CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1 |c:3| Show InChI InChI=1S/C13H9ClN2O5S/c14-9-6-22(20,21)12-8(5-7-3-1-2-4-15-7)11(17)16(12)10(9)13(18)19/h1-5,12H,6H2,(H,18,19)/p-1/b8-5-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50339211

(CHEMBL1689482)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCNC(=O)COCC(=O)Nc2ccc(CCC(=O)N3CCC3=O)cc2)NC1=O)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(C)=O)C(C)C |r,wU:8.8,39.41,4.3,64.68,49.56,2.2,146.162,162.171,175.184,106.112,132.140,20.21,wD:56.60,100.108,117.123,157.166,99.105,136.149,167.180,35.102,30.136,(38.18,-23.64,;36.84,-24.41,;35.51,-23.65,;35.5,-22.12,;34.18,-24.43,;30.24,-26.69,;28.91,-25.91,;27.57,-26.67,;28.93,-24.38,;30.25,-23.63,;30.28,-22.09,;31.61,-21.35,;31.63,-19.82,;30.31,-19.03,;30.33,-17.49,;28.97,-19.79,;28.96,-21.32,;27.6,-23.59,;26.28,-24.35,;26.26,-25.88,;24.93,-23.56,;24.95,-22.03,;23.63,-21.24,;23.49,-19.72,;22,-19.37,;21.21,-20.7,;22.22,-21.85,;23.6,-24.33,;23.65,-25.82,;25,-26.56,;22.29,-26.58,;22.28,-28.13,;23.62,-28.93,;23.61,-30.5,;24.92,-31.32,;24.92,-32.86,;26.24,-33.64,;26.24,-35.17,;24.91,-35.93,;27.57,-35.94,;27.56,-37.49,;26.21,-38.25,;24.82,-37.62,;23.78,-38.76,;24.55,-40.1,;26.06,-39.78,;28.91,-35.19,;30.21,-35.95,;30.21,-37.49,;31.56,-35.19,;33.01,-35.66,;33.92,-34.43,;33.02,-33.19,;31.56,-33.66,;30.22,-32.91,;28.91,-33.67,;30.22,-31.37,;28.89,-30.61,;27.56,-31.38,;26.23,-30.62,;27.57,-32.92,;31.56,-30.58,;31.54,-29.05,;30.2,-28.3,;32.87,-28.28,;34.2,-29.05,;34.19,-30.59,;35.51,-31.36,;35.51,-32.91,;36.86,-33.7,;38.21,-32.92,;38.21,-31.37,;39.56,-33.7,;40.9,-32.93,;42.25,-33.71,;43.6,-32.93,;43.6,-31.38,;44.95,-33.72,;46.29,-32.94,;47.63,-33.73,;48.99,-32.96,;48.99,-31.4,;50.34,-30.62,;51.68,-31.4,;53.03,-30.62,;53.03,-29.07,;54.38,-31.4,;54.77,-32.9,;56.28,-32.5,;55.88,-31,;56.66,-29.65,;47.63,-30.62,;46.29,-31.39,;32.9,-26.73,;34.2,-25.98,;35.52,-26.73,;23.59,-33.63,;23.59,-35.15,;22.26,-32.86,;20.93,-33.63,;20.93,-35.16,;19.6,-35.94,;22.26,-35.93,;19.6,-32.85,;19.6,-31.32,;18.26,-33.63,;16.93,-32.85,;16.93,-31.32,;15.59,-30.55,;14.27,-31.32,;12.94,-30.55,;11.6,-31.33,;10.28,-30.56,;11.61,-32.86,;15.59,-33.63,;14.26,-32.87,;15.6,-35.16,;14.28,-35.93,;14.29,-37.49,;12.94,-38.27,;11.59,-37.5,;10.25,-38.28,;10.26,-39.84,;8.91,-40.62,;11.61,-40.61,;12.95,-39.82,;12.94,-35.17,;11.61,-35.95,;12.94,-33.64,;20.97,-25.81,;19.63,-26.56,;19.63,-28.1,;18.29,-25.8,;16.95,-26.57,;15.64,-25.82,;15.63,-24.29,;14.28,-26.59,;14.3,-28.12,;12.98,-28.91,;11.63,-28.16,;10.33,-28.94,;11.62,-26.62,;12.98,-25.83,;11.48,-25.42,;12.98,-24.3,;14.32,-23.53,;11.65,-23.52,;11.66,-21.98,;13,-21.22,;14.32,-22,;15.65,-21.24,;15.67,-19.7,;14.33,-18.93,;13,-19.69,;10.32,-24.28,;8.99,-23.51,;8.99,-21.96,;7.65,-24.28,;7.65,-25.82,;6.32,-23.51,;4.98,-24.28,;4.98,-25.83,;3.64,-23.51,;3.64,-21.96,;2.31,-24.28,;.98,-23.51,;.98,-21.96,;-.37,-24.28,;-.37,-25.83,;.98,-26.6,;2.32,-25.82,;.98,-28.14,;-1.7,-23.51,;-3.04,-24.25,;-3.06,-25.8,;-4.37,-23.46,;-4.35,-21.93,;-5.71,-24.22,;-7.03,-23.44,;-8.37,-24.2,;-7.02,-21.9,;18.28,-24.27,;16.95,-23.5,;19.61,-23.5,)| Show InChI InChI=1S/C120H168N30O32S2/c1-13-64(6)99-116(178)134-79(23-17-18-43-126-92(155)56-182-57-93(156)133-74-33-26-70(27-34-74)32-40-94(157)150-46-42-95(150)158)106(168)142-86(47-62(2)3)119(181)149-45-20-25-91(149)114(176)140-84(52-76-55-125-61-129-76)109(171)143-89(112(174)147-100(68(10)151)117(179)135-80(24-19-44-127-120(122)123)105(167)136-81(101(121)163)48-72-28-35-77(153)36-29-72)59-184-183-58-88(111(173)139-83(51-75-54-124-60-128-75)108(170)138-82(110(172)146-99)49-73-30-37-78(154)38-31-73)144-115(177)98(63(4)5)145-113(175)90(39-41-96(159)160)148(12)118(180)87(50-71-21-15-14-16-22-71)141-104(166)67(9)131-102(164)66(8)132-107(169)85(53-97(161)162)137-103(165)65(7)130-69(11)152/h14-16,21-22,26-31,33-38,54-55,60-68,79-91,98-100,151,153-154H,13,17-20,23-25,32,39-53,56-59H2,1-12H3,(H2,121,163)(H,124,128)(H,125,129)(H,126,155)(H,130,152)(H,131,164)(H,132,169)(H,133,156)(H,134,178)(H,135,179)(H,136,167)(H,137,165)(H,138,170)(H,139,173)(H,140,176)(H,141,166)(H,142,168)(H,143,171)(H,144,177)(H,145,175)(H,146,172)(H,147,174)(H,159,160)(H,161,162)(H4,122,123,127)/t64-,65-,66-,67-,68+,79-,80+,81+,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,98-,99-,100+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PlGF-1-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Mus musculus) | BDBM50339193
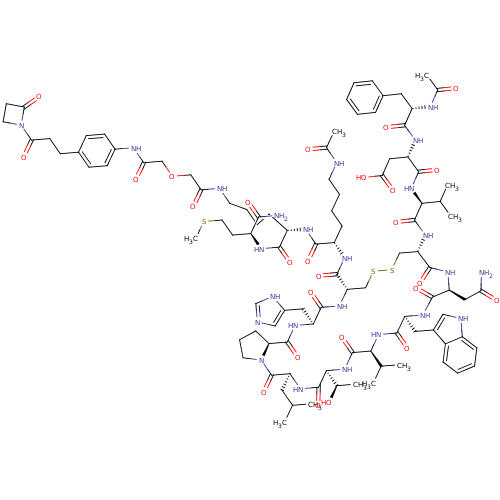
(CHEMBL1689463)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCCNC(=O)COCC(=O)Nc1ccc(CCC(=O)N2CCC2=O)cc1)NC(=O)[C@H](CCCCNC(C)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2ccccc2)NC(C)=O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N1)C(N)=O |r| Show InChI InChI=1S/C102H144N24O26S3/c1-54(2)41-75-102(151)125-38-20-27-78(125)98(147)118-72(44-64-48-105-53-109-64)92(141)120-76(96(145)114-69(25-16-18-36-106-58(8)128)90(139)113-68(89(138)112-67(88(104)137)35-40-153-10)26-17-19-37-107-80(131)49-152-50-81(132)111-63-31-28-60(29-32-63)30-33-82(133)126-39-34-83(126)134)51-154-155-52-77(121-99(148)85(55(3)4)122-95(144)74(46-84(135)136)117-91(140)70(110-59(9)129)42-61-21-12-11-13-22-61)97(146)116-73(45-79(103)130)93(142)115-71(43-62-47-108-66-24-15-14-23-65(62)66)94(143)123-86(56(5)6)100(149)124-87(57(7)127)101(150)119-75/h11-15,21-24,28-29,31-32,47-48,53-57,67-78,85-87,108,127H,16-20,25-27,30,33-46,49-52H2,1-10H3,(H2,103,130)(H2,104,137)(H,105,109)(H,106,128)(H,107,131)(H,110,129)(H,111,132)(H,112,138)(H,113,139)(H,114,145)(H,115,142)(H,116,146)(H,117,140)(H,118,147)(H,119,150)(H,120,141)(H,121,148)(H,122,144)(H,123,143)(H,124,149)(H,135,136)/t57-,67+,68+,69+,70+,71+,72+,73+,74+,75+,76+,77+,78+,85+,86+,87+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PlGF-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Mus musculus) | BDBM50339201
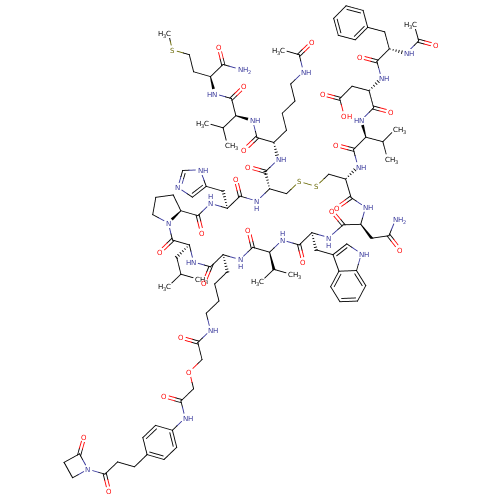
(CHEMBL1689470)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCCNC(C)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2ccccc2)NC(C)=O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCNC(=O)COCC(=O)Nc2ccc(CCC(=O)N3CCC3=O)cc2)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C103H146N24O25S3/c1-55(2)42-76-103(151)126-39-21-28-79(126)99(147)119-73(45-65-49-106-54-110-65)93(141)121-77(97(145)114-70(27-17-19-37-107-59(9)128)91(139)123-86(56(3)4)100(148)113-68(89(105)137)36-41-153-11)52-154-155-53-78(122-102(150)88(58(7)8)125-96(144)75(47-85(135)136)118-92(140)71(111-60(10)129)43-62-22-13-12-14-23-62)98(146)117-74(46-80(104)130)94(142)116-72(44-63-48-109-67-25-16-15-24-66(63)67)95(143)124-87(57(5)6)101(149)115-69(90(138)120-76)26-18-20-38-108-81(131)50-152-51-82(132)112-64-32-29-61(30-33-64)31-34-83(133)127-40-35-84(127)134/h12-16,22-25,29-30,32-33,48-49,54-58,68-79,86-88,109H,17-21,26-28,31,34-47,50-53H2,1-11H3,(H2,104,130)(H2,105,137)(H,106,110)(H,107,128)(H,108,131)(H,111,129)(H,112,132)(H,113,148)(H,114,145)(H,115,149)(H,116,142)(H,117,146)(H,118,140)(H,119,147)(H,120,138)(H,121,141)(H,122,150)(H,123,139)(H,124,143)(H,125,144)(H,135,136)/t68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,86-,87-,88-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PlGF-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Staphylococcus aureus) | BDBM50079695
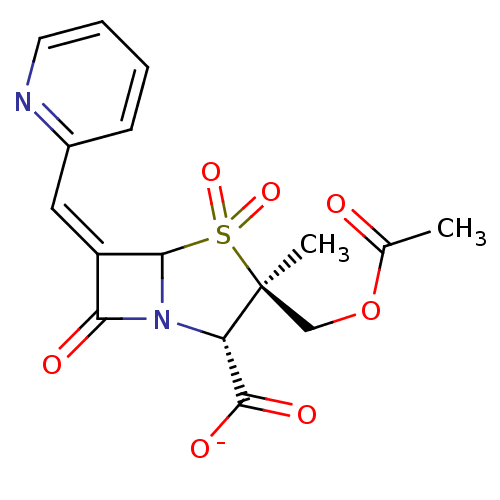
(CHEMBL294203 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...)Show SMILES CC(=O)OC[C@@]1(C)[C@@H](N2C(\C(=C/c3ccccn3)C2=O)S1(=O)=O)C([O-])=O Show InChI InChI=1S/C16H16N2O7S/c1-9(19)25-8-16(2)12(15(21)22)18-13(20)11(14(18)26(16,23)24)7-10-5-3-4-6-17-10/h3-7,12,14H,8H2,1-2H3,(H,21,22)/p-1/b11-7-/t12-,14?,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus |
Bioorg Med Chem Lett 9: 1997-2002 (1999)
BindingDB Entry DOI: 10.7270/Q2TQ60RF |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Homo sapiens (Human)) | BDBM50339193

(CHEMBL1689463)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCCNC(=O)COCC(=O)Nc1ccc(CCC(=O)N2CCC2=O)cc1)NC(=O)[C@H](CCCCNC(C)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2ccccc2)NC(C)=O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N1)C(N)=O |r| Show InChI InChI=1S/C102H144N24O26S3/c1-54(2)41-75-102(151)125-38-20-27-78(125)98(147)118-72(44-64-48-105-53-109-64)92(141)120-76(96(145)114-69(25-16-18-36-106-58(8)128)90(139)113-68(89(138)112-67(88(104)137)35-40-153-10)26-17-19-37-107-80(131)49-152-50-81(132)111-63-31-28-60(29-32-63)30-33-82(133)126-39-34-83(126)134)51-154-155-52-77(121-99(148)85(55(3)4)122-95(144)74(46-84(135)136)117-91(140)70(110-59(9)129)42-61-21-12-11-13-22-61)97(146)116-73(45-79(103)130)93(142)115-71(43-62-47-108-66-24-15-14-23-65(62)66)94(143)123-86(56(5)6)100(149)124-87(57(7)127)101(150)119-75/h11-15,21-24,28-29,31-32,47-48,53-57,67-78,85-87,108,127H,16-20,25-27,30,33-46,49-52H2,1-10H3,(H2,103,130)(H2,104,137)(H,105,109)(H,106,128)(H,107,131)(H,110,129)(H,111,132)(H,112,138)(H,113,139)(H,114,145)(H,115,142)(H,116,146)(H,117,140)(H,118,147)(H,119,150)(H,120,141)(H,121,148)(H,122,144)(H,123,143)(H,124,149)(H,135,136)/t57-,67+,68+,69+,70+,71+,72+,73+,74+,75+,76+,77+,78+,85+,86+,87+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PlGF-1-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50197002

(CHEMBL268201 | N-[4-(3,5-dioxo-hexyl)-phenyl]-3-{2...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1ccno1)-c1ncco1)C(=O)Cc1ccc(OCCOCCOCCOCCOCCC(=O)Nc2ccc(CCC(=O)CC(C)=O)cc2)cc1 Show InChI InChI=1S/C51H57N5O13S/c1-37(57)33-43(58)15-9-38-7-13-42(14-8-38)54-48(59)20-23-63-25-26-64-27-28-65-29-30-66-31-32-67-44-16-10-39(11-17-44)34-50(60)56(2)36-41-35-40(51-52-22-24-68-51)12-18-45(41)46-5-3-4-6-47(46)70(61,62)55-49-19-21-53-69-49/h3-8,10-14,16-19,21-22,24,35,55H,9,15,20,23,25-34,36H2,1-2H3,(H,54,59) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CovX Research LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET1 from human ETA receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 501-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.009
BindingDB Entry DOI: 10.7270/Q2DZ07ZF |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114504
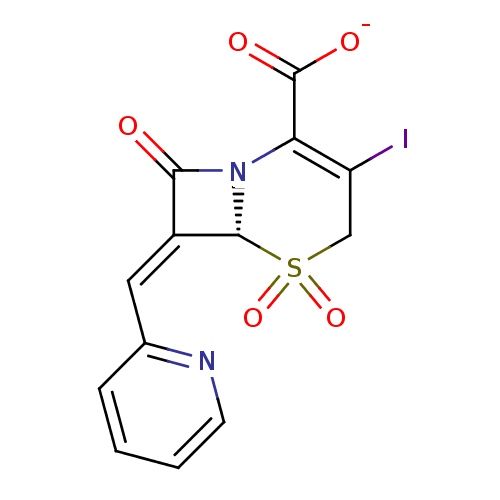
(CHEMBL45084 | Sodium; (R)-3-iodo-5,5,8-trioxo-7-[1...)Show SMILES [O-]C(=O)C1=C(I)CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1 |c:3| Show InChI InChI=1S/C13H9IN2O5S/c14-9-6-22(20,21)12-8(5-7-3-1-2-4-15-7)11(17)16(12)10(9)13(18)19/h1-5,12H,6H2,(H,18,19)/p-1/b8-5-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114514
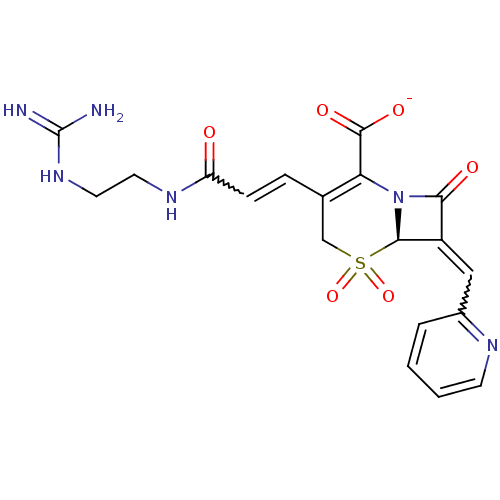
(CHEMBL44932 | Sodium; (R)-3-[(E)-2-(2-guanidino-et...)Show SMILES NC(=N)NCCNC(=O)C=CC1=C(N2[C@@H](C(=Cc3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |w:9.8,16.16,t:11| Show InChI InChI=1S/C19H20N6O6S/c20-19(21)24-8-7-23-14(26)5-4-11-10-32(30,31)17-13(9-12-3-1-2-6-22-12)16(27)25(17)15(11)18(28)29/h1-6,9,17H,7-8,10H2,(H,23,26)(H,28,29)(H4,20,21,24)/p-1/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088835
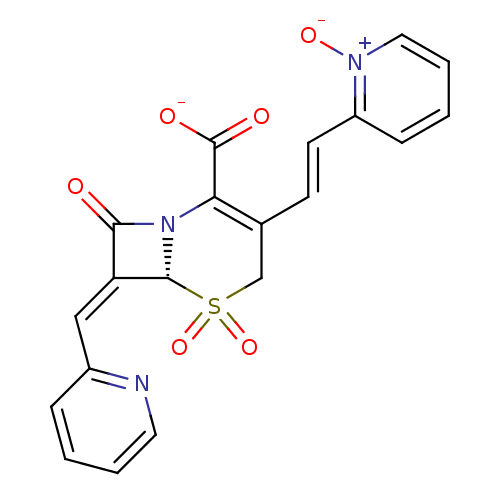
(CHEMBL433726 | Sodium; (R)-5,5,8-trioxo-3-[(E)-2-(...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1)\C=C\c1cccc[n+]1[O-] |t:3| Show InChI InChI=1S/C20H15N3O6S/c24-18-16(11-14-5-1-3-9-21-14)19-23(18)17(20(25)26)13(12-30(19,28)29)7-8-15-6-2-4-10-22(15)27/h1-11,19H,12H2,(H,25,26)/p-1/b8-7+,16-11-/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibition of class A TEM-1 beta-lactamase derived from Enterobacter cloacae |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50114518
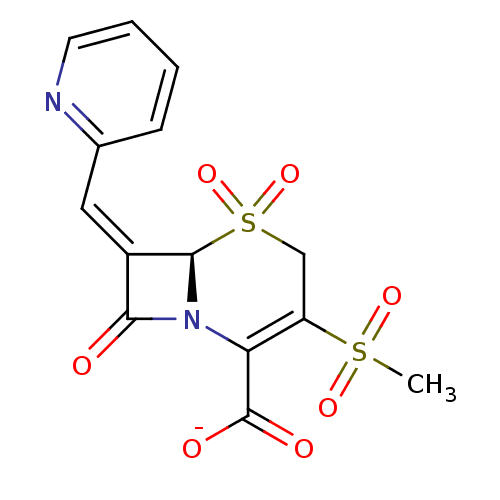
(CHEMBL297805 | Sodium; (R)-3-methanesulfonyl-5,5,8...)Show SMILES CS(=O)(=O)C1=C(N2[C@@H](\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |t:4| Show InChI InChI=1S/C14H12N2O7S2/c1-24(20,21)10-7-25(22,23)13-9(6-8-4-2-3-5-15-8)12(17)16(13)11(10)14(18)19/h2-6,13H,7H2,1H3,(H,18,19)/p-1/b9-6-/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against class A TEM-1 beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50197000

(CHEMBL429019 | N-(35-{[4-(3,5-dioxohexyl)phenyl]ca...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1ccno1)-c1ncco1)C(=O)CCCC(=O)NCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCC(=O)Nc1ccc(CCC(=O)CC(C)=O)cc1 Show InChI InChI=1S/C62H86N6O20S/c1-49(69)46-54(70)16-12-50-10-14-53(15-11-50)66-59(72)19-23-76-26-28-78-30-32-80-34-36-82-38-40-84-42-44-86-45-43-85-41-39-83-37-35-81-33-31-79-29-27-77-24-21-63-58(71)8-5-9-61(73)68(2)48-52-47-51(62-64-22-25-87-62)13-17-55(52)56-6-3-4-7-57(56)89(74,75)67-60-18-20-65-88-60/h3-4,6-7,10-11,13-15,17-18,20,22,25,47,67H,5,8-9,12,16,19,21,23-24,26-46,48H2,1-2H3,(H,63,71)(H,66,72) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CovX Research LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET1 from human ETA receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 501-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.009
BindingDB Entry DOI: 10.7270/Q2DZ07ZF |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50197004
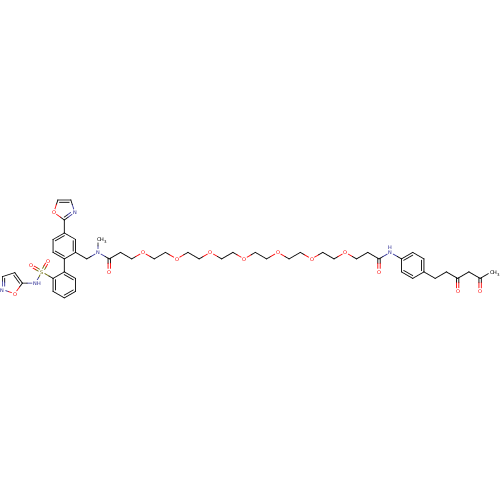
(3-{2-[2-(2-{2-[2-(2-{2-[4-(3,5-dioxo-hexyl)-phenyl...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1ccno1)-c1ncco1)C(=O)CCOCCOCCOCCOCCOCCOCCOCCC(=O)Nc1ccc(CCC(=O)CC(C)=O)cc1 Show InChI InChI=1S/C50H63N5O15S/c1-38(56)35-43(57)13-9-39-7-11-42(12-8-39)53-47(58)16-20-62-23-25-64-27-29-66-31-33-68-34-32-67-30-28-65-26-24-63-21-17-49(59)55(2)37-41-36-40(50-51-19-22-69-50)10-14-44(41)45-5-3-4-6-46(45)71(60,61)54-48-15-18-52-70-48/h3-8,10-12,14-15,18-19,22,36,54H,9,13,16-17,20-21,23-35,37H2,1-2H3,(H,53,58) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CovX Research LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET1 from human ETA receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 501-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.009
BindingDB Entry DOI: 10.7270/Q2DZ07ZF |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50114514

(CHEMBL44932 | Sodium; (R)-3-[(E)-2-(2-guanidino-et...)Show SMILES NC(=N)NCCNC(=O)C=CC1=C(N2[C@@H](C(=Cc3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |w:9.8,16.16,t:11| Show InChI InChI=1S/C19H20N6O6S/c20-19(21)24-8-7-23-14(26)5-4-11-10-32(30,31)17-13(9-12-3-1-2-6-22-12)16(27)25(17)15(11)18(28)29/h1-6,9,17H,7-8,10H2,(H,23,26)(H,28,29)(H4,20,21,24)/p-1/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against class A TEM-1 beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
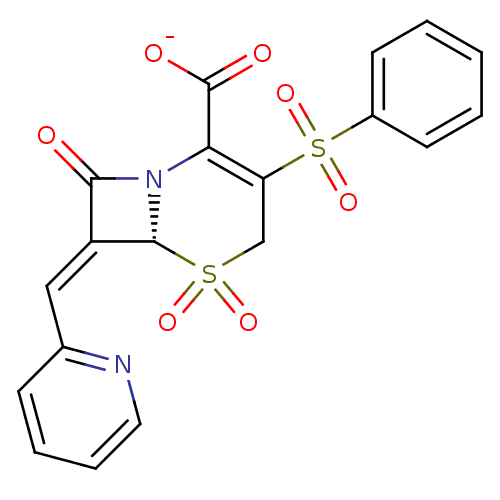
Beta-lactamase
(Escherichia coli) | BDBM50114516
(CHEMBL295322 | Sodium; (R)-3-benzenesulfonyl-5,5,8...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1)S(=O)(=O)c1ccccc1 |t:3| Show InChI InChI=1S/C19H14N2O7S2/c22-17-14(10-12-6-4-5-9-20-12)18-21(17)16(19(23)24)15(11-29(18,25)26)30(27,28)13-7-2-1-3-8-13/h1-10,18H,11H2,(H,23,24)/p-1/b14-10-/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against class A TEM-1 beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088831

(CHEMBL353613 | Sodium; 3-((E)-2-carbamoyl-vinyl)-5...)Show SMILES NC(=O)\C=C\C1=C(N2C(\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |t:5| Show InChI InChI=1S/C16H13N3O6S/c17-12(20)5-4-9-8-26(24,25)15-11(7-10-3-1-2-6-18-10)14(21)19(15)13(9)16(22)23/h1-7,15H,8H2,(H2,17,20)(H,22,23)/p-1/b5-4+,11-7- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibition of GC1 extended spectrum class C beta-lactamase |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088827
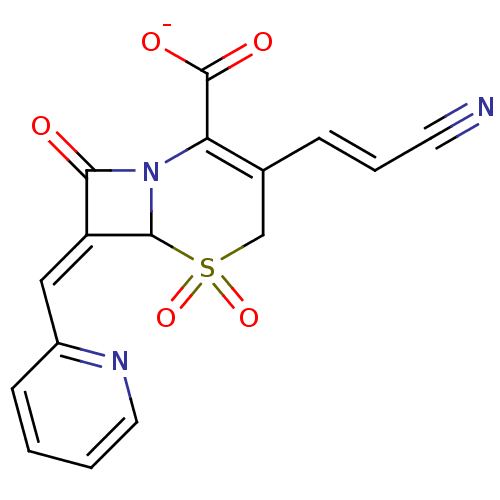
(CHEMBL355558 | Sodium; 3-((E)-2-cyano-vinyl)-5,5,8...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)C2N1C(=O)\C2=C\c1ccccn1)\C=C\C#N |t:3| Show InChI InChI=1S/C16H11N3O5S/c17-6-3-4-10-9-25(23,24)15-12(8-11-5-1-2-7-18-11)14(20)19(15)13(10)16(21)22/h1-5,7-8,15H,9H2,(H,21,22)/p-1/b4-3+,12-8- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition against Class C beta-lactamase derived from Enterobacter cloacae P99 |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114503
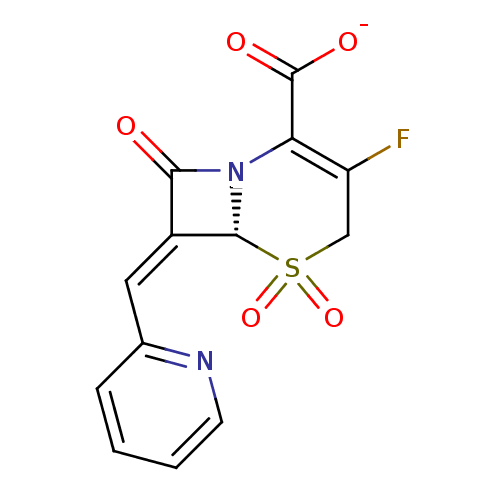
(CHEMBL46478 | Sodium; (R)-3-fluoro-5,5,8-trioxo-7-...)Show SMILES [O-]C(=O)C1=C(F)CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1 |c:3| Show InChI InChI=1S/C13H9FN2O5S/c14-9-6-22(20,21)12-8(5-7-3-1-2-4-15-7)11(17)16(12)10(9)13(18)19/h1-5,12H,6H2,(H,18,19)/p-1/b8-5-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50114521
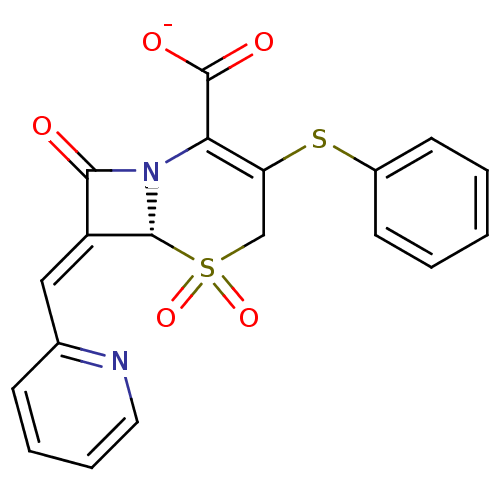
(CHEMBL42528 | Sodium; (R)-5,5,8-trioxo-3-phenylsul...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1)Sc1ccccc1 |t:3| Show InChI InChI=1S/C19H14N2O5S2/c22-17-14(10-12-6-4-5-9-20-12)18-21(17)16(19(23)24)15(11-28(18,25)26)27-13-7-2-1-3-8-13/h1-10,18H,11H2,(H,23,24)/p-1/b14-10-/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against class A TEM-1 beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088827

(CHEMBL355558 | Sodium; 3-((E)-2-cyano-vinyl)-5,5,8...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)C2N1C(=O)\C2=C\c1ccccn1)\C=C\C#N |t:3| Show InChI InChI=1S/C16H11N3O5S/c17-6-3-4-10-9-25(23,24)15-12(8-11-5-1-2-7-18-11)14(20)19(15)13(10)16(21)22/h1-5,7-8,15H,9H2,(H,21,22)/p-1/b4-3+,12-8- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition against the GC1 extended spectrum Class C beta-lactamase |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088827

(CHEMBL355558 | Sodium; 3-((E)-2-cyano-vinyl)-5,5,8...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)C2N1C(=O)\C2=C\c1ccccn1)\C=C\C#N |t:3| Show InChI InChI=1S/C16H11N3O5S/c17-6-3-4-10-9-25(23,24)15-12(8-11-5-1-2-7-18-11)14(20)19(15)13(10)16(21)22/h1-5,7-8,15H,9H2,(H,21,22)/p-1/b4-3+,12-8- | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibition of class A TEM-1 beta-lactamase derived from Enterobacter cloacae |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Staphylococcus aureus) | BDBM50079686

(CHEMBL292093 | disodium (2S,3R,5R,6Z)-3-methyl-6-(...)Show SMILES C[C@]1(COC(=O)Cc2ccccc2)[C@@H](N2C(\C(=C/C([O-])=O)C2=O)S1(=O)=O)C([O-])=O Show InChI InChI=1S/C18H17NO9S/c1-18(9-28-13(22)7-10-5-3-2-4-6-10)14(17(24)25)19-15(23)11(8-12(20)21)16(19)29(18,26)27/h2-6,8,14,16H,7,9H2,1H3,(H,20,21)(H,24,25)/p-2/b11-8-/t14-,16?,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus |
Bioorg Med Chem Lett 9: 1997-2002 (1999)
BindingDB Entry DOI: 10.7270/Q2TQ60RF |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088825
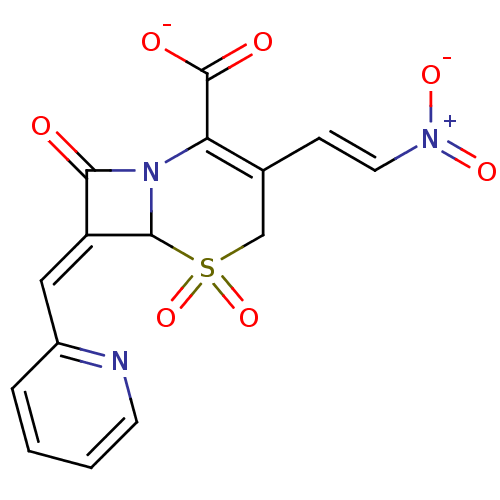
(CHEMBL353422 | Sodium; 3-((E)-2-nitro-vinyl)-5,5,8...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)C2N1C(=O)\C2=C\c1ccccn1)\C=C\[N+]([O-])=O |t:3| Show InChI InChI=1S/C15H11N3O7S/c19-13-11(7-10-3-1-2-5-16-10)14-18(13)12(15(20)21)9(4-6-17(22)23)8-26(14,24)25/h1-7,14H,8H2,(H,20,21)/p-1/b6-4+,11-7- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition against the GC1 extended spectrum Class C beta-lactamase |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088834
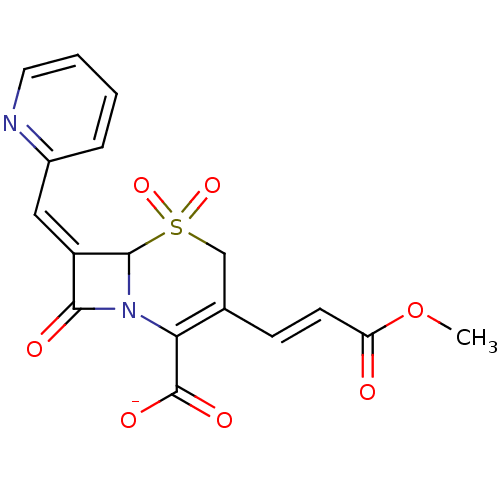
(CHEMBL169392 | Sodium; 3-((E)-2-methoxycarbonyl-vi...)Show SMILES COC(=O)\C=C\C1=C(N2C(\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |t:6| Show InChI InChI=1S/C17H14N2O7S/c1-26-13(20)6-5-10-9-27(24,25)16-12(8-11-4-2-3-7-18-11)15(21)19(16)14(10)17(22)23/h2-8,16H,9H2,1H3,(H,22,23)/p-1/b6-5+,12-8- | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibition of class A TEM-1 beta-lactamase derived from Enterobacter cloacae |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50196999

(CHEMBL413001 | N-[4-(3,5-dioxo-hexyl)-phenyl]-3-{2...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1ccno1)-c1ncco1)C(=O)CCc1ccc(OCCOCCOCCOCCOCCC(=O)Nc2ccc(CCC(=O)CC(C)=O)cc2)cc1 Show InChI InChI=1S/C52H59N5O13S/c1-38(58)35-44(59)16-9-39-7-14-43(15-8-39)55-49(60)22-25-64-27-28-65-29-30-66-31-32-67-33-34-68-45-17-10-40(11-18-45)12-20-51(61)57(2)37-42-36-41(52-53-24-26-69-52)13-19-46(42)47-5-3-4-6-48(47)71(62,63)56-50-21-23-54-70-50/h3-8,10-11,13-15,17-19,21,23-24,26,36,56H,9,12,16,20,22,25,27-35,37H2,1-2H3,(H,55,60) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21.6 | n/a | n/a | n/a | n/a | n/a | n/a |
CovX Research LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET1 from human ETA receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 501-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.009
BindingDB Entry DOI: 10.7270/Q2DZ07ZF |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114501
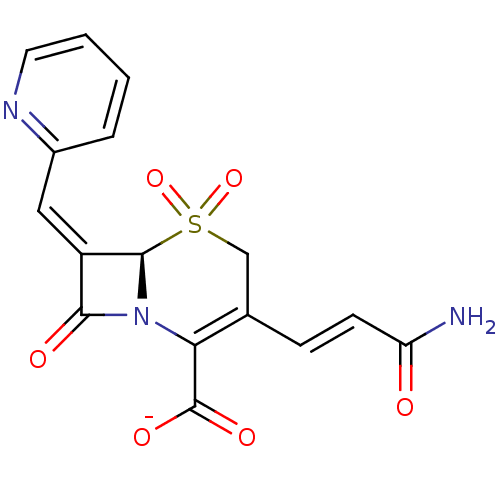
(CHEMBL289979 | Sodium; (R)-3-((E)-2-carbamoyl-viny...)Show SMILES NC(=O)\C=C\C1=C(N2[C@@H](\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |t:5| Show InChI InChI=1S/C16H13N3O6S/c17-12(20)5-4-9-8-26(24,25)15-11(7-10-3-1-2-6-18-10)14(21)19(15)13(9)16(22)23/h1-7,15H,8H2,(H2,17,20)(H,22,23)/p-1/b5-4+,11-7-/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114521

(CHEMBL42528 | Sodium; (R)-5,5,8-trioxo-3-phenylsul...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1)Sc1ccccc1 |t:3| Show InChI InChI=1S/C19H14N2O5S2/c22-17-14(10-12-6-4-5-9-20-12)18-21(17)16(19(23)24)15(11-28(18,25)26)27-13-7-2-1-3-8-13/h1-10,18H,11H2,(H,23,24)/p-1/b14-10-/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Staphylococcus aureus) | BDBM50079689
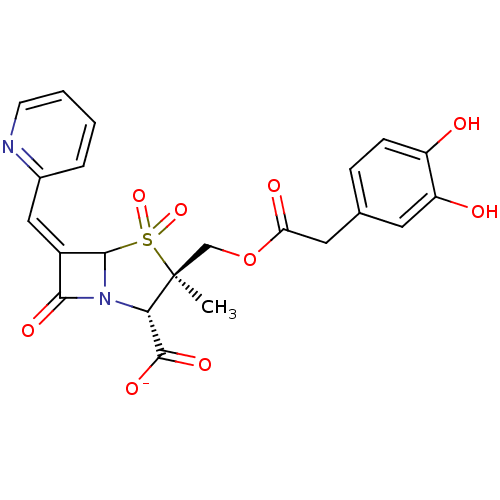
(CHEMBL303053 | Sodium; (2S,3R)-3-[2-(3,4-dihydroxy...)Show SMILES C[C@]1(COC(=O)Cc2ccc(O)c(O)c2)[C@@H](N2C(\C(=C/c3ccccn3)C2=O)S1(=O)=O)C([O-])=O Show InChI InChI=1S/C22H20N2O9S/c1-22(11-33-17(27)9-12-5-6-15(25)16(26)8-12)18(21(29)30)24-19(28)14(20(24)34(22,31)32)10-13-4-2-3-7-23-13/h2-8,10,18,20,25-26H,9,11H2,1H3,(H,29,30)/p-1/b14-10-/t18-,20?,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus |
Bioorg Med Chem Lett 9: 1997-2002 (1999)
BindingDB Entry DOI: 10.7270/Q2TQ60RF |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088831

(CHEMBL353613 | Sodium; 3-((E)-2-carbamoyl-vinyl)-5...)Show SMILES NC(=O)\C=C\C1=C(N2C(\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |t:5| Show InChI InChI=1S/C16H13N3O6S/c17-12(20)5-4-9-8-26(24,25)15-11(7-10-3-1-2-6-18-10)14(21)19(15)13(9)16(22)23/h1-7,15H,8H2,(H2,17,20)(H,22,23)/p-1/b5-4+,11-7- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Affinity for alpha4-beta1 integrin from HL60 cell lysate |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088817
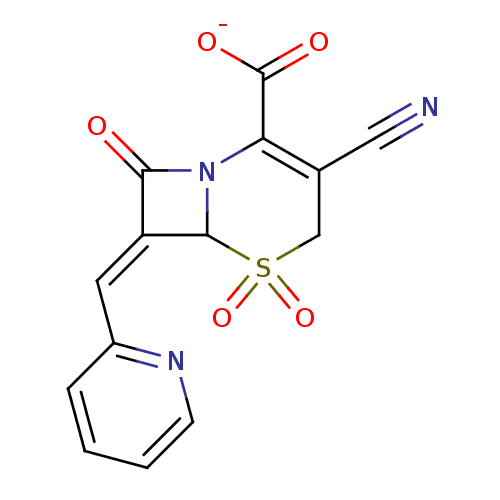
(CHEMBL355288 | Sodium; 3-cyano-5,5,8-trioxo-7-[1-p...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)C2N1C(=O)\C2=C\c1ccccn1)C#N |t:3| Show InChI InChI=1S/C14H9N3O5S/c15-6-8-7-23(21,22)13-10(5-9-3-1-2-4-16-9)12(18)17(13)11(8)14(19)20/h1-5,13H,7H2,(H,19,20)/p-1/b10-5- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibition of class C beta-lactamase derived from Enterobacter cloacae P99 |
Bioorg Med Chem Lett 10: 853-7 (2000)
BindingDB Entry DOI: 10.7270/Q2HQ3Z41 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Staphylococcus aureus) | BDBM50033680
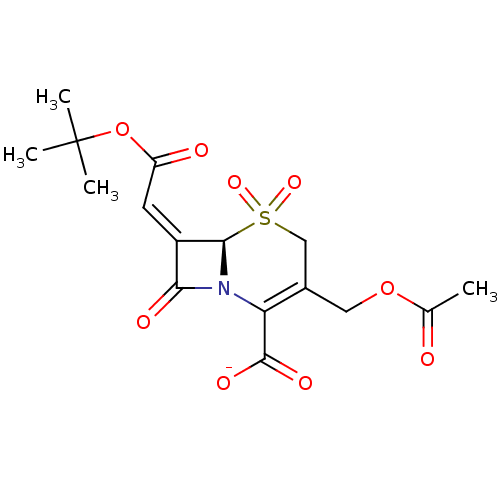
(CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...)Show SMILES CC(=O)OCC1=C(N2[C@@H](\C(=C/C(=O)OC(C)(C)C)C2=O)S(=O)(=O)C1)C([O-])=O |t:5| Show InChI InChI=1S/C16H19NO9S/c1-8(18)25-6-9-7-27(23,24)14-10(5-11(19)26-16(2,3)4)13(20)17(14)12(9)15(21)22/h5,14H,6-7H2,1-4H3,(H,21,22)/p-1/b10-5-/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus |
Bioorg Med Chem Lett 10: 847-51 (2000)
BindingDB Entry DOI: 10.7270/Q2NG4PVB |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114508

(CHEMBL46958 | Disodium; (R)-3-carboxy-5,5,8-trioxo...)Show SMILES [O-]C(=O)C1=C(N2[C@@H](\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |t:3| Show InChI InChI=1S/C14H10N2O7S/c17-11-8(5-7-3-1-2-4-15-7)12-16(11)10(14(20)21)9(13(18)19)6-24(12,22)23/h1-5,12H,6H2,(H,18,19)(H,20,21)/p-2/b8-5-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114506
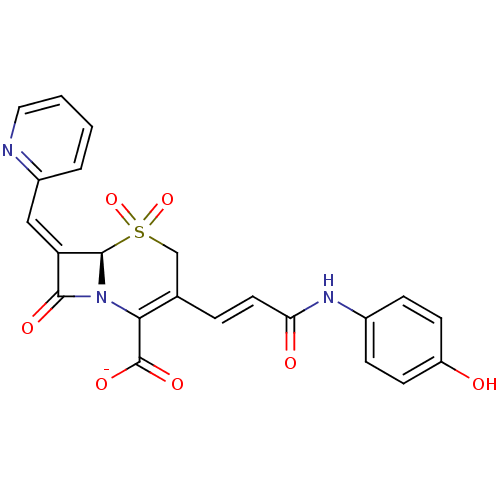
(CHEMBL44278 | Sodium; (R)-3-[(E)-2-(4-hydroxy-phen...)Show SMILES Oc1ccc(NC(=O)\C=C\C2=C(N3[C@@H](\C(=C/c4ccccn4)C3=O)S(=O)(=O)C2)C([O-])=O)cc1 |t:10| Show InChI InChI=1S/C22H17N3O7S/c26-16-7-5-14(6-8-16)24-18(27)9-4-13-12-33(31,32)21-17(11-15-3-1-2-10-23-15)20(28)25(21)19(13)22(29)30/h1-11,21,26H,12H2,(H,24,27)(H,29,30)/p-1/b9-4+,17-11-/t21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114525

(CHEMBL296738 | Sodium; (R)-5,5,8-trioxo-3-phenyl-7...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1)c1ccccc1 |t:3| Show InChI InChI=1S/C19H14N2O5S/c22-17-14(10-13-8-4-5-9-20-13)18-21(17)16(19(23)24)15(11-27(18,25)26)12-6-2-1-3-7-12/h1-10,18H,11H2,(H,23,24)/p-1/b14-10-/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50088813
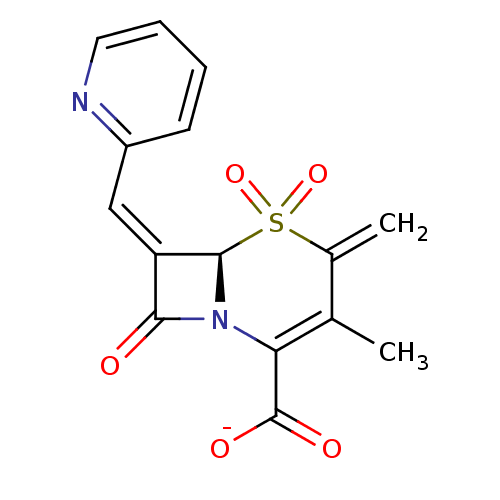
(CHEMBL166730 | Sodium; (R)-3-methyl-4-methylene-5,...)Show SMILES CC1=C(N2[C@@H](\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1=C)C([O-])=O |t:1| Show InChI InChI=1S/C15H12N2O5S/c1-8-9(2)23(21,22)14-11(7-10-5-3-4-6-16-10)13(18)17(14)12(8)15(19)20/h3-7,14H,2H2,1H3,(H,19,20)/p-1/b11-7-/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibition of Class C beta-lactamase from E. cloacae strain P99 |
Bioorg Med Chem Lett 10: 847-51 (2000)
BindingDB Entry DOI: 10.7270/Q2NG4PVB |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50114523
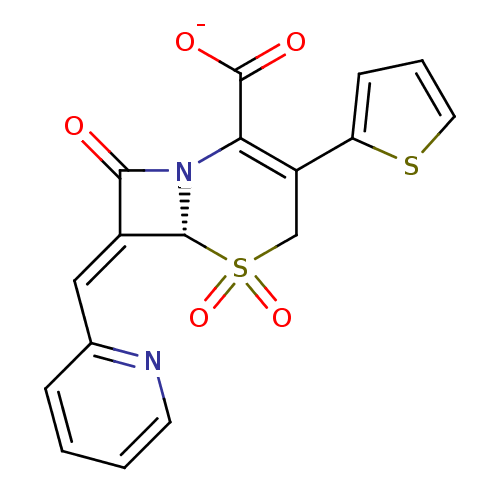
(CHEMBL45273 | Sodium; (R)-5,5,8-trioxo-7-[1-pyridi...)Show SMILES [O-]C(=O)C1=C(CS(=O)(=O)[C@H]2N1C(=O)\C2=C\c1ccccn1)c1cccs1 |t:3| Show InChI InChI=1S/C17H12N2O5S2/c20-15-11(8-10-4-1-2-6-18-10)16-19(15)14(17(21)22)12(9-26(16,23)24)13-5-3-7-25-13/h1-8,16H,9H2,(H,21,22)/p-1/b11-8-/t16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Staphylococcus aureus) | BDBM50079694

(CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...)Show SMILES C[C@]1(COC(=O)Cc2ccccc2)[C@@H](N2C(\C(=C/c3ccccn3)C2=O)S1(=O)=O)C([O-])=O Show InChI InChI=1S/C22H20N2O7S/c1-22(13-31-17(25)11-14-7-3-2-4-8-14)18(21(27)28)24-19(26)16(20(24)32(22,29)30)12-15-9-5-6-10-23-15/h2-10,12,18,20H,11,13H2,1H3,(H,27,28)/p-1/b16-12-/t18-,20?,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus |
Bioorg Med Chem Lett 9: 1997-2002 (1999)
BindingDB Entry DOI: 10.7270/Q2TQ60RF |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50197005

(CHEMBL436821 | N-[4-{2-[2-(2-{2-[4-(3,5-dioxo-hexy...)Show SMILES CN(Cc1cc(OCCOCCOCCOCCC(=O)Nc2ccc(CCC(=O)CC(C)=O)cc2)ccc1-c1ccccc1S(=O)(=O)Nc1ccno1)C(=O)CC(C)(C)C Show InChI InChI=1S/C44H56N4O11S/c1-32(49)28-36(50)15-12-33-10-13-35(14-11-33)46-41(51)19-21-55-22-23-56-24-25-57-26-27-58-37-16-17-38(34(29-37)31-48(5)43(52)30-44(2,3)4)39-8-6-7-9-40(39)60(53,54)47-42-18-20-45-59-42/h6-11,13-14,16-18,20,29,47H,12,15,19,21-28,30-31H2,1-5H3,(H,46,51) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44.9 | n/a | n/a | n/a | n/a | n/a | n/a |
CovX Research LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET1 from human ETA receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 501-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.009
BindingDB Entry DOI: 10.7270/Q2DZ07ZF |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50114515
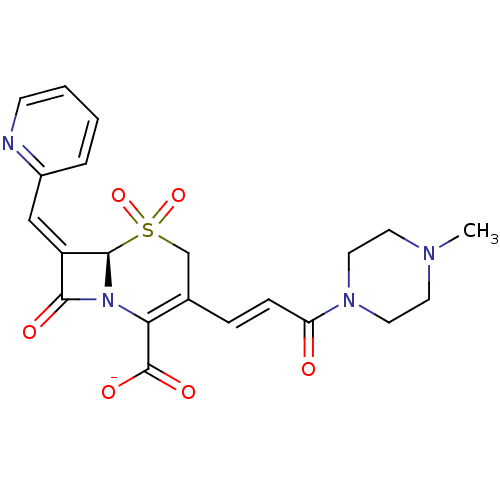
(CHEMBL416447 | Sodium; (R)-3-[(E)-3-(4-methyl-pipe...)Show SMILES CN1CCN(CC1)C(=O)\C=C\C1=C(N2[C@@H](\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |t:12| Show InChI InChI=1S/C21H22N4O6S/c1-23-8-10-24(11-9-23)17(26)6-5-14-13-32(30,31)20-16(12-15-4-2-3-7-22-15)19(27)25(20)18(14)21(28)29/h2-7,12,20H,8-11,13H2,1H3,(H,28,29)/p-1/b6-5+,16-12-/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against class A TEM-1 beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |
Placenta growth factor
(Mus musculus) | BDBM50339197
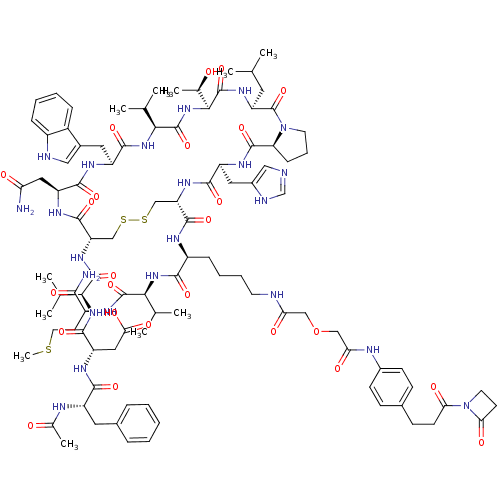
(CHEMBL1689466)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCCNC(=O)COCC(=O)Nc1ccc(CCC(=O)N2CCC2=O)cc1)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2ccccc2)NC(C)=O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C99H139N23O25S3/c1-51(2)38-71-99(146)121-35-19-25-74(121)94(141)113-68(41-61-45-102-50-105-61)88(135)115-72(92(139)109-65(86(133)117-81(52(3)4)95(142)108-64(85(101)132)33-37-148-11)24-17-18-34-103-76(126)46-147-47-77(127)107-60-29-26-57(27-30-60)28-31-78(128)122-36-32-79(122)129)48-149-150-49-73(116-96(143)82(53(5)6)118-91(138)70(43-80(130)131)112-87(134)66(106-56(10)124)39-58-20-13-12-14-21-58)93(140)111-69(42-75(100)125)89(136)110-67(40-59-44-104-63-23-16-15-22-62(59)63)90(137)119-83(54(7)8)97(144)120-84(55(9)123)98(145)114-71/h12-16,20-23,26-27,29-30,44-45,50-55,64-74,81-84,104,123H,17-19,24-25,28,31-43,46-49H2,1-11H3,(H2,100,125)(H2,101,132)(H,102,105)(H,103,126)(H,106,124)(H,107,127)(H,108,142)(H,109,139)(H,110,136)(H,111,140)(H,112,134)(H,113,141)(H,114,145)(H,115,135)(H,116,143)(H,117,133)(H,118,138)(H,119,137)(H,120,144)(H,130,131)/t55-,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,81+,82+,83+,84+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PlGF-VEGFR-1 interaction by ELISA |
J Med Chem 54: 1256-65 (2011)
Article DOI: 10.1021/jm101226k
BindingDB Entry DOI: 10.7270/Q2BK1CN1 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
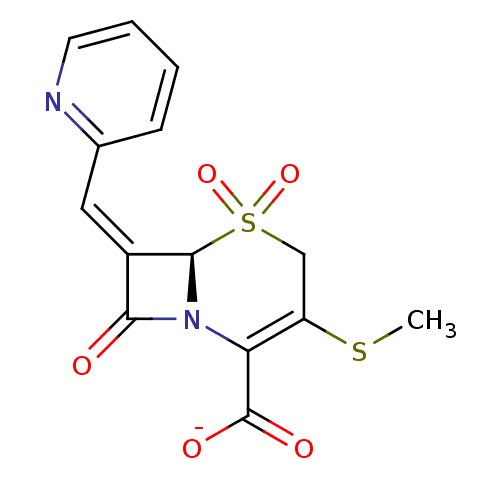
(Enterobacter cloacae) | BDBM50114522
(CHEMBL297671 | Sodium; (R)-3-methylsulfanyl-5,5,8-...)Show SMILES CSC1=C(N2[C@@H](\C(=C/c3ccccn3)C2=O)S(=O)(=O)C1)C([O-])=O |t:2| Show InChI InChI=1S/C14H12N2O5S2/c1-22-10-7-23(20,21)13-9(6-8-4-2-3-5-15-8)12(17)16(13)11(10)14(18)19/h2-6,13H,7H2,1H3,(H,18,19)/p-1/b9-6-/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Class C beta-lactamase |
Bioorg Med Chem Lett 12: 1663-6 (2002)
BindingDB Entry DOI: 10.7270/Q2MC8ZB3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































