 Found 157 hits with Last Name = 'gerwick' and Initial = 'wh'
Found 157 hits with Last Name = 'gerwick' and Initial = 'wh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
J Nat Prod 74: 2313-7 (2011)
Article DOI: 10.1021/np200610t
BindingDB Entry DOI: 10.7270/Q21N81J3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544
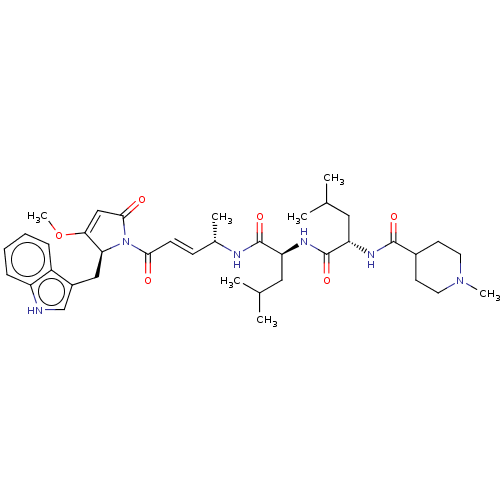
(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543
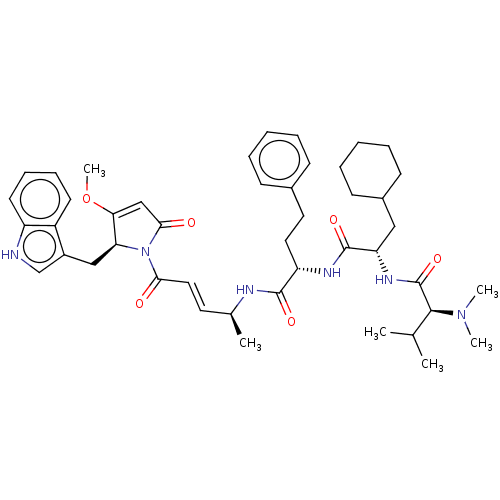
(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602545
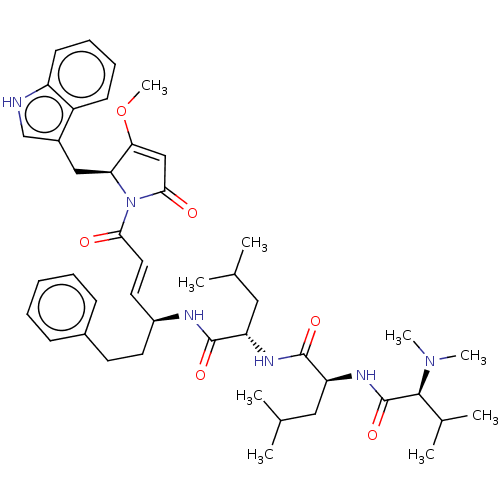
(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50033762
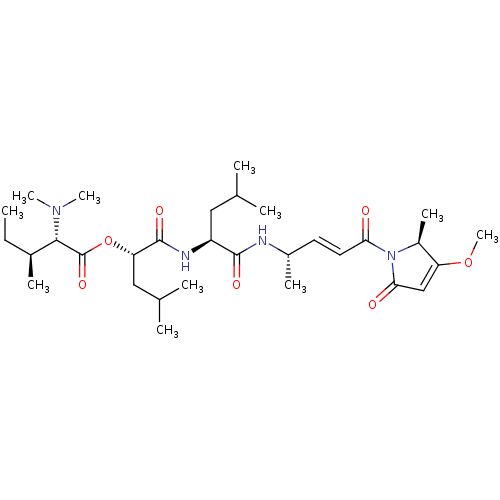
(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
J Nat Prod 74: 2313-7 (2011)
Article DOI: 10.1021/np200610t
BindingDB Entry DOI: 10.7270/Q21N81J3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542
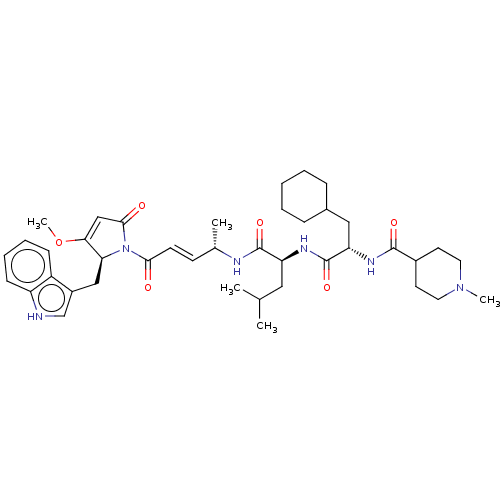
(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50257325

(CHEMBL4090056)Show SMILES CCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCS(C)(=O)=O)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H45N3O7S/c1-8-9-10-11-20(29)28-21(17(4)5)24(32)26-18(12-13-36(7,33)34)23(31)27-19(14-16(2)3)22(30)25(6)15-35-25/h16-19,21H,8-15H2,1-7H3,(H,26,32)(H,27,31)(H,28,29)/t18-,19-,21-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pediatrics, School of Medicine, University of California, San Diego , La Jolla, California 92093, United States.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human 20S proteasome beta5 subunit using suc-LLVY-AMC as substrate after 4 hrs at 30 mins time interval by fluorescence as... |
J Med Chem 60: 6721-6732 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00671
BindingDB Entry DOI: 10.7270/Q2CR5WSK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM22988
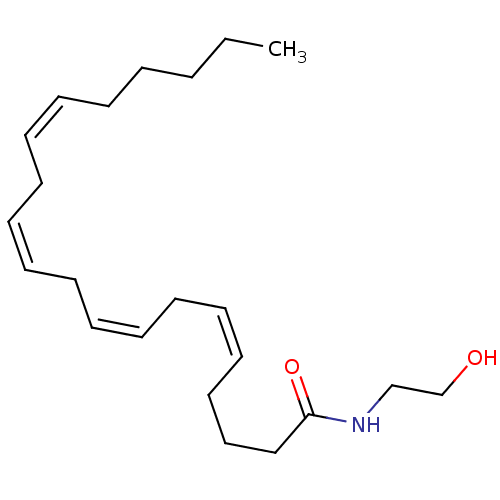
((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...)Show InChI InChI=1S/C22H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(25)23-20-21-24/h6-7,9-10,12-13,15-16,24H,2-5,8,11,14,17-21H2,1H3,(H,23,25)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in CD rat brain membranes |
J Nat Prod 66: 1364-8 (2003)
Article DOI: 10.1021/np030242n
BindingDB Entry DOI: 10.7270/Q2BZ65TG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108987
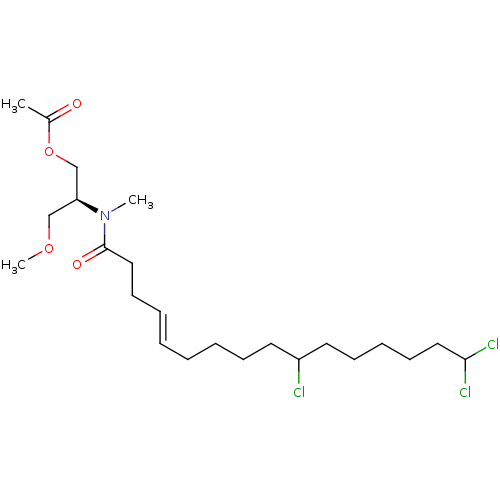
(CHEMBL3597331)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCC(Cl)Cl |r| Show InChI InChI=1S/C23H40Cl3NO4/c1-19(28)31-18-21(17-30-3)27(2)23(29)16-12-7-5-4-6-9-13-20(24)14-10-8-11-15-22(25)26/h5,7,20-22H,4,6,8-18H2,1-3H3/b7-5+/t20?,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108989
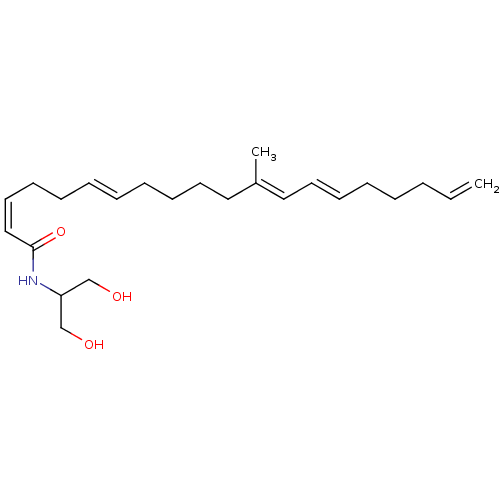
(CHEMBL3597332)Show SMILES C\C(CCCC\C=C\CC\C=C/C(=O)NC(CO)CO)=C/C=C/CCCC=C Show InChI InChI=1S/C24H39NO3/c1-3-4-5-6-11-14-17-22(2)18-15-12-9-7-8-10-13-16-19-24(28)25-23(20-26)21-27/h3,7-8,11,14,16-17,19,23,26-27H,1,4-6,9-10,12-13,15,18,20-21H2,2H3,(H,25,28)/b8-7+,14-11+,19-16-,22-17+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108988
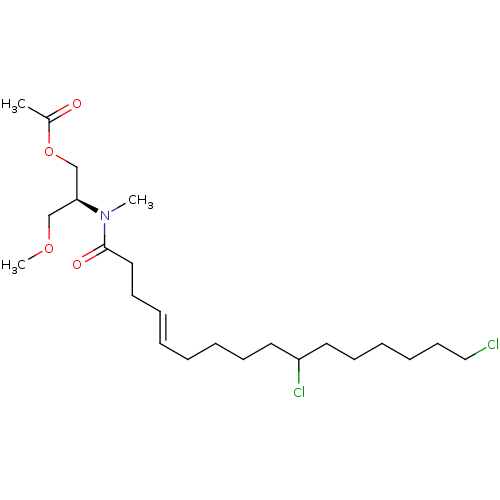
(CHEMBL3597330)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCCCl |r| Show InChI InChI=1S/C23H41Cl2NO4/c1-20(27)30-19-22(18-29-3)26(2)23(28)16-12-7-5-4-6-10-14-21(25)15-11-8-9-13-17-24/h5,7,21-22H,4,6,8-19H2,1-3H3/b7-5+/t21?,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50108987

(CHEMBL3597331)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCC(Cl)Cl |r| Show InChI InChI=1S/C23H40Cl3NO4/c1-19(28)31-18-21(17-30-3)27(2)23(29)16-12-7-5-4-6-9-13-20(24)14-10-8-11-15-22(25)26/h5,7,20-22H,4,6,8-18H2,1-3H3/b7-5+/t20?,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 861 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50108988

(CHEMBL3597330)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCCCl |r| Show InChI InChI=1S/C23H41Cl2NO4/c1-20(27)30-19-22(18-29-3)26(2)23(28)16-12-7-5-4-6-10-14-21(25)15-11-8-9-13-17-24/h5,7,21-22H,4,6,8-19H2,1-3H3/b7-5+/t21?,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50257327

(CHEMBL4067938)Show SMILES CCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C26H47N3O5/c1-8-10-12-14-21(30)29-22(18(5)6)25(33)27-19(13-11-9-2)24(32)28-20(15-17(3)4)23(31)26(7)16-34-26/h17-20,22H,8-16H2,1-7H3,(H,27,33)(H,28,32)(H,29,30)/t19-,20-,22-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pediatrics, School of Medicine, University of California, San Diego , La Jolla, California 92093, United States.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human 20S proteasome beta5 subunit using suc-LLVY-AMC as substrate after 4 hrs at 30 mins time interval by fluorescence as... |
J Med Chem 60: 6721-6732 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00671
BindingDB Entry DOI: 10.7270/Q2CR5WSK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50359305

(CHEMBL1928588)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N[C@@H](CO)C(O)=O |r| Show InChI InChI=1S/C23H37NO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(26)24-21(20-25)23(27)28/h6-7,9-10,12-13,15-16,21,25H,2-5,8,11,14,17-20H2,1H3,(H,24,26)(H,27,28)/b7-6-,10-9-,13-12-,16-15-/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins |
J Nat Prod 74: 2313-7 (2011)
Article DOI: 10.1021/np200610t
BindingDB Entry DOI: 10.7270/Q21N81J3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50359305

(CHEMBL1928588)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N[C@@H](CO)C(O)=O |r| Show InChI InChI=1S/C23H37NO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(26)24-21(20-25)23(27)28/h6-7,9-10,12-13,15-16,21,25H,2-5,8,11,14,17-20H2,1H3,(H,24,26)(H,27,28)/b7-6-,10-9-,13-12-,16-15-/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins |
J Nat Prod 74: 2313-7 (2011)
Article DOI: 10.1021/np200610t
BindingDB Entry DOI: 10.7270/Q21N81J3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50250766

(CHEMBL466777 | Semiplenamide G)Show SMILES CCCCCCCCCCCCCCC[C@H]1O[C@]1(C)C(=O)NC(C)COC(C)=O |r| Show InChI InChI=1S/C24H45NO4/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-22-24(4,29-22)23(27)25-20(2)19-28-21(3)26/h20,22H,5-19H2,1-4H3,(H,25,27)/t20?,22-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in CD rat brain membranes |
J Nat Prod 66: 1364-8 (2003)
Article DOI: 10.1021/np030242n
BindingDB Entry DOI: 10.7270/Q2BZ65TG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50250763

(CHEMBL465162 | Semiplenamide B)Show SMILES CCCCCCCCCCCCC\C=C\CC\C=C(/C)C(=O)NCCOC(C)=O Show InChI InChI=1S/C25H45NO3/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-23(2)25(28)26-21-22-29-24(3)27/h16-17,20H,4-15,18-19,21-22H2,1-3H3,(H,26,28)/b17-16+,23-20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in CD rat brain membranes |
J Nat Prod 66: 1364-8 (2003)
Article DOI: 10.1021/np030242n
BindingDB Entry DOI: 10.7270/Q2BZ65TG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50250762

(CHEMBL459704 | semiplenamide A)Show InChI InChI=1S/C23H43NO2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(2)23(26)24-20-21-25/h15-16,19,25H,3-14,17-18,20-21H2,1-2H3,(H,24,26)/b16-15+,22-19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in CD rat brain membranes |
J Nat Prod 66: 1364-8 (2003)
Article DOI: 10.1021/np030242n
BindingDB Entry DOI: 10.7270/Q2BZ65TG |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50257326

(CHEMBL4086345)Show SMILES CCCCCC(=O)N[C@H](C(C)C)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C26H47N3O5/c1-8-10-12-14-21(30)29-22(18(5)6)25(33)27-19(13-11-9-2)24(32)28-20(15-17(3)4)23(31)26(7)16-34-26/h17-20,22H,8-16H2,1-7H3,(H,27,33)(H,28,32)(H,29,30)/t19-,20-,22+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pediatrics, School of Medicine, University of California, San Diego , La Jolla, California 92093, United States.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human 20S proteasome beta5 subunit using suc-LLVY-AMC as substrate after 4 hrs at 30 mins time interval by fluorescence as... |
J Med Chem 60: 6721-6732 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00671
BindingDB Entry DOI: 10.7270/Q2CR5WSK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603782
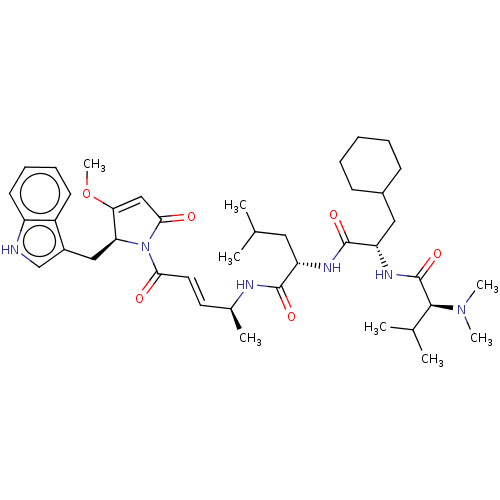
(CHEMBL4596414)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505559

(CHEMBL4441303)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)\C=C\C(=O)N1[C@@H](CC(C)C)C(OC)=CC1=O |r,c:48| Show InChI InChI=1S/C40H62N4O7/c1-12-28(8)37(43(9)10)40(49)51-34(22-27(6)7)39(48)42-31(20-25(2)3)38(47)41-30(23-29-16-14-13-15-17-29)18-19-35(45)44-32(21-26(4)5)33(50-11)24-36(44)46/h13-19,24-28,30-32,34,37H,12,20-23H2,1-11H3,(H,41,47)(H,42,48)/b19-18+/t28-,30+,31-,32-,34-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603794

(CHEMBL5184737)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc(cc1)C#N)C(=O)\C=C\[C@@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603779
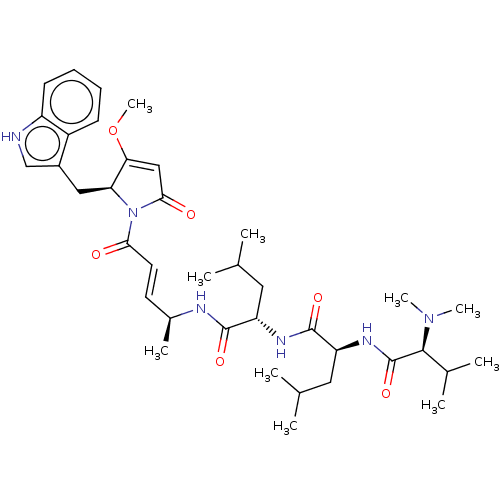
(CHEMBL3558756)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50494415
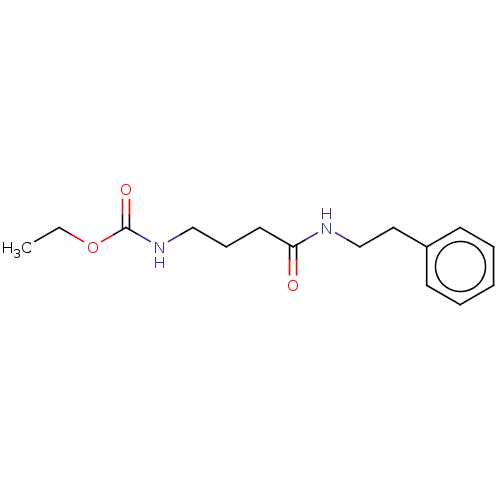
(CHEMBL3086767)Show InChI InChI=1S/C15H22N2O3/c1-2-20-15(19)17-11-6-9-14(18)16-12-10-13-7-4-3-5-8-13/h3-5,7-8H,2,6,9-12H2,1H3,(H,16,18)(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.112 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 after 30 mins by fluorescence assay |
J Nat Prod 76: 2026-33 (2013)
Article DOI: 10.1021/np400198r
BindingDB Entry DOI: 10.7270/Q21J9DQ7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50494415

(CHEMBL3086767)Show InChI InChI=1S/C15H22N2O3/c1-2-20-15(19)17-11-6-9-14(18)16-12-10-13-7-4-3-5-8-13/h3-5,7-8H,2,6,9-12H2,1H3,(H,16,18)(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.119 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 after 30 mins by fluorescence assay |
J Nat Prod 76: 2026-33 (2013)
Article DOI: 10.1021/np400198r
BindingDB Entry DOI: 10.7270/Q21J9DQ7 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603788

(CHEMBL5171874)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccccc1)C(=O)\C=C\[C@@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603795

(CHEMBL5175270)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc(cc1)C#N)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data