

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343759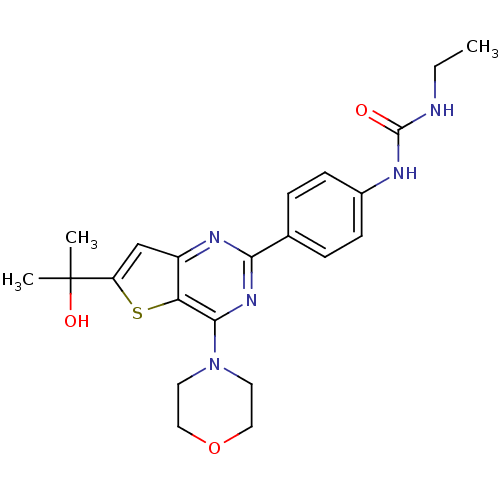 (1-ethyl-3-(4-(6-(2-hydroxypropan-2-yl)-4-morpholin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human N-terminal FLAG-tagged mTOR (1362-end residues) in presence of [gamma33P]ATP after 40 mins | Eur J Med Chem 129: 135-150 (2017) Article DOI: 10.1016/j.ejmech.2017.02.015 BindingDB Entry DOI: 10.7270/Q2QN692K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438750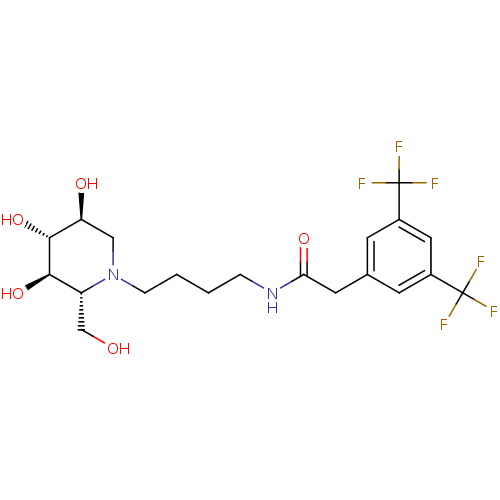 (CHEMBL2414884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50341385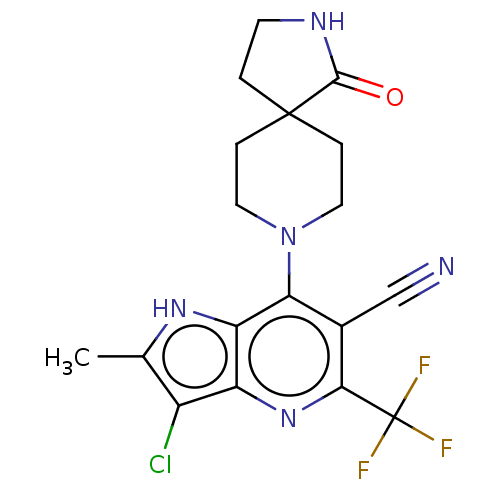 (CHEMBL4161567) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327358 (CHEMBL4168403) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438752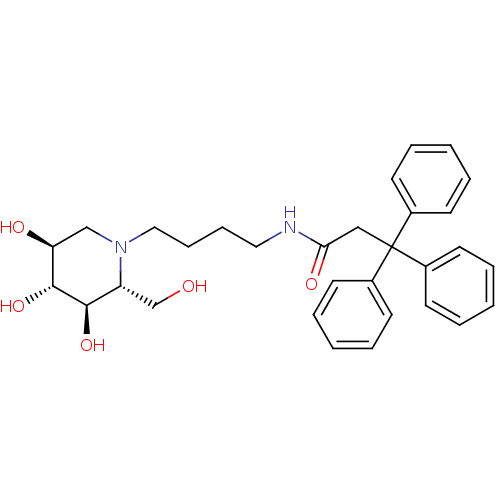 (CHEMBL2414882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438754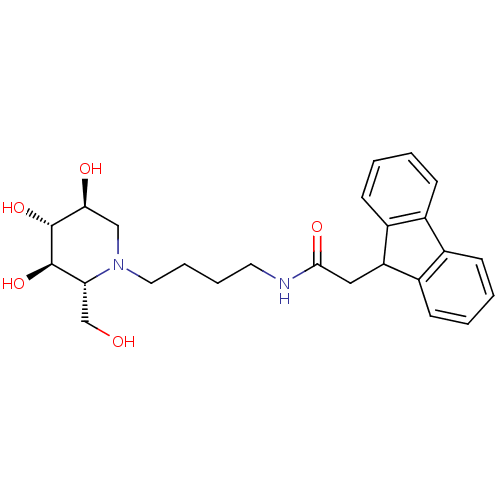 (CHEMBL2414880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327363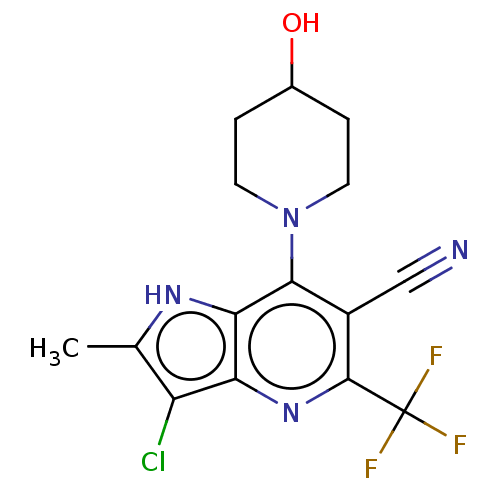 (CHEMBL4176684) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327360 (CHEMBL4168827) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327362 (CHEMBL4159390) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50341383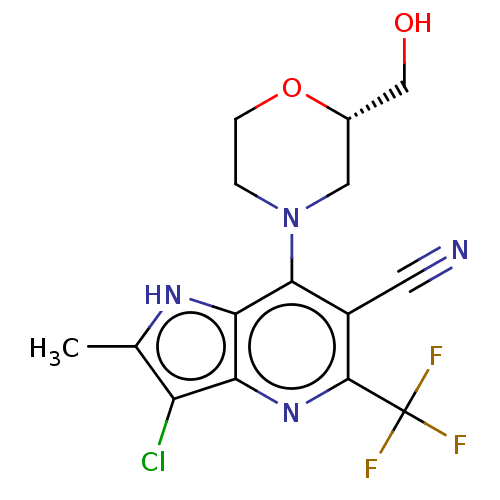 (CHEMBL4175607) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438753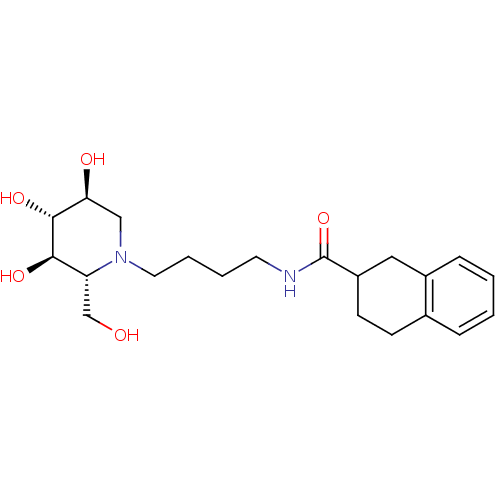 (CHEMBL2414881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438748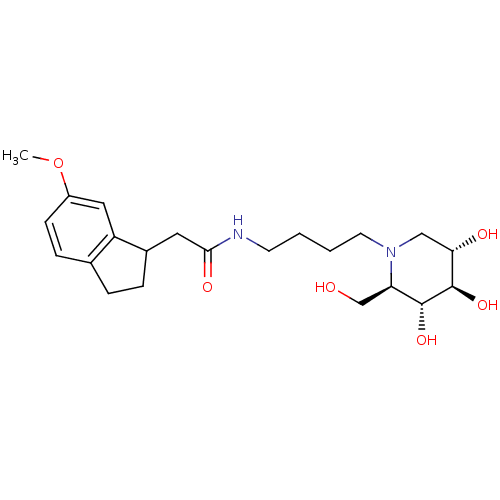 (CHEMBL2414879) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Non-competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358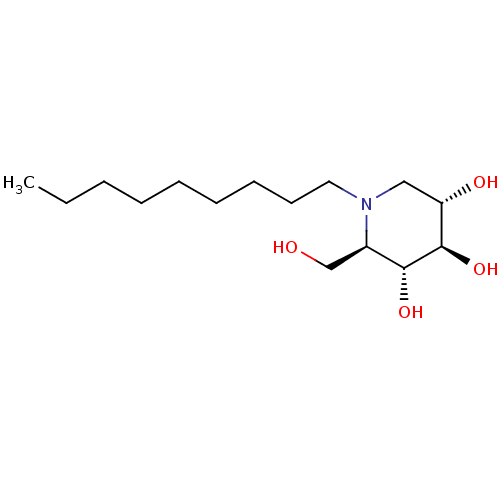 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327357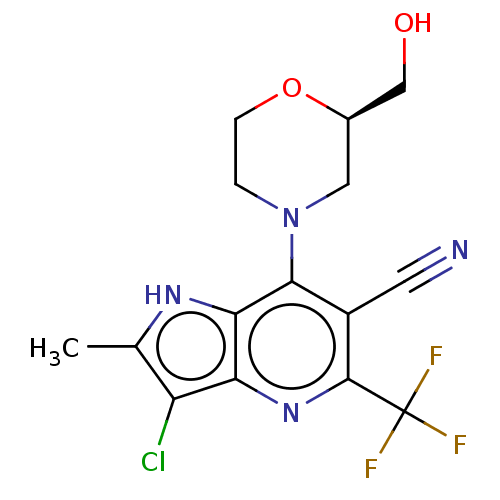 (CHEMBL4164986) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327355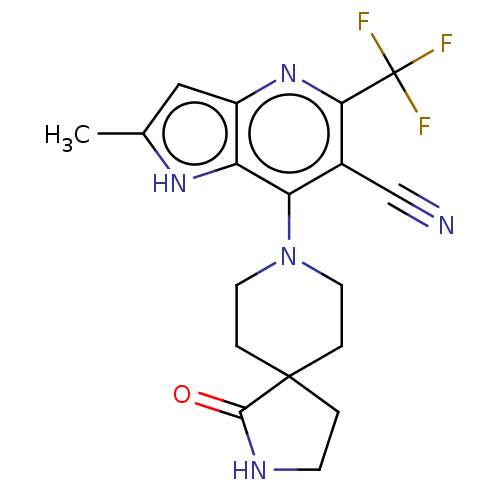 (CHEMBL4164691) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327359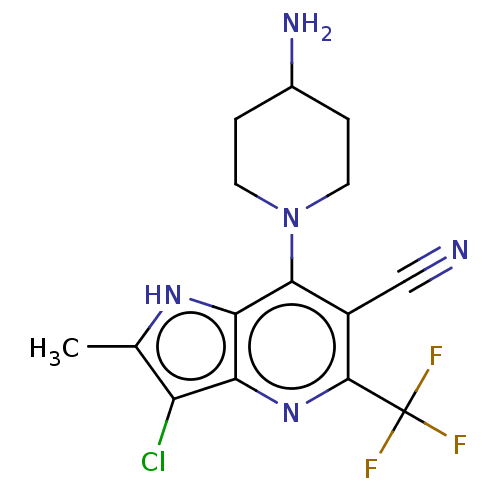 (CHEMBL4160507) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438751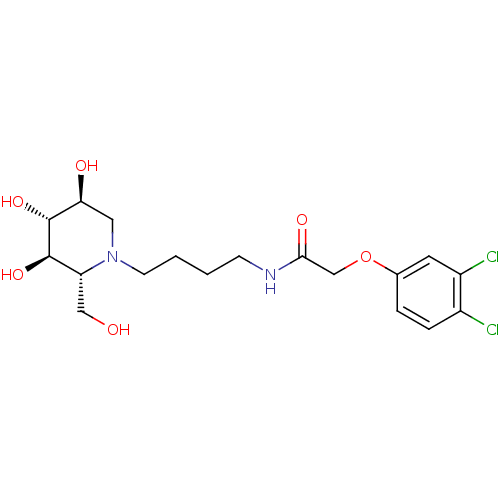 (CHEMBL2414883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438749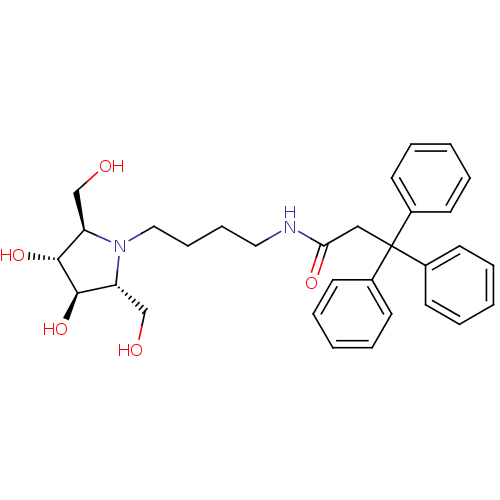 (CHEMBL2414888) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327361 (CHEMBL4172179) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50327356 (CHEMBL4172584) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2A (Homo sapiens) | BDBM50341384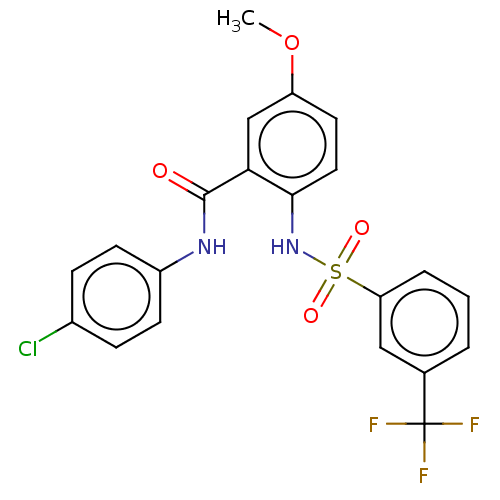 (CHEMBL4170023) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... | ACS Med Chem Lett 9: 440-445 (2018) Article DOI: 10.1021/acsmedchemlett.8b00013 BindingDB Entry DOI: 10.7270/Q26T0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 phosphorylation at 0.1 to 1000 nM after 1 hr by... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 ITD mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 ITD phosphorylation at 0.1 to 1000 n... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 D835Y mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 D835Y phosphorylation at 0.1 to 10... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM26198 (4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of protein kinase Jak 2 | Bioorg Med Chem Lett 12: 1219-23 (2002) BindingDB Entry DOI: 10.7270/Q2JW8D67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM26198 (4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Tyrosine kinase 2 kinase | Bioorg Med Chem Lett 12: 1219-23 (2002) BindingDB Entry DOI: 10.7270/Q2JW8D67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582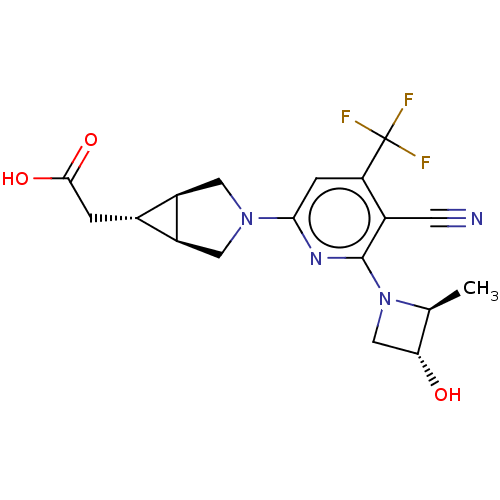 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human C-src using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma ATP by hotspot kinase assay | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50348452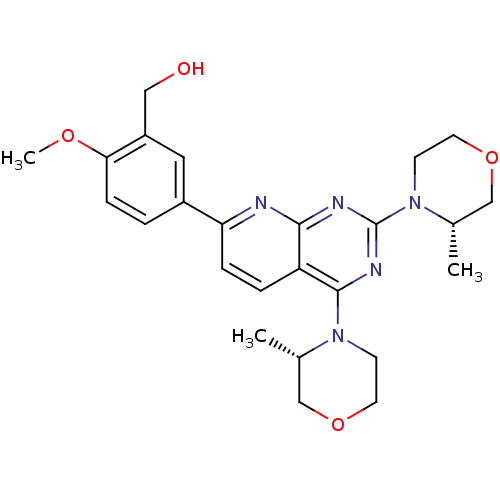 (AZD-8055 | CHEMBL1801204 | US9102670, 1a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human N-terminal FLAG-tagged mTOR (1362-end residues) in presence of [gamma33P]ATP after 40 mins | Eur J Med Chem 129: 135-150 (2017) Article DOI: 10.1016/j.ejmech.2017.02.015 BindingDB Entry DOI: 10.7270/Q2QN692K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma ATP by hotspot kinase assay | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM26198 (4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of protein kinase Jak 3 | Bioorg Med Chem Lett 12: 1219-23 (2002) BindingDB Entry DOI: 10.7270/Q2JW8D67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM35615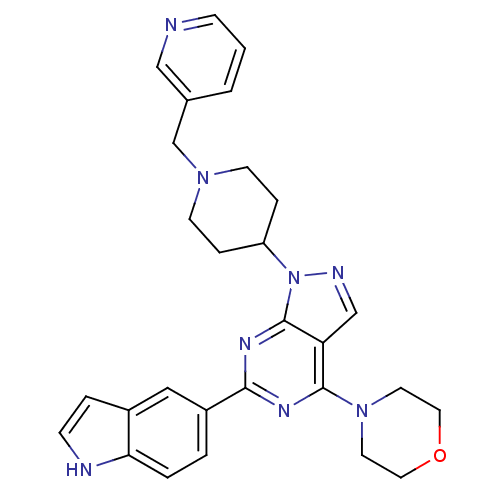 (pyrazolo pyrimidine, 5u) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of mTOR (unknown origin) | Eur J Med Chem 129: 135-150 (2017) Article DOI: 10.1016/j.ejmech.2017.02.015 BindingDB Entry DOI: 10.7270/Q2QN692K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM50380246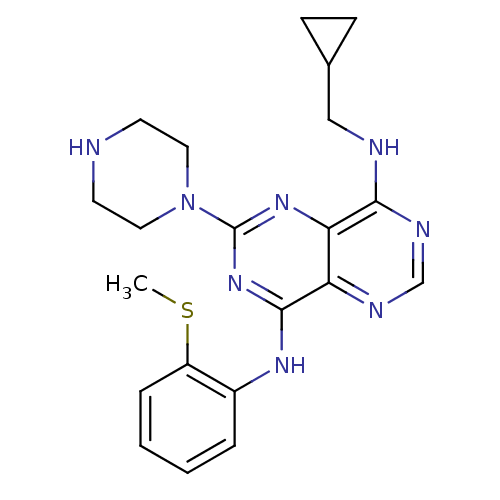 (CHEMBL2017214) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of KHK (unknown origin) using D-fructose as substrate after 60 mins in presence of ATP by LC-MS analysis | J Med Chem 60: 7835-7849 (2017) Article DOI: 10.1021/acs.jmedchem.7b00947 BindingDB Entry DOI: 10.7270/Q2H997CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319586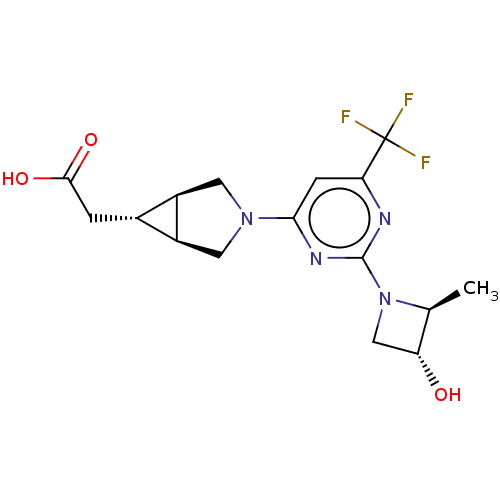 (US10174007, Example 5 | US10787438, Example 5 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Mus musculus) | BDBM26198 (4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of murine Jak 1 protein kinase | Bioorg Med Chem Lett 12: 1219-23 (2002) BindingDB Entry DOI: 10.7270/Q2JW8D67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519727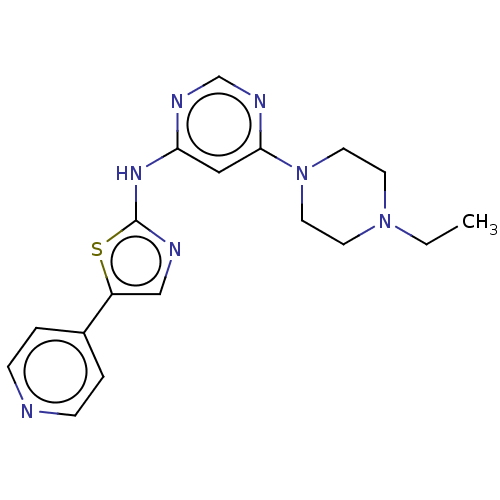 (CHEMBL4448198) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GST-tagged FLT3 (Y567 to S993 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519722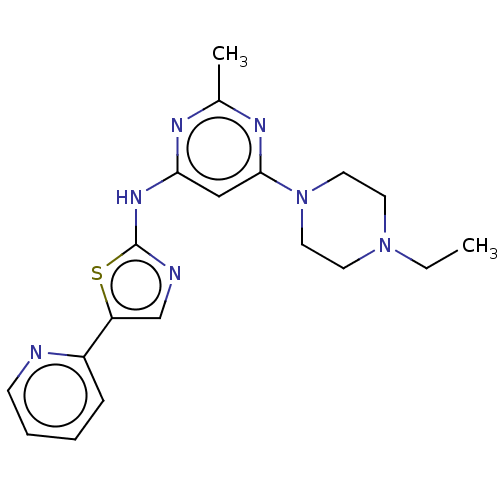 (CHEMBL4475875) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GST-tagged FLT3 (Y567 to S993 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50519732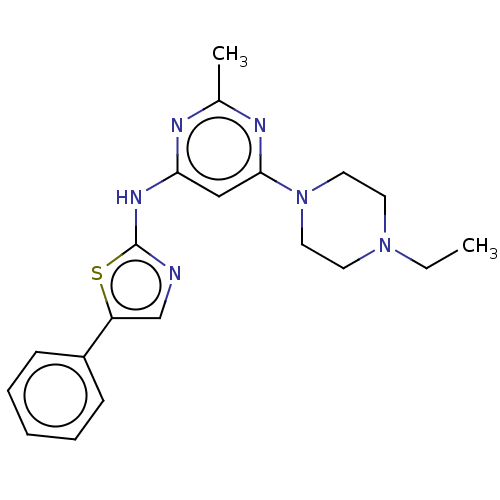 (CHEMBL4483070) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6x-His-tagged c-KIT (547 to 935 residues)/(694 to 753 residues deletion) (unknown origin) expressed in baculovir... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using 8 mM fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50519727 (CHEMBL4448198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6x-His-tagged c-KIT (547 to 935 residues)/(694 to 753 residues deletion) (unknown origin) expressed in baculovir... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GST-tagged FLT3 (Y567 to S993 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM25045 (3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description In vitro agonist potency was evaluated in HEK293 cells transfected with human melanocortin receptor (hMC1R) | Eur J Med Chem 129: 135-150 (2017) Article DOI: 10.1016/j.ejmech.2017.02.015 BindingDB Entry DOI: 10.7270/Q2QN692K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM4814 (CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GST-tagged FLT3 (Y567 to S993 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519729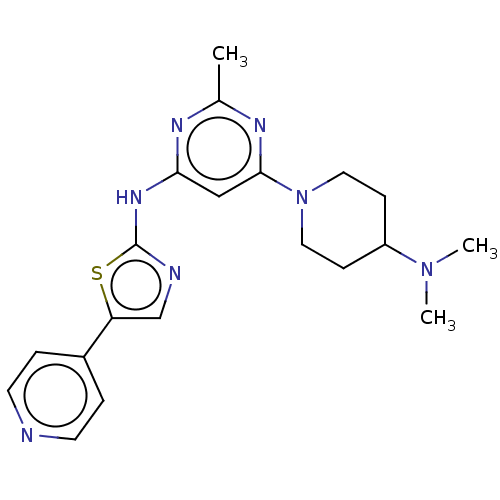 (CHEMBL4438245) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GST-tagged FLT3 (Y567 to S993 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519724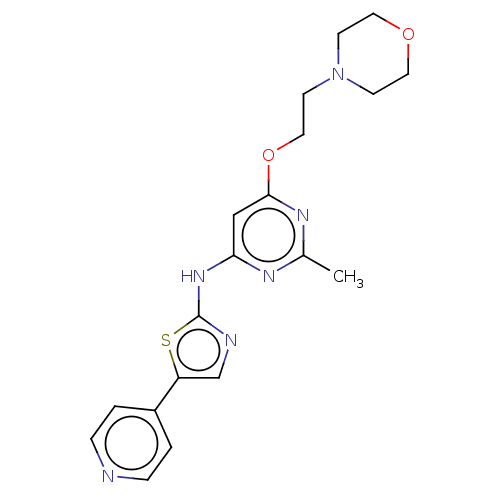 (CHEMBL4521178) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GST-tagged FLT3 (Y567 to S993 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519733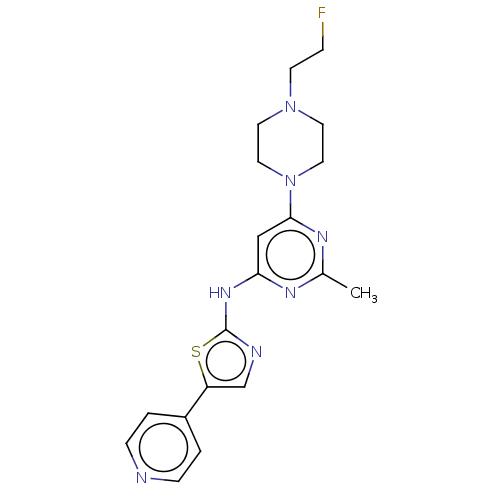 (CHEMBL4446778) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GST-tagged FLT3 (Y567 to S993 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50095991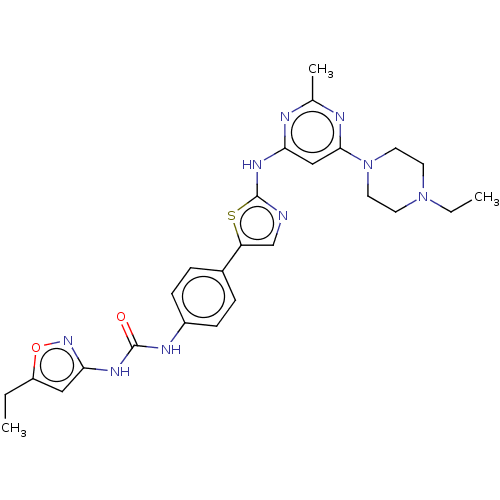 (CHEMBL3593290) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GST-tagged FLT3 (Y567 to S993 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 213 total ) | Next | Last >> |