

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11542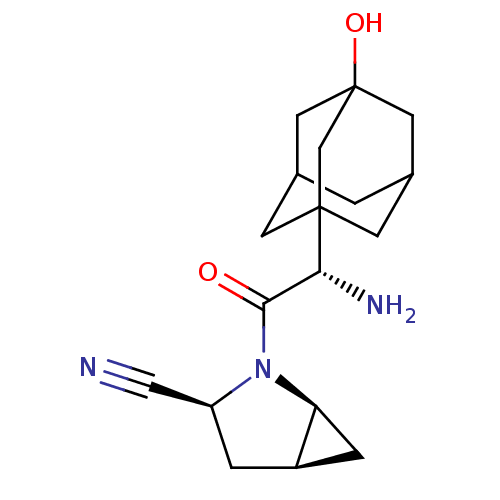 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11541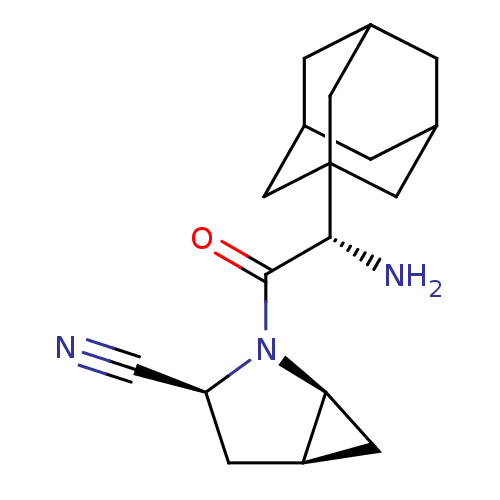 ((1S,3S,5S)-2-[(2S)-2-(adamantan-1-yl)-2-aminoacety...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11530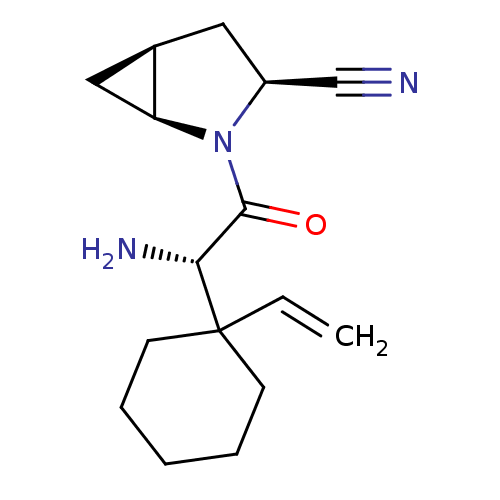 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11544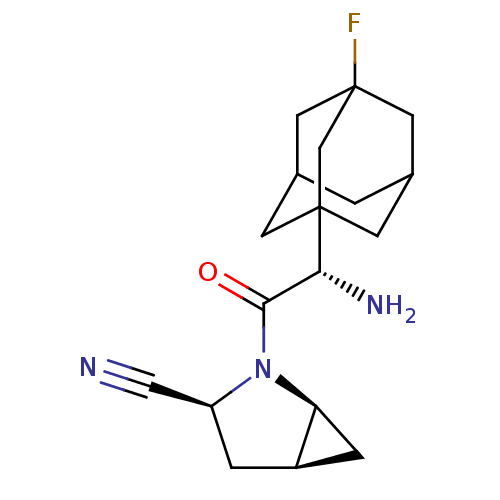 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-fluoroadamantan-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106836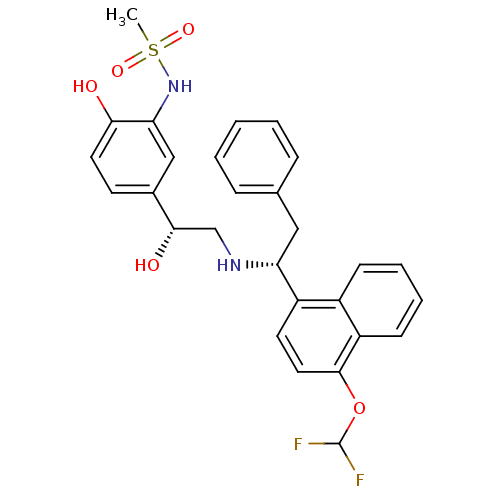 (CHEMBL105758 | N-(5-{(R)-2-[(R)-1-(4-Difluorometho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to CHO cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol | Bioorg Med Chem Lett 11: 3041-4 (2001) BindingDB Entry DOI: 10.7270/Q2794402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50287059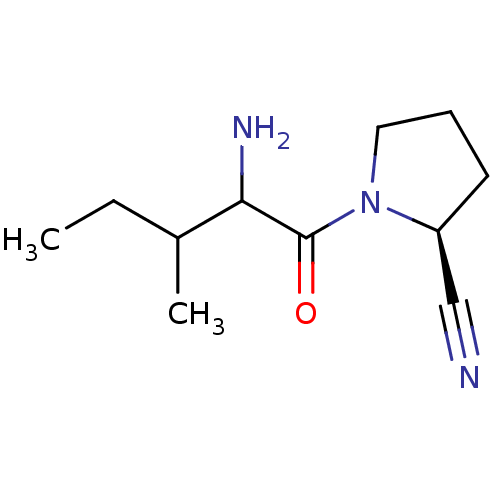 ((S)-1-(2-Amino-3-methyl-pentanoyl)-pyrrolidine-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068673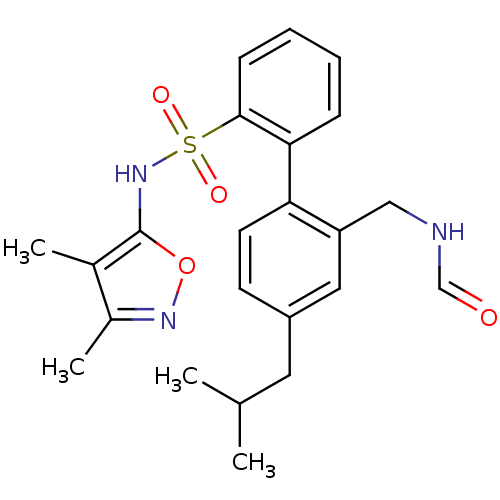 (2'-Formylaminomethyl-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11543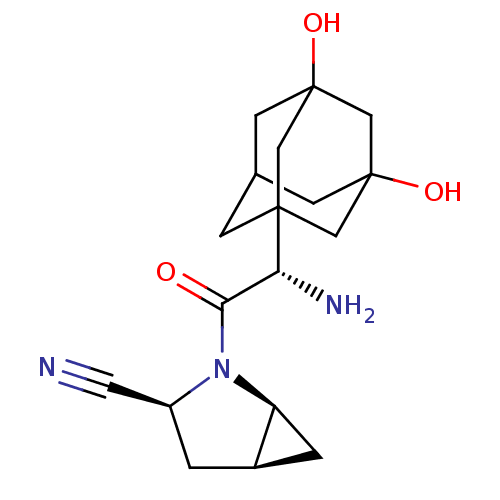 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068706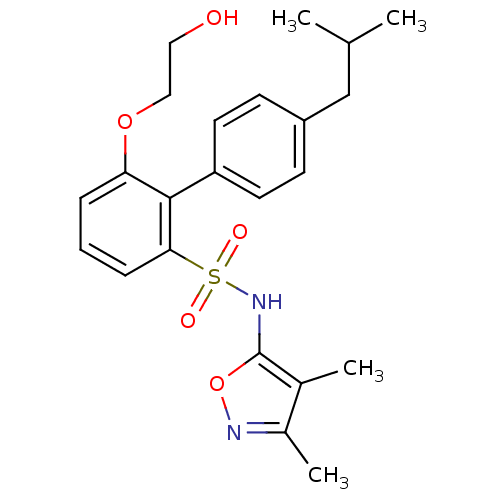 (6-(2-Hydroxy-ethoxy)-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214244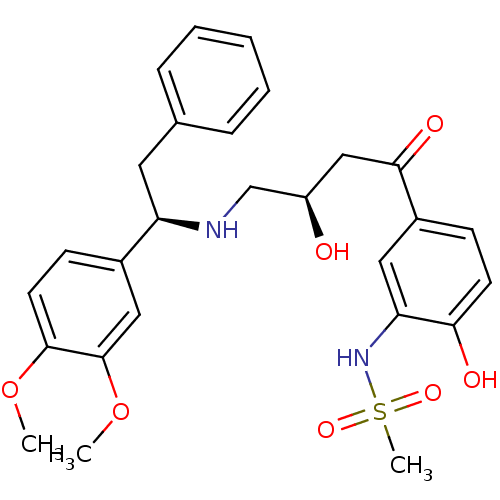 (CHEMBL250978 | N-(5-((R)-4-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11529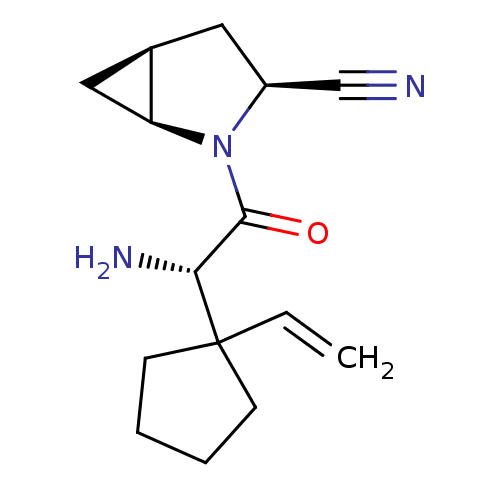 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50145999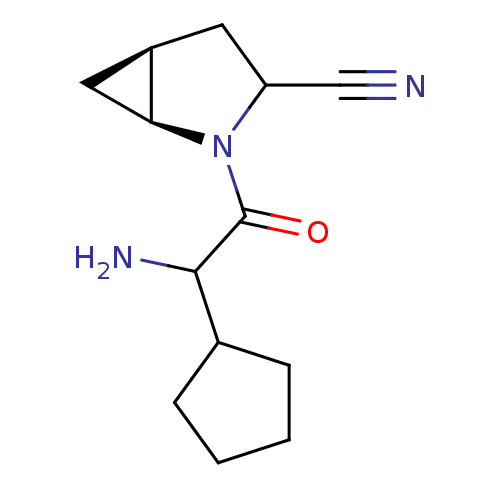 ((1S,5S)-2-(2-Amino-2-cyclopentyl-acetyl)-2-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068674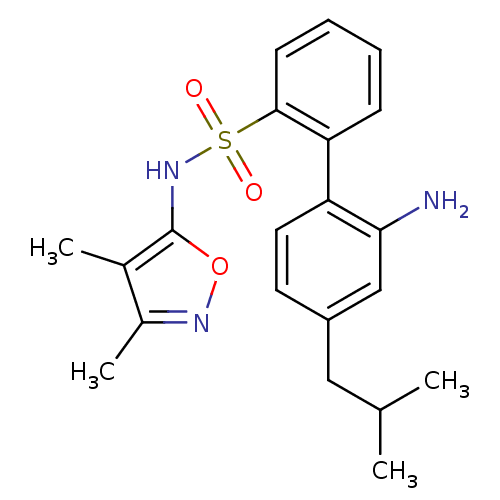 (2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214246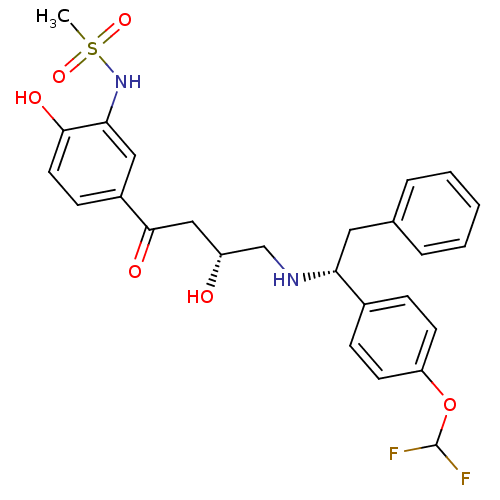 (CHEMBL401135 | N-(5-((R)-4-((R)-1-(4-(difluorometh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068713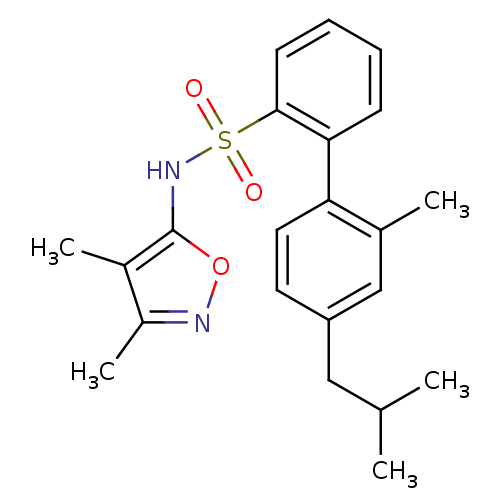 (4'-Isobutyl-2'-methyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068720 (6-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11535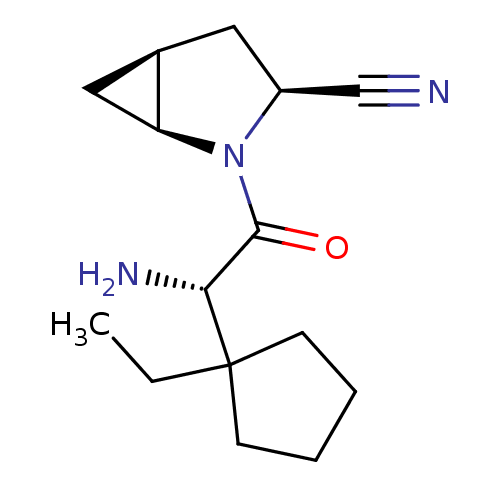 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethylcyclopentyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214213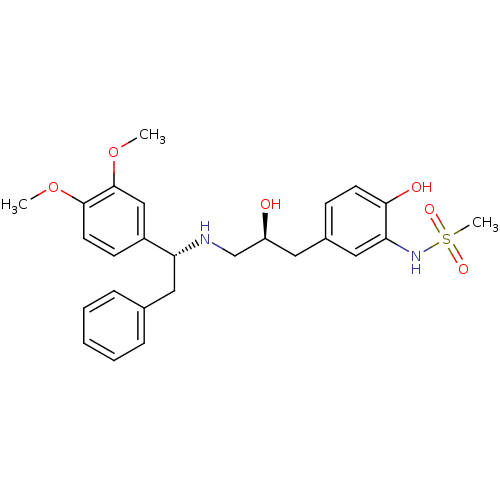 (CHEMBL399329 | N-(5-((S)-3-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214234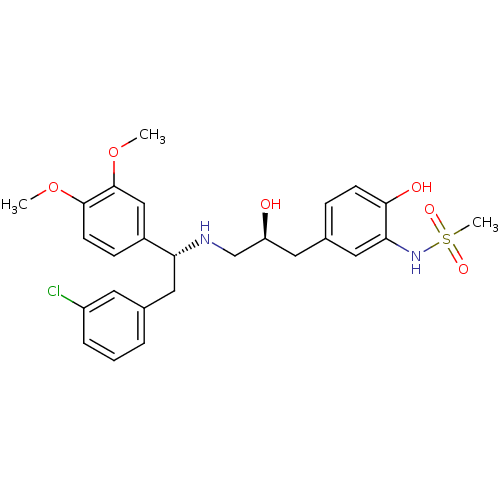 (CHEMBL250755 | N-(5-((S)-3-((R)-2-(3-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068740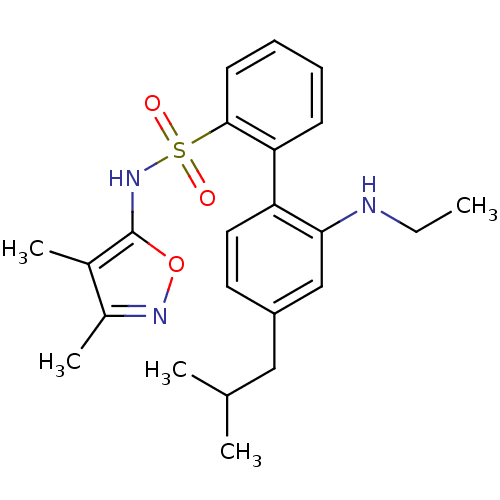 (2'-Ethylamino-4'-isobutyl-biphenyl-2-sulfonic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068697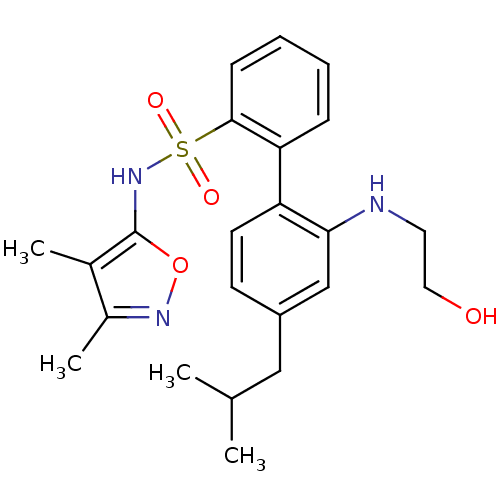 (2'-(2-Hydroxy-ethylamino)-4'-isobutyl-biphenyl-2-s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146002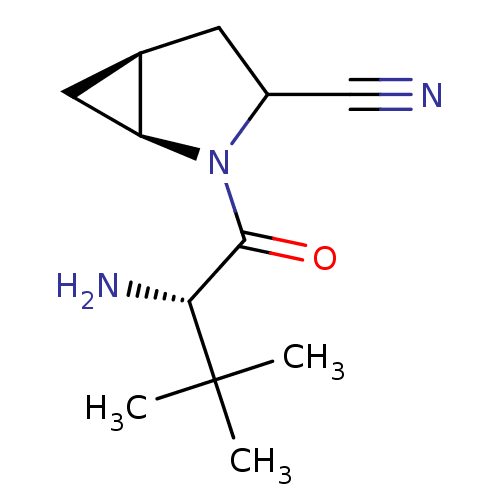 ((S)-2-((S)-2-Amino-3-methyl-3-(S)-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214240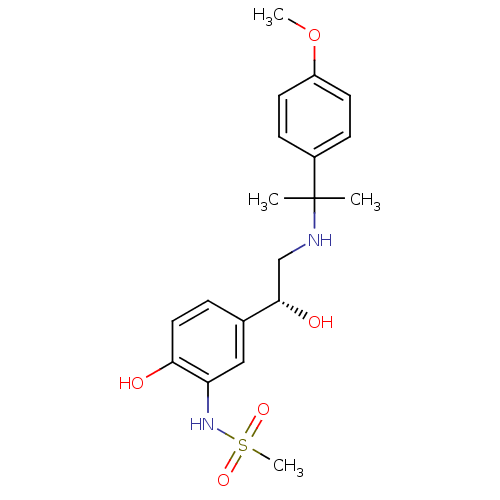 ((R)-N-(2-hydroxy-5-(1-hydroxy-2-(2-(4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214242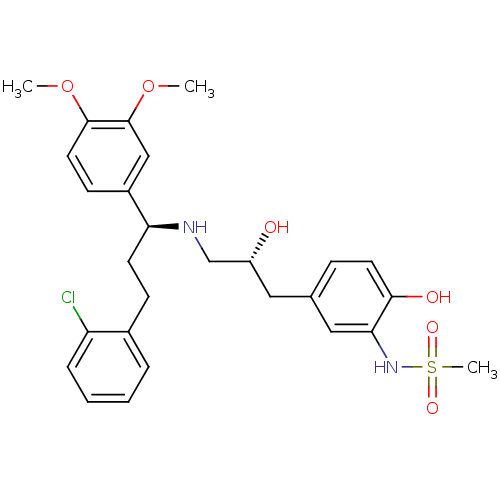 (CHEMBL447786 | N-(5-((R)-3-((S)-3-(2-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214248 (CHEMBL437578 | N-(5-((S)-3-((R)-2-(2-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146015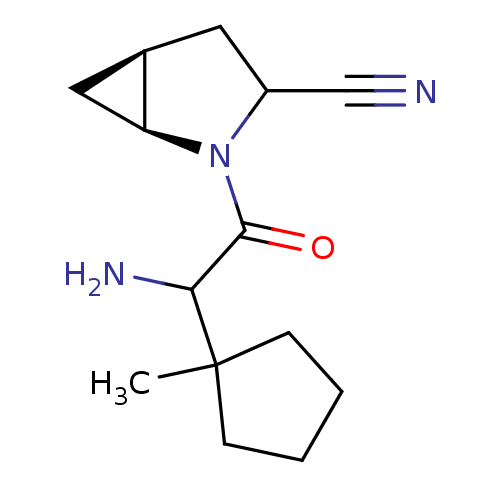 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclopentyl)-acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11533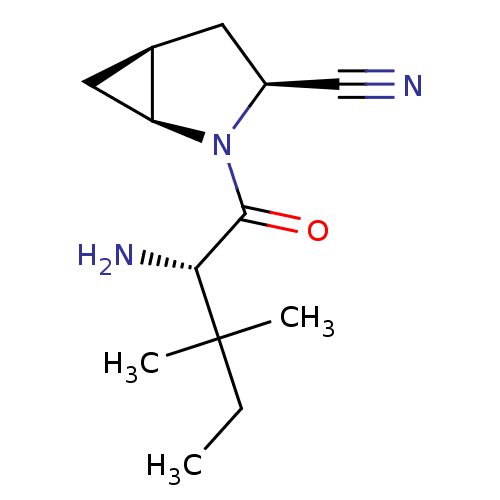 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpentanoyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.10 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11538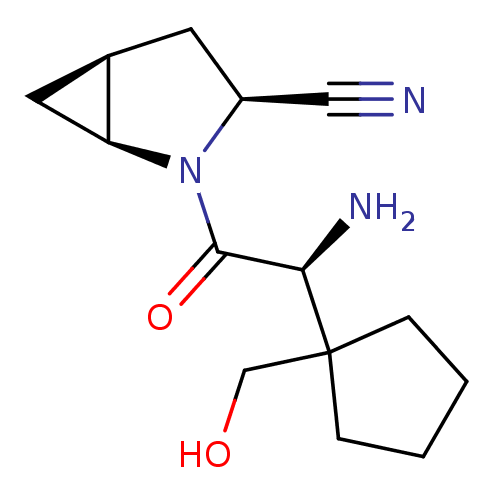 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11539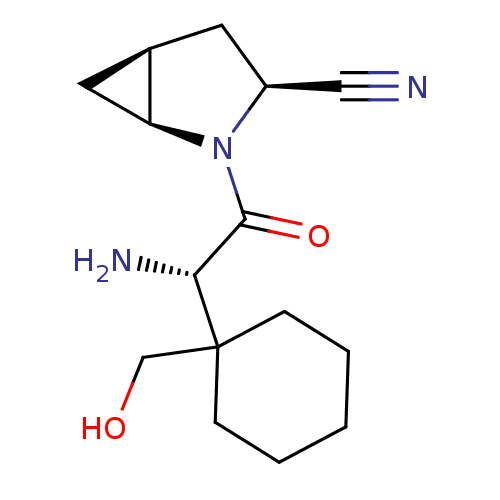 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50151003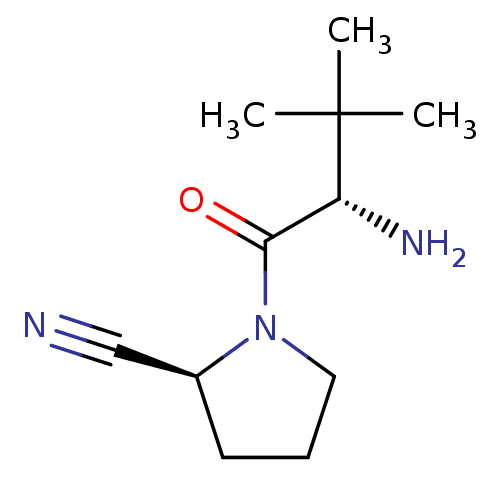 ((S)-1-((S)-2-Amino-3,3-dimethyl-butyryl)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214237 (CHEMBL400947 | N-(2-hydroxy-5-((S)-2-hydroxy-3-((R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146009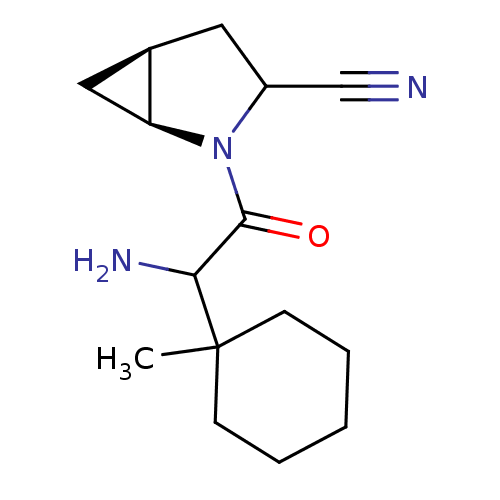 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclohexyl)-acetyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068736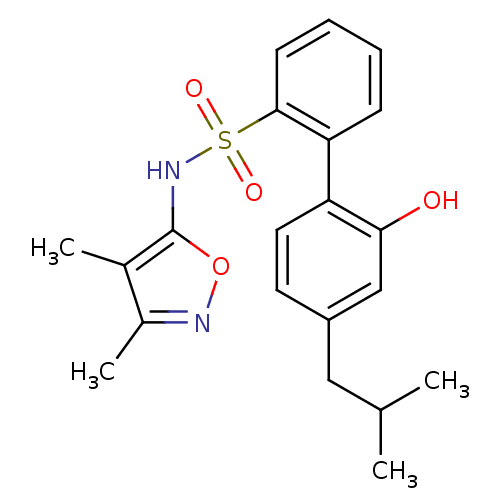 (2'-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106852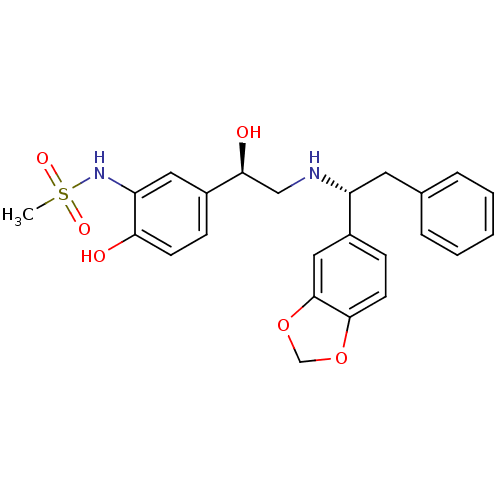 (CHEMBL321518 | N-{5-[(R)-2-((R)-1-Benzo[1,3]dioxol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to CHO cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol | Bioorg Med Chem Lett 11: 3041-4 (2001) BindingDB Entry DOI: 10.7270/Q2794402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148497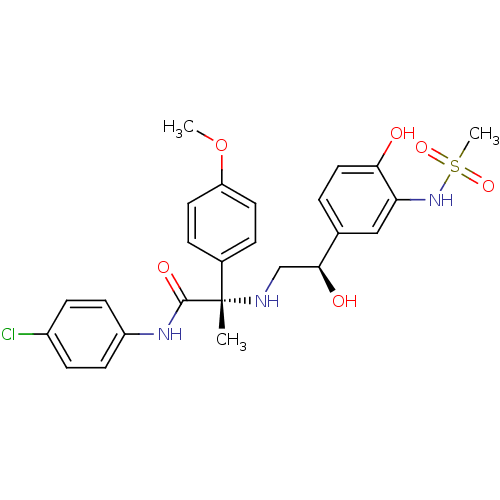 ((S)-N-(4-Chloro-phenyl)-2-[2-hydroxy-2-((R)-4-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214228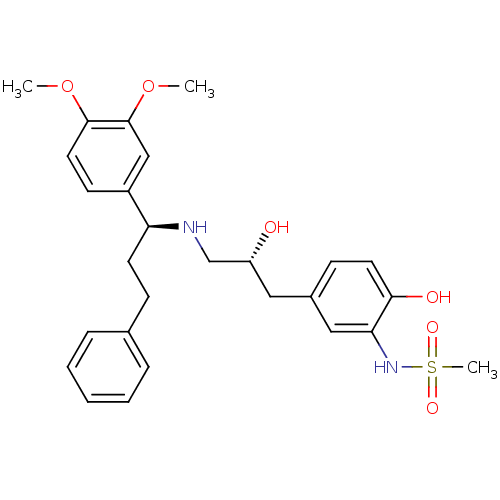 (CHEMBL400067 | N-(5-((R)-3-((S)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106833 (CHEMBL317648 | N-(5-{(R)-2-[(R)-1-(4-Difluorometho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to CHO cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol | Bioorg Med Chem Lett 11: 3041-4 (2001) BindingDB Entry DOI: 10.7270/Q2794402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214225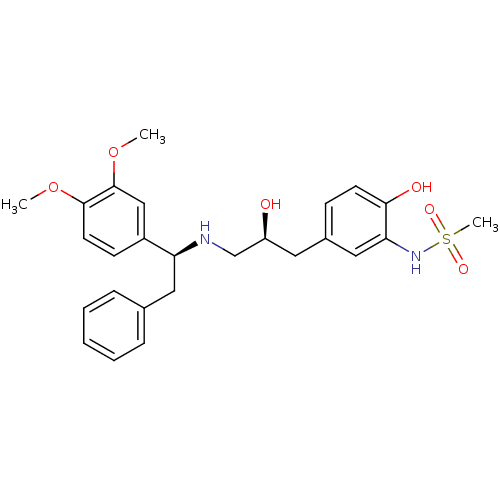 (CHEMBL398557 | N-(5-((S)-3-((S)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11532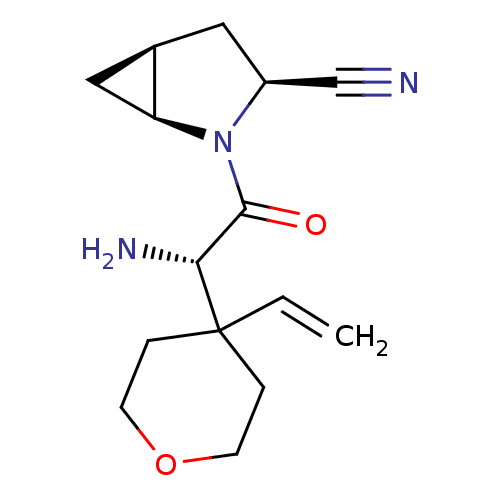 ((1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethenyloxan-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11531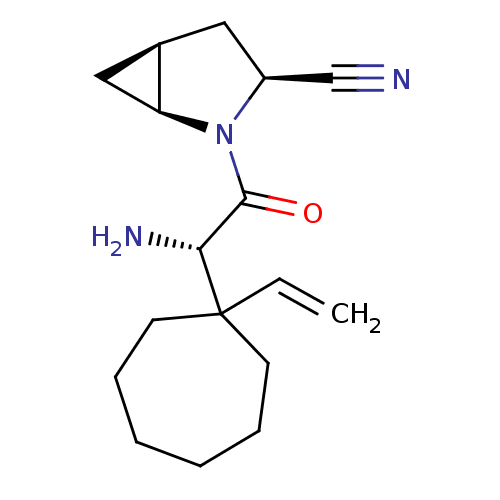 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcycloheptyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106810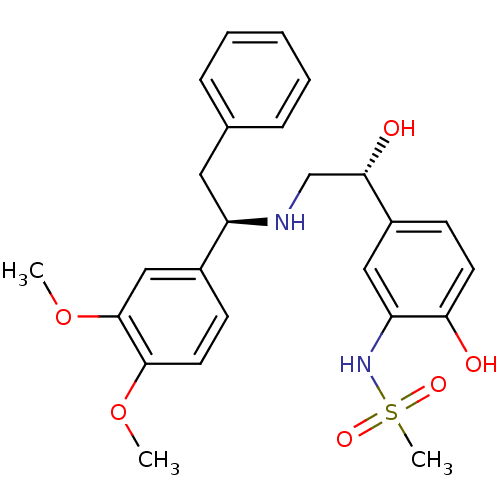 (CHEMBL317621 | N-(5-((R)-2-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against CHO cells transfected with human beta-3 adrenergic receptor in the presence of [125I]iodocyanopindolol | Bioorg Med Chem Lett 11: 3035-9 (2001) BindingDB Entry DOI: 10.7270/Q2MS3T97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146010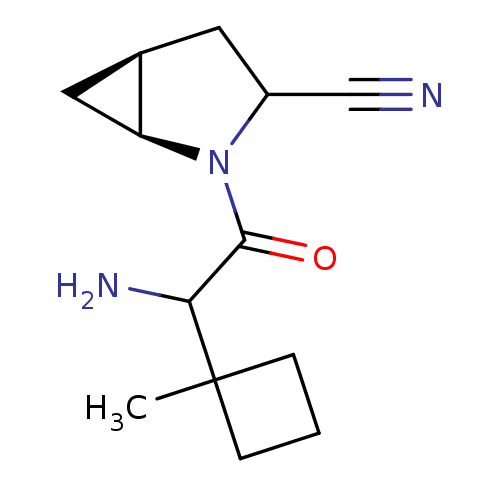 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclobutyl)-acetyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148487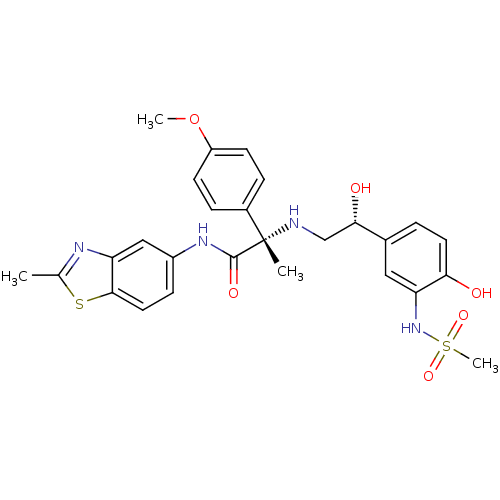 ((S)-2-[2-Hydroxy-2-((R)-4-hydroxy-3-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106810 (CHEMBL317621 | N-(5-((R)-2-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11528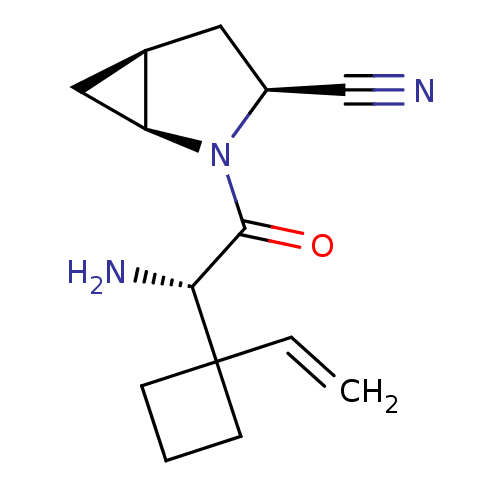 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclobutyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214227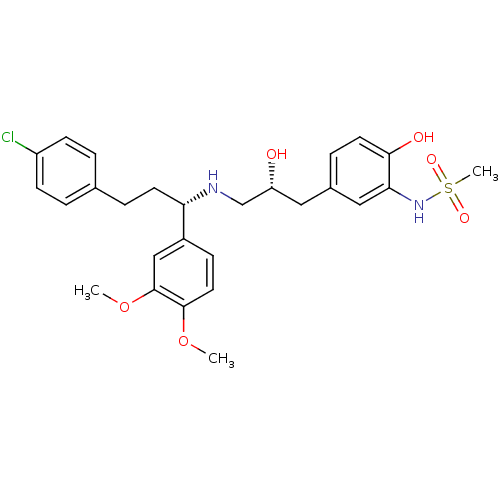 (CHEMBL251764 | N-(5-((R)-3-((S)-3-(4-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146007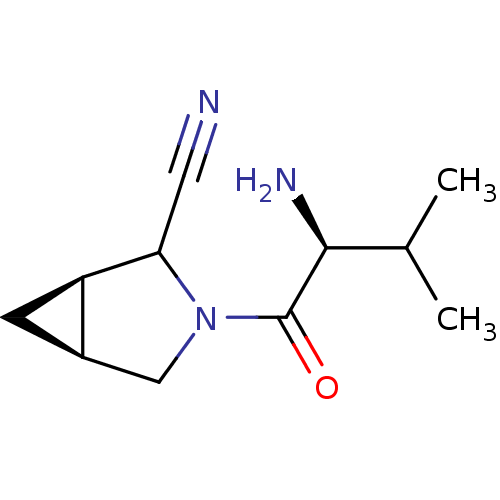 ((1R,5S)-3-((S)-2-Amino-3-methyl-butyryl)-3-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50145997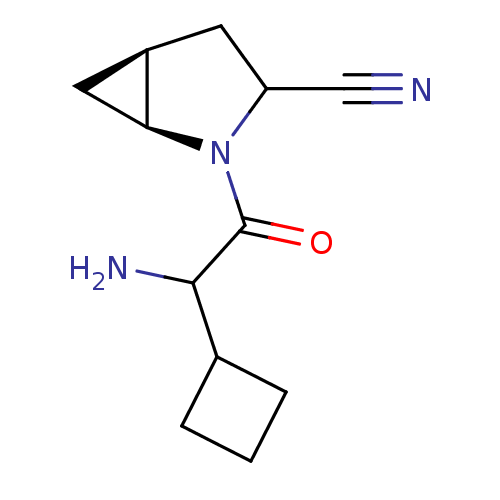 ((1S,5S)-2-(2-Amino-2-cyclobutyl-acetyl)-2-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068685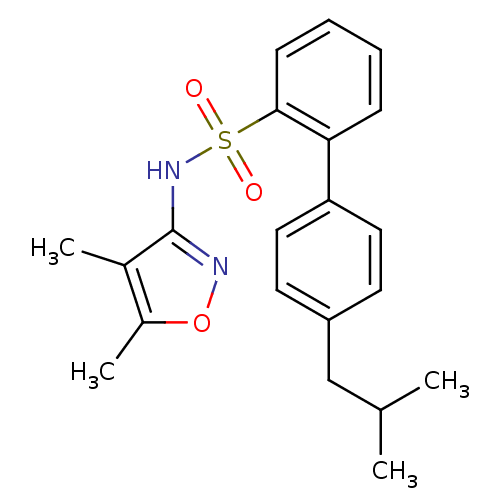 (4'-Isobutyl-biphenyl-2-sulfonic acid (4,5-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068704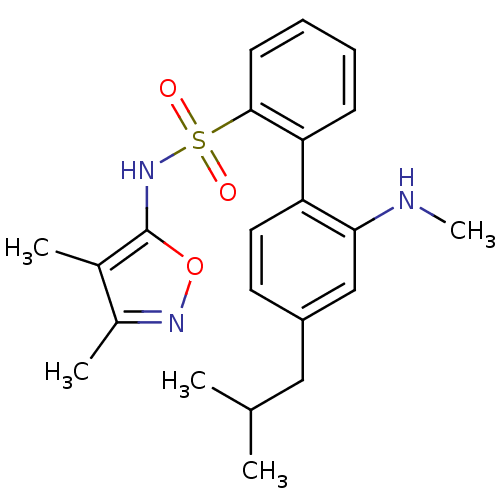 (4'-Isobutyl-2'-methylamino-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 395 total ) | Next | Last >> |