 Found 79 hits with Last Name = 'advenier' and Initial = 'c'
Found 79 hits with Last Name = 'advenier' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM86055
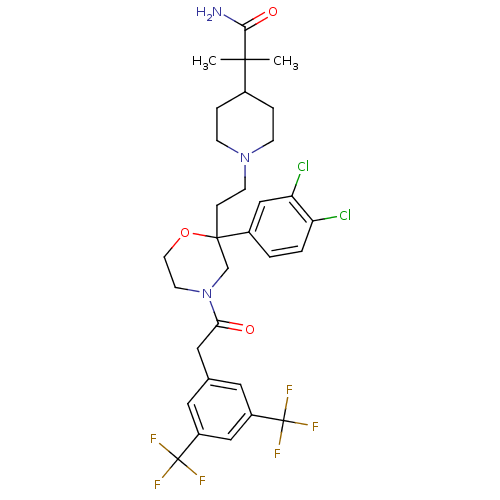
(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(mouse) | BDBM85083
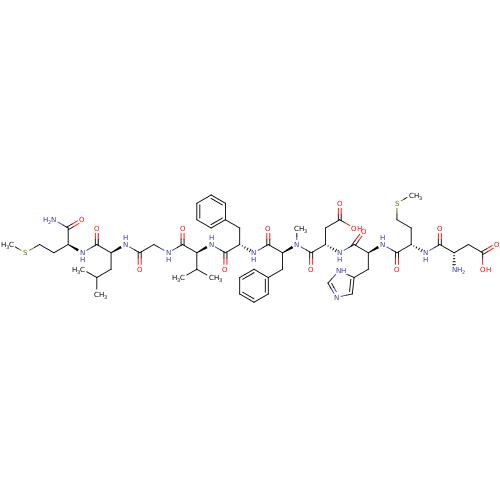
(NKB [MePhe7])Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C56H81N13O14S2/c1-31(2)22-39(51(78)63-37(48(58)75)18-20-84-6)62-44(70)29-60-55(82)47(32(3)4)68-53(80)40(23-33-14-10-8-11-15-33)66-54(81)43(24-34-16-12-9-13-17-34)69(5)56(83)42(27-46(73)74)67-52(79)41(25-35-28-59-30-61-35)65-50(77)38(19-21-85-7)64-49(76)36(57)26-45(71)72/h8-17,28,30-32,36-43,47H,18-27,29,57H2,1-7H3,(H2,58,75)(H,59,61)(H,60,82)(H,62,70)(H,63,78)(H,64,76)(H,65,77)(H,66,81)(H,67,79)(H,68,80)(H,71,72)(H,73,74)/t36-,37-,38-,39-,40-,41-,42-,43-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Gerbil) | BDBM85083

(NKB [MePhe7])Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C56H81N13O14S2/c1-31(2)22-39(51(78)63-37(48(58)75)18-20-84-6)62-44(70)29-60-55(82)47(32(3)4)68-53(80)40(23-33-14-10-8-11-15-33)66-54(81)43(24-34-16-12-9-13-17-34)69(5)56(83)42(27-46(73)74)67-52(79)41(25-35-28-59-30-61-35)65-50(77)38(19-21-85-7)64-49(76)36(57)26-45(71)72/h8-17,28,30-32,36-43,47H,18-27,29,57H2,1-7H3,(H2,58,75)(H,59,61)(H,60,82)(H,62,70)(H,63,78)(H,64,76)(H,65,77)(H,66,81)(H,67,79)(H,68,80)(H,71,72)(H,73,74)/t36-,37-,38-,39-,40-,41-,42-,43-,47-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281767

(Acetic acid 1-{3-(3,4-dichloro-phenyl)-4-[ethyl-(t...)Show SMILES CCN(CC(CCN1CCC(CC1)(OC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1cccs1 Show InChI InChI=1S/C30H34Cl2N2O3S/c1-3-34(29(36)28-10-7-19-38-28)21-24(23-11-12-26(31)27(32)20-23)13-16-33-17-14-30(15-18-33,37-22(2)35)25-8-5-4-6-9-25/h4-12,19-20,24H,3,13-18,21H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281769
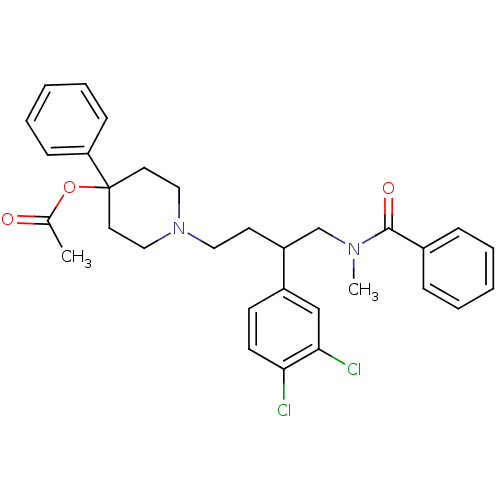
(Acetic acid 1-[4-(benzoyl-methyl-amino)-3-(3,4-dic...)Show SMILES CN(CC(CCN1CCC(CC1)(OC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H34Cl2N2O3/c1-23(36)38-31(27-11-7-4-8-12-27)16-19-35(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-34(2)30(37)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM85083

(NKB [MePhe7])Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C56H81N13O14S2/c1-31(2)22-39(51(78)63-37(48(58)75)18-20-84-6)62-44(70)29-60-55(82)47(32(3)4)68-53(80)40(23-33-14-10-8-11-15-33)66-54(81)43(24-34-16-12-9-13-17-34)69(5)56(83)42(27-46(73)74)67-52(79)41(25-35-28-59-30-61-35)65-50(77)38(19-21-85-7)64-49(76)36(57)26-45(71)72/h8-17,28,30-32,36-43,47H,18-27,29,57H2,1-7H3,(H2,58,75)(H,59,61)(H,60,82)(H,62,70)(H,63,78)(H,64,76)(H,65,77)(H,66,81)(H,67,79)(H,68,80)(H,71,72)(H,73,74)/t36-,37-,38-,39-,40-,41-,42-,43-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM85083

(NKB [MePhe7])Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C56H81N13O14S2/c1-31(2)22-39(51(78)63-37(48(58)75)18-20-84-6)62-44(70)29-60-55(82)47(32(3)4)68-53(80)40(23-33-14-10-8-11-15-33)66-54(81)43(24-34-16-12-9-13-17-34)69(5)56(83)42(27-46(73)74)67-52(79)41(25-35-28-59-30-61-35)65-50(77)38(19-21-85-7)64-49(76)36(57)26-45(71)72/h8-17,28,30-32,36-43,47H,18-27,29,57H2,1-7H3,(H2,58,75)(H,59,61)(H,60,82)(H,62,70)(H,63,78)(H,64,76)(H,65,77)(H,66,81)(H,67,79)(H,68,80)(H,71,72)(H,73,74)/t36-,37-,38-,39-,40-,41-,42-,43-,47-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM81942
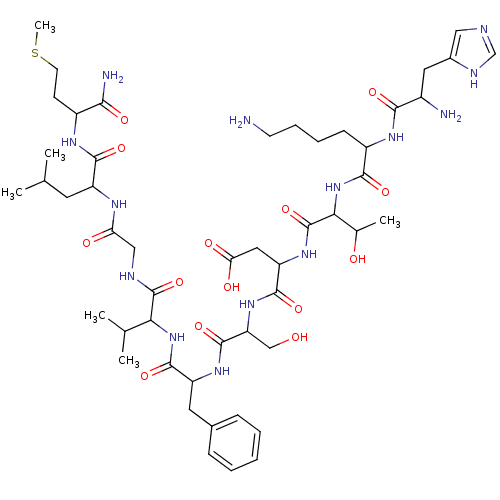
(CAS_55582 | NKA | NSC_55582 | Neurokinin alpha | S...)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(NC(=O)C(Cc1ccccc1)NC(=O)C(CO)NC(=O)C(CC(O)=O)NC(=O)C(NC(=O)C(CCCCN)NC(=O)C(N)Cc1cnc[nH]1)C(C)O)C(C)C)C(N)=O Show InChI InChI=1S/C50H80N14O14S/c1-26(2)18-34(45(73)58-32(42(53)70)15-17-79-6)57-38(67)23-55-49(77)40(27(3)4)63-47(75)35(19-29-12-8-7-9-13-29)60-48(76)37(24-65)62-46(74)36(21-39(68)69)61-50(78)41(28(5)66)64-44(72)33(14-10-11-16-51)59-43(71)31(52)20-30-22-54-25-56-30/h7-9,12-13,22,25-28,31-37,40-41,65-66H,10-11,14-21,23-24,51-52H2,1-6H3,(H2,53,70)(H,54,56)(H,55,77)(H,57,67)(H,58,73)(H,59,71)(H,60,76)(H,61,78)(H,62,74)(H,63,75)(H,64,72)(H,68,69) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50071484
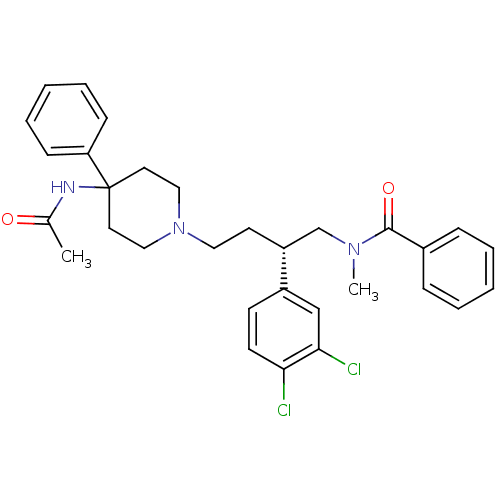
(CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37)/t26-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50335566
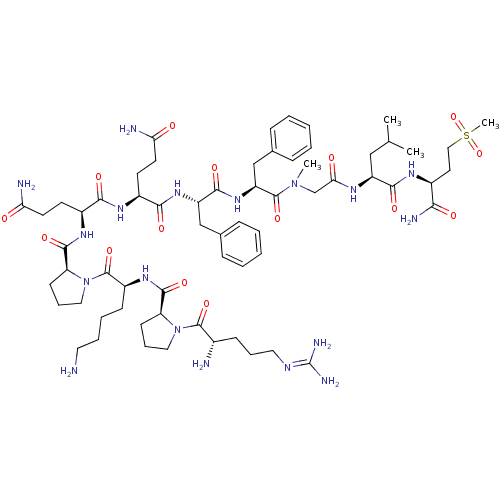
(CHEMBL1651026 | Substance P [Sar9,Met(O2)11])Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]S([#6])(=O)=O)-[#6](-[#7])=O |r| Show InChI InChI=1S/C64H100N18O15S/c1-38(2)34-46(57(89)74-42(54(69)86)28-33-98(4,96)97)73-53(85)37-80(3)62(94)48(36-40-18-9-6-10-19-40)79-58(90)47(35-39-16-7-5-8-17-39)78-56(88)43(24-26-51(67)83)75-55(87)44(25-27-52(68)84)76-59(91)50-23-15-32-82(50)63(95)45(21-11-12-29-65)77-60(92)49-22-14-31-81(49)61(93)41(66)20-13-30-72-64(70)71/h5-10,16-19,38,41-50H,11-15,20-37,65-66H2,1-4H3,(H2,67,83)(H2,68,84)(H2,69,86)(H,73,85)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,88)(H,79,90)(H4,70,71,72)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM81942

(CAS_55582 | NKA | NSC_55582 | Neurokinin alpha | S...)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(NC(=O)C(Cc1ccccc1)NC(=O)C(CO)NC(=O)C(CC(O)=O)NC(=O)C(NC(=O)C(CCCCN)NC(=O)C(N)Cc1cnc[nH]1)C(C)O)C(C)C)C(N)=O Show InChI InChI=1S/C50H80N14O14S/c1-26(2)18-34(45(73)58-32(42(53)70)15-17-79-6)57-38(67)23-55-49(77)40(27(3)4)63-47(75)35(19-29-12-8-7-9-13-29)60-48(76)37(24-65)62-46(74)36(21-39(68)69)61-50(78)41(28(5)66)64-44(72)33(14-10-11-16-51)59-43(71)31(52)20-30-22-54-25-56-30/h7-9,12-13,22,25-28,31-37,40-41,65-66H,10-11,14-21,23-24,51-52H2,1-6H3,(H2,53,70)(H,54,56)(H,55,77)(H,57,67)(H,58,73)(H,59,71)(H,60,76)(H,61,78)(H,62,74)(H,63,75)(H,64,72)(H,68,69) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281771
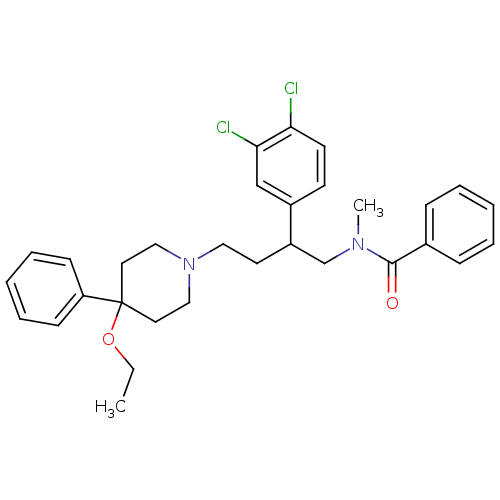
(CHEMBL173841 | N-[2-(3,4-Dichloro-phenyl)-4-(4-eth...)Show SMILES CCOC1(CCN(CCC(CN(C)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C31H36Cl2N2O2/c1-3-37-31(27-12-8-5-9-13-27)17-20-35(21-18-31)19-16-26(25-14-15-28(32)29(33)22-25)23-34(2)30(36)24-10-6-4-7-11-24/h4-15,22,26H,3,16-21,23H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50129513
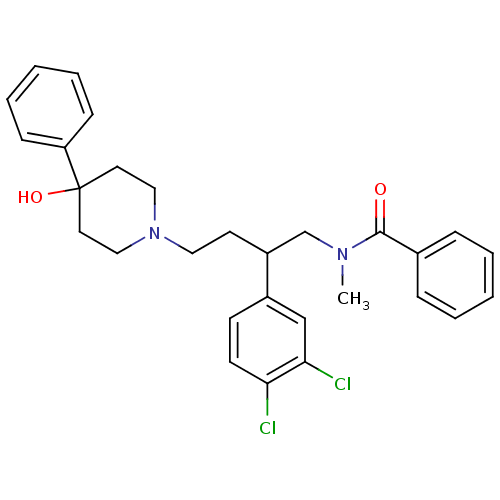
(CHEMBL71397 | N-[2-(3,4-Dichloro-phenyl)-4-(4-hydr...)Show SMILES CN(CC(CCN1CCC(O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C29H32Cl2N2O2/c1-32(28(34)22-8-4-2-5-9-22)21-24(23-12-13-26(30)27(31)20-23)14-17-33-18-15-29(35,16-19-33)25-10-6-3-7-11-25/h2-13,20,24,35H,14-19,21H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281768

(CHEMBL176588 | Thiophene-3-carboxylic acid [2-(3,4...)Show SMILES CN(CC(CCN1CCC(O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccsc1 Show InChI InChI=1S/C27H30Cl2N2O2S/c1-30(26(32)22-10-16-34-19-22)18-21(20-7-8-24(28)25(29)17-20)9-13-31-14-11-27(33,12-15-31)23-5-3-2-4-6-23/h2-8,10,16-17,19,21,33H,9,11-15,18H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(HAMSTER) | BDBM50071484

(CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of [125I]-NKA binding to neurokinin NK2 receptor from hamster urinary bladder membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281766

(CHEMBL173008 | Thiophene-2-carboxylic acid [2-(3,4...)Show SMILES CN(CC(CCN1CCC(O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1cccs1 Show InChI InChI=1S/C27H30Cl2N2O2S/c1-30(26(32)25-8-5-17-34-25)19-21(20-9-10-23(28)24(29)18-20)11-14-31-15-12-27(33,13-16-31)22-6-3-2-4-7-22/h2-10,17-18,21,33H,11-16,19H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281773

(CHEMBL176853 | Naphthalene-1-carboxylic acid [2-(3...)Show SMILES CN(CC(CCN1CCC(O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C33H34Cl2N2O2/c1-36(32(38)29-13-7-9-24-8-5-6-12-28(24)29)23-26(25-14-15-30(34)31(35)22-25)16-19-37-20-17-33(39,18-21-37)27-10-3-2-4-11-27/h2-15,22,26,39H,16-21,23H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM81942

(CAS_55582 | NKA | NSC_55582 | Neurokinin alpha | S...)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(NC(=O)C(Cc1ccccc1)NC(=O)C(CO)NC(=O)C(CC(O)=O)NC(=O)C(NC(=O)C(CCCCN)NC(=O)C(N)Cc1cnc[nH]1)C(C)O)C(C)C)C(N)=O Show InChI InChI=1S/C50H80N14O14S/c1-26(2)18-34(45(73)58-32(42(53)70)15-17-79-6)57-38(67)23-55-49(77)40(27(3)4)63-47(75)35(19-29-12-8-7-9-13-29)60-48(76)37(24-65)62-46(74)36(21-39(68)69)61-50(78)41(28(5)66)64-44(72)33(14-10-11-16-51)59-43(71)31(52)20-30-22-54-25-56-30/h7-9,12-13,22,25-28,31-37,40-41,65-66H,10-11,14-21,23-24,51-52H2,1-6H3,(H2,53,70)(H,54,56)(H,55,77)(H,57,67)(H,58,73)(H,59,71)(H,60,76)(H,61,78)(H,62,74)(H,63,75)(H,64,72)(H,68,69) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281770
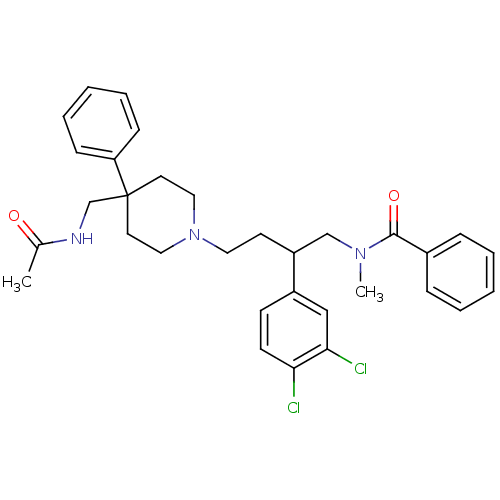
(CHEMBL367542 | N-[4-[4-(Acetylamino-methyl)-4-phen...)Show SMILES CN(CC(CCN1CCC(CNC(C)=O)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C32H37Cl2N3O2/c1-24(38)35-23-32(28-11-7-4-8-12-28)16-19-37(20-17-32)18-15-27(26-13-14-29(33)30(34)21-26)22-36(2)31(39)25-9-5-3-6-10-25/h3-14,21,27H,15-20,22-23H2,1-2H3,(H,35,38) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281772

(CHEMBL368372 | N-[2-(3,4-Dichloro-phenyl)-4-(4-hyd...)Show SMILES CN(CC(CCN1CCC(CO)(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C30H34Cl2N2O2/c1-33(29(36)23-8-4-2-5-9-23)21-25(24-12-13-27(31)28(32)20-24)14-17-34-18-15-30(22-35,16-19-34)26-10-6-3-7-11-26/h2-13,20,25,35H,14-19,21-22H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50281774
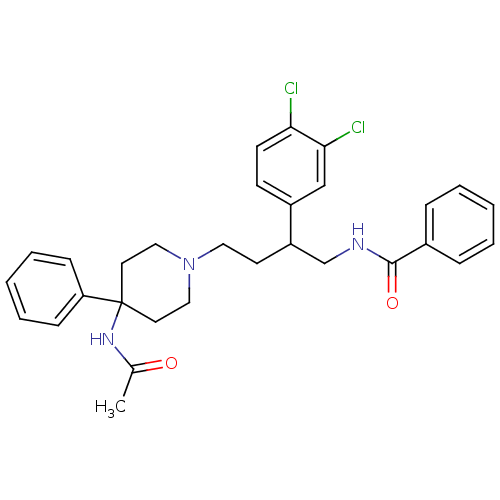
(CHEMBL366382 | N-[4-(4-Acetylamino-4-phenyl-piperi...)Show SMILES CC(=O)NC1(CCN(CCC(CNC(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C30H33Cl2N3O2/c1-22(36)34-30(26-10-6-3-7-11-26)15-18-35(19-16-30)17-14-25(24-12-13-27(31)28(32)20-24)21-33-29(37)23-8-4-2-5-9-23/h2-13,20,25H,14-19,21H2,1H3,(H,33,37)(H,34,36) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50129520
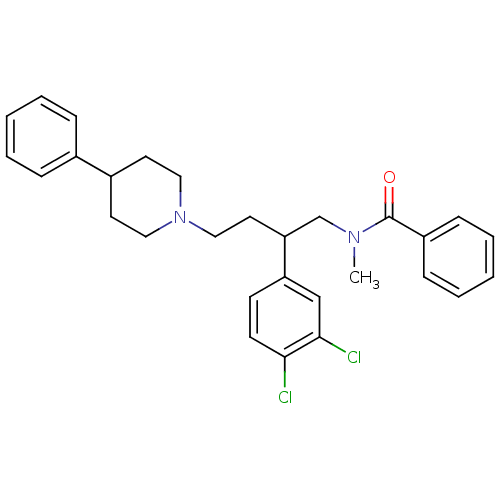
(CHEMBL69335 | N-[2-(3,4-Dichloro-phenyl)-4-(4-phen...)Show SMILES CN(CC(CCN1CCC(CC1)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C29H32Cl2N2O/c1-32(29(34)24-10-6-3-7-11-24)21-26(25-12-13-27(30)28(31)20-25)16-19-33-17-14-23(15-18-33)22-8-4-2-5-9-22/h2-13,20,23,26H,14-19,21H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NKA binding to neurokinin NK2 receptor from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Gerbil) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 544 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50071484

(CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37)/t26-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 945 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of [125I]-NKA binding to Tachykinin receptor 2 from rat duodenum membranes |
Bioorg Med Chem Lett 3: 925-930 (1993)
Article DOI: 10.1016/S0960-894X(00)80694-0
BindingDB Entry DOI: 10.7270/Q2N29WW1 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(mouse) | BDBM86055

(SSR240600)Show SMILES CC(C)(C1CCN(CCC2(CN(CCO2)C(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C31H35Cl2F6N3O3/c1-28(2,27(40)44)20-5-8-41(9-6-20)10-7-29(21-3-4-24(32)25(33)17-21)18-42(11-12-45-29)26(43)15-19-13-22(30(34,35)36)16-23(14-19)31(37,38)39/h3-4,13-14,16-17,20H,5-12,15,18H2,1-2H3,(H2,40,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1171-9 (2002)
Article DOI: 10.1124/jpet.102.040162
BindingDB Entry DOI: 10.7270/Q23J3BJH |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50017392
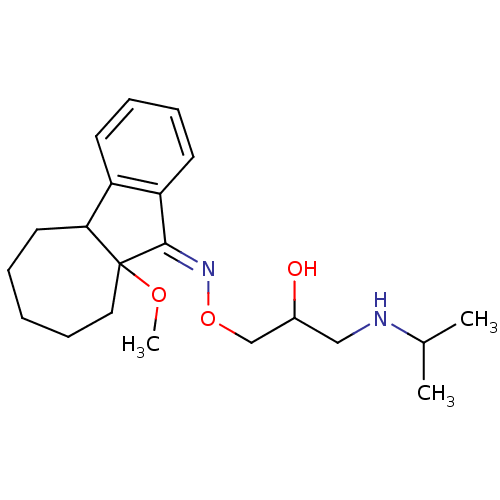
(9a-Methoxy-5,6,7,8,9,9a-hexahydro-4bH-benzo[a]azul...)Show InChI InChI=1S/C21H32N2O3/c1-15(2)22-13-16(24)14-26-23-20-18-10-7-6-9-17(18)19-11-5-4-8-12-21(19,20)25-3/h6-7,9-10,15-16,19,22,24H,4-5,8,11-14H2,1-3H3/b23-20- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-2 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig trachea(IT) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50017384
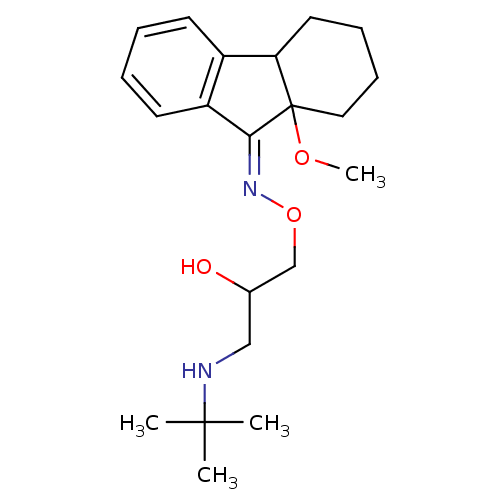
(9a-Methoxy-1,2,3,4,4a,9a-hexahydro-fluoren-9-one O...)Show InChI InChI=1S/C21H32N2O3/c1-20(2,3)22-13-15(24)14-26-23-19-17-10-6-5-9-16(17)18-11-7-8-12-21(18,19)25-4/h5-6,9-10,15,18,22,24H,7-8,11-14H2,1-4H3/b23-19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-2 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig trachea(IT) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(GUINEA PIG) | BDBM50017383

(9a-Methoxy-5,6,7,8,9,9a-hexahydro-4bH-benzo[a]azul...)Show InChI InChI=1S/C22H34N2O3/c1-21(2,3)23-14-16(25)15-27-24-20-18-11-8-7-10-17(18)19-12-6-5-9-13-22(19,20)26-4/h7-8,10-11,16,19,23,25H,5-6,9,12-15H2,1-4H3/b24-20- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-1 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig atria(IA) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50017388

(9a-Methoxy-1,2,3,4,4a,9a-hexahydro-fluoren-9-one O...)Show InChI InChI=1S/C20H30N2O3/c1-14(2)21-12-15(23)13-25-22-19-17-9-5-4-8-16(17)18-10-6-7-11-20(18,19)24-3/h4-5,8-9,14-15,18,21,23H,6-7,10-13H2,1-3H3/b22-19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-2 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig trachea(IT) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(GUINEA PIG) | BDBM50017385

(9a-Hydroxy-5,6,7,8,9,9a-hexahydro-4bH-benzo[a]azul...)Show InChI InChI=1S/C21H32N2O3/c1-20(2,3)22-13-15(24)14-26-23-19-17-10-7-6-9-16(17)18-11-5-4-8-12-21(18,19)25/h6-7,9-10,15,18,22,24-25H,4-5,8,11-14H2,1-3H3/b23-19- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-1 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig atria(IA) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50017385

(9a-Hydroxy-5,6,7,8,9,9a-hexahydro-4bH-benzo[a]azul...)Show InChI InChI=1S/C21H32N2O3/c1-20(2,3)22-13-15(24)14-26-23-19-17-10-7-6-9-16(17)18-11-5-4-8-12-21(18,19)25/h6-7,9-10,15,18,22,24-25H,4-5,8,11-14H2,1-3H3/b23-19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-2 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig trachea(IT) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50017383

(9a-Methoxy-5,6,7,8,9,9a-hexahydro-4bH-benzo[a]azul...)Show InChI InChI=1S/C22H34N2O3/c1-21(2,3)23-14-16(25)15-27-24-20-18-11-8-7-10-17(18)19-12-6-5-9-13-22(19,20)26-4/h7-8,10-11,16,19,23,25H,5-6,9,12-15H2,1-4H3/b24-20- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-2 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig trachea(IT) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50017396
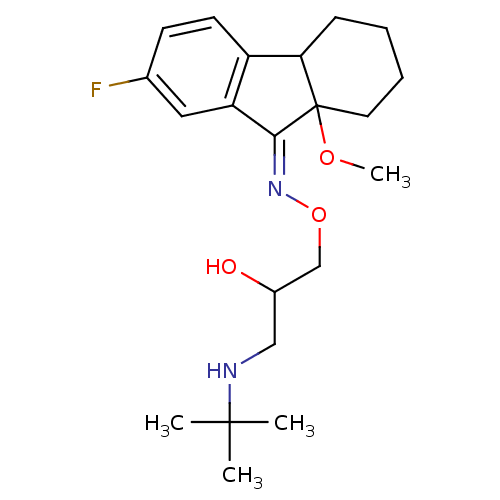
(7-Fluoro-9a-methoxy-1,2,3,4,4a,9a-hexahydro-fluore...)Show SMILES COC12CCCCC1c1ccc(F)cc1\C2=N\OCC(O)CNC(C)(C)C Show InChI InChI=1S/C21H31FN2O3/c1-20(2,3)23-12-15(25)13-27-24-19-17-11-14(22)8-9-16(17)18-7-5-6-10-21(18,19)26-4/h8-9,11,15,18,23,25H,5-7,10,12-13H2,1-4H3/b24-19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-2 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig trachea(IT) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50017386

(7-Fluoro-9a-methoxy-1,2,3,4,4a,9a-hexahydro-fluore...)Show InChI InChI=1S/C20H29FN2O3/c1-13(2)22-11-15(24)12-26-23-19-17-10-14(21)7-8-16(17)18-6-4-5-9-20(18,19)25-3/h7-8,10,13,15,18,22,24H,4-6,9,11-12H2,1-3H3/b23-19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Nancy I
Curated by ChEMBL
| Assay Description
Beta-2 adrenergic receptor blocking was assessed from 50% inhibition of the isoproterenol submaximal response on isolated Guinea pig trachea(IT) |
J Med Chem 32: 315-20 (1989)
BindingDB Entry DOI: 10.7270/Q2RX9B26 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data