

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452874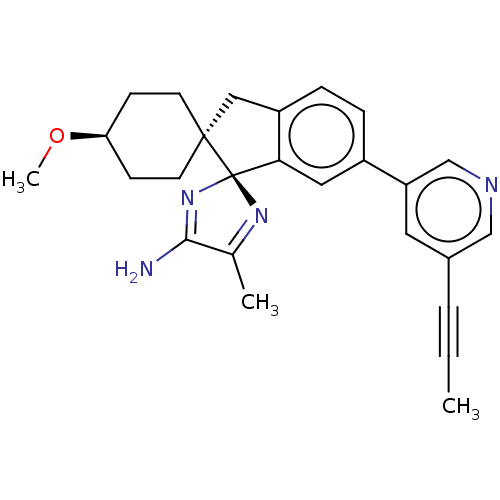 (CHEMBL4217620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant BACE-1 (1 to 460 residue) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116459 BindingDB Entry DOI: 10.7270/Q2057KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50403793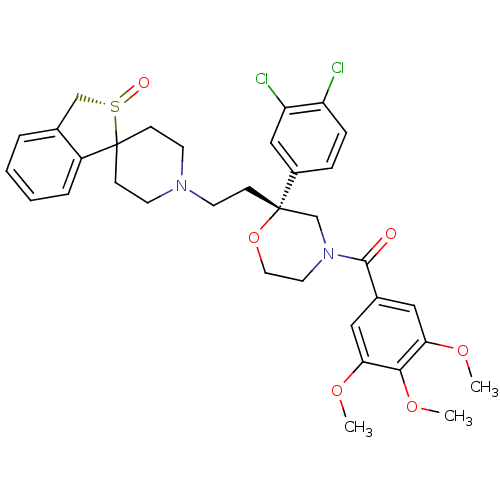 (CHEMBL2115415) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was evaluated in vitro for its ability to displace [3H]-SP from Tachykinin receptor 3 of male guinea pig lung membrane | Bioorg Med Chem Lett 10: 1665-8 (2000) BindingDB Entry DOI: 10.7270/Q2QZ297D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217526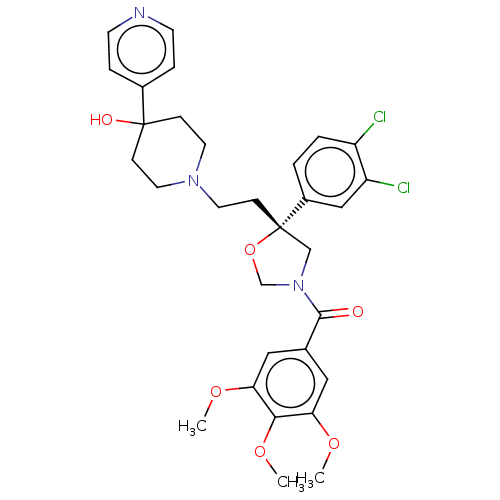 (CHEMBL366972) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50090485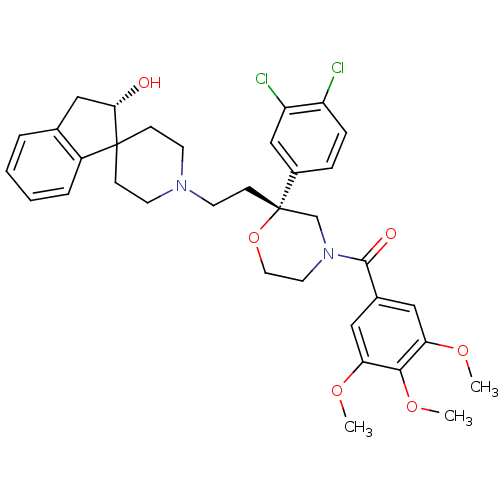 (CHEMBL295615 | spiro[(2-hydroxy)indane-1,40-piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was evaluated in vitro for its ability to displace [3H]-SP from Tachykinin receptor 3 of male guinea pig lung membrane | Bioorg Med Chem Lett 10: 1665-8 (2000) BindingDB Entry DOI: 10.7270/Q2QZ297D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217519 (CHEMBL354709) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50057477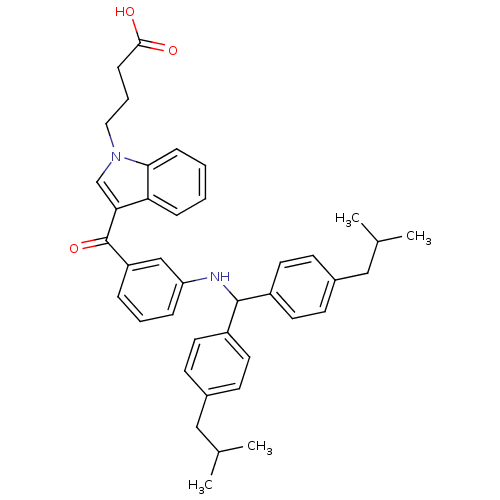 (4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Steroid 5-alpha-reductase type 1 in COS-1 cells was determined | Bioorg Med Chem Lett 8: 561-6 (1999) BindingDB Entry DOI: 10.7270/Q2571B5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50403793 (CHEMBL2115415) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was evaluated in vitro for its ability to displace [3H]-SP from Tachykinin receptor 2 of male guinea pig lung membrane | Bioorg Med Chem Lett 10: 1665-8 (2000) BindingDB Entry DOI: 10.7270/Q2QZ297D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217520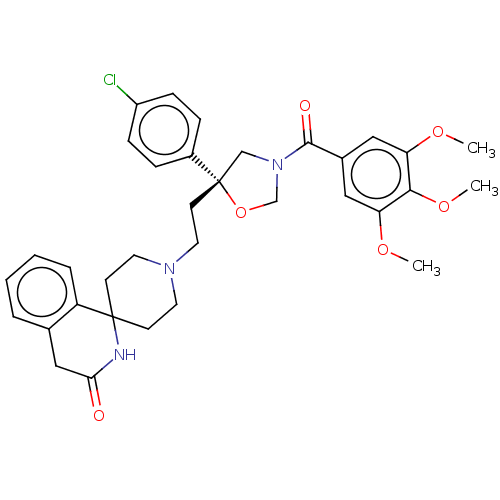 (CHEMBL354645) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120757 (US8703761, 1-29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217517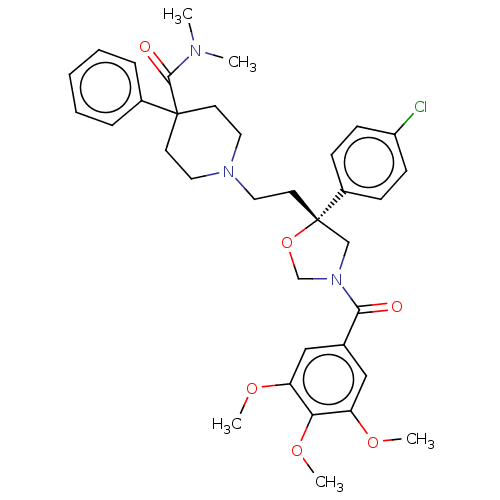 (CHEMBL353158) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription activator BRG1 (Homo sapiens (Human)) | BDBM50469320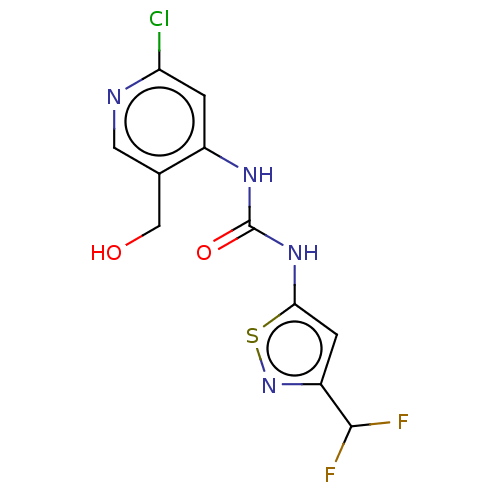 (CHEMBL4293567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469320 (CHEMBL4293567) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469324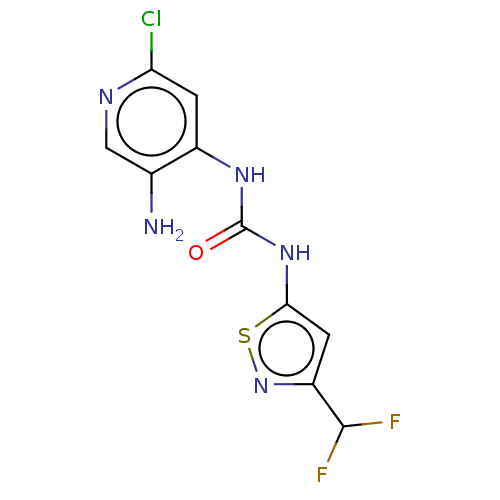 (CHEMBL4286345) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription activator BRG1 (Homo sapiens (Human)) | BDBM50469330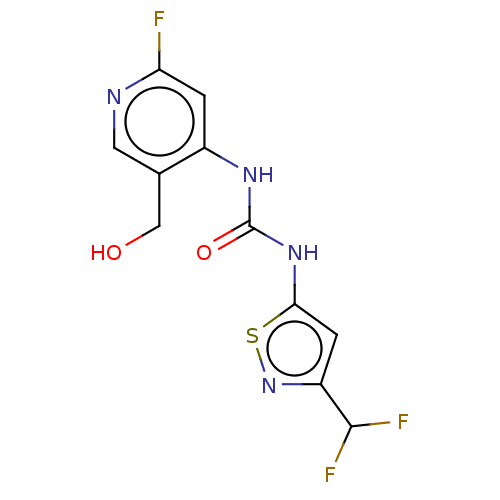 (CHEMBL4295096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription activator BRG1 (Homo sapiens (Human)) | BDBM50469324 (CHEMBL4286345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469331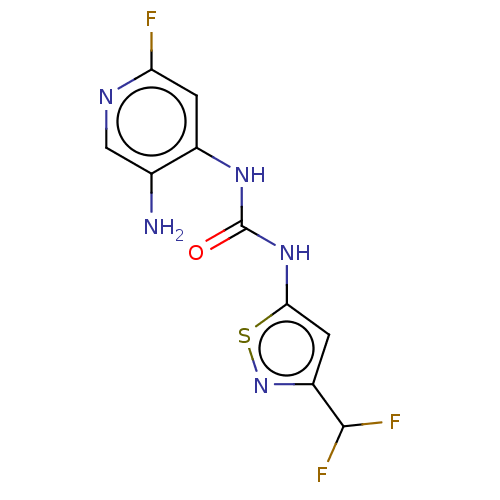 (CHEMBL4282980) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217511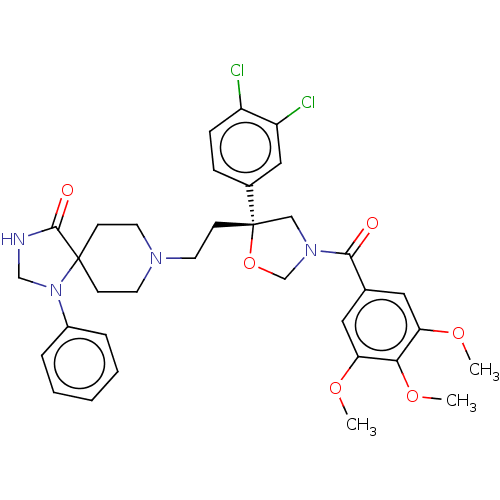 (CHEMBL172342) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469330 (CHEMBL4295096) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469329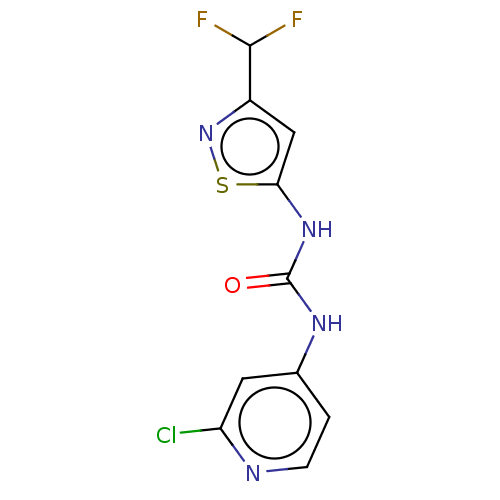 (CHEMBL4278436) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription activator BRG1 (Homo sapiens (Human)) | BDBM50469331 (CHEMBL4282980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217506 (CHEMBL170936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217509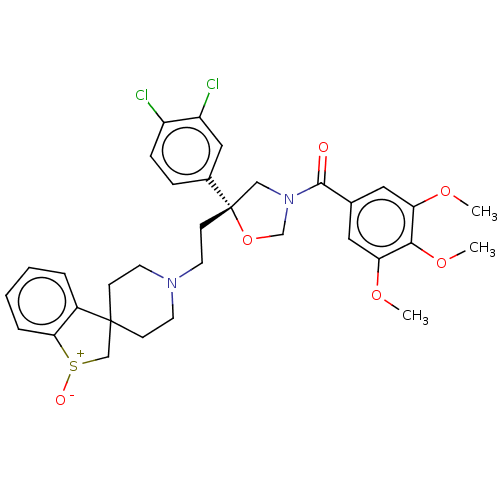 (CHEMBL354731) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription activator BRG1 (Homo sapiens (Human)) | BDBM50469329 (CHEMBL4278436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217508 (CHEMBL367509) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217504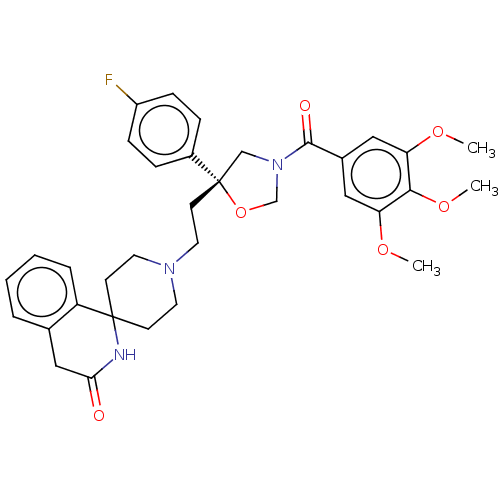 (CHEMBL353284) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217505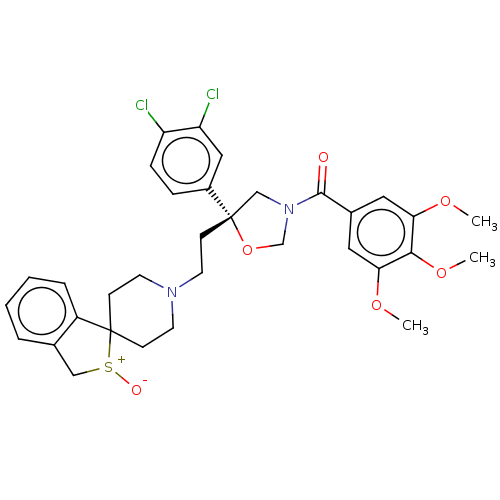 (CHEMBL414377) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217513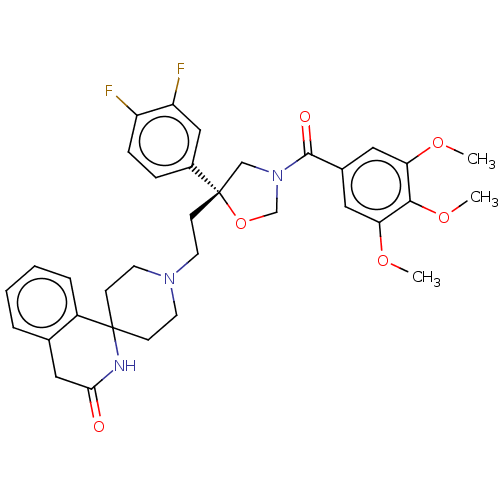 (CHEMBL171658) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50090485 (CHEMBL295615 | spiro[(2-hydroxy)indane-1,40-piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was evaluated in vitro for its ability to displace [3H]-SP from Tachykinin receptor 2 of male guinea pig lung membrane | Bioorg Med Chem Lett 10: 1665-8 (2000) BindingDB Entry DOI: 10.7270/Q2QZ297D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217528 (CHEMBL353596) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50143817 (CHEMBL3758618) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant 6His-tagged DAGT1 expressed in fall armyworm Sf9 cells using diolein and oleoyl-CoA incubated for 30 mins by LC/MS/MS... | Bioorg Med Chem Lett 26: 1245-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.025 BindingDB Entry DOI: 10.7270/Q2N29ZSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217509 (CHEMBL354731) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217505 (CHEMBL414377) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217522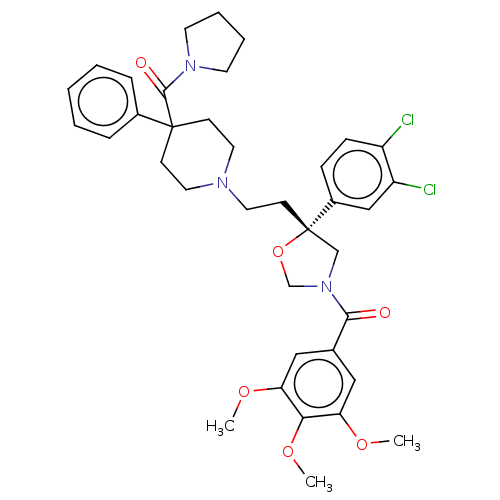 (CHEMBL353394) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120755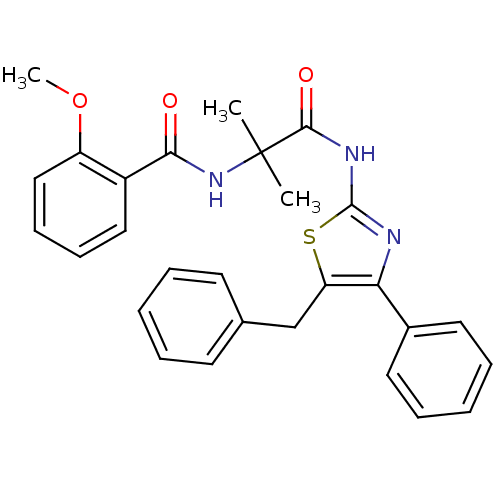 (US8703761, 1-8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217507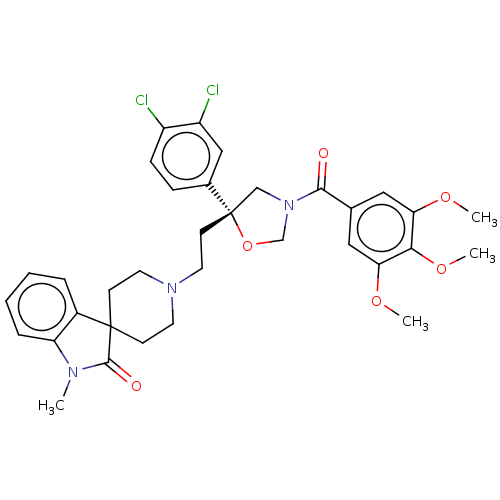 (CHEMBL171704) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217527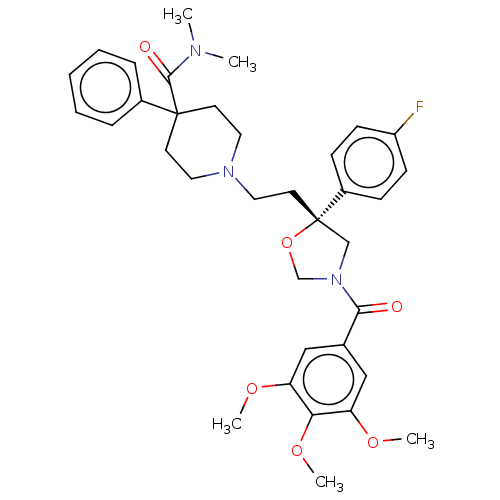 (CHEMBL172227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239481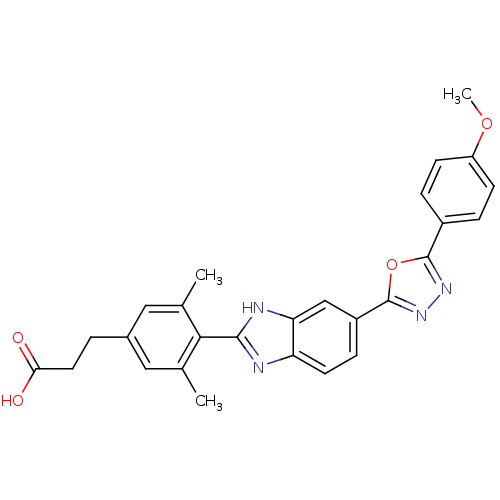 (CHEMBL4061308) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217530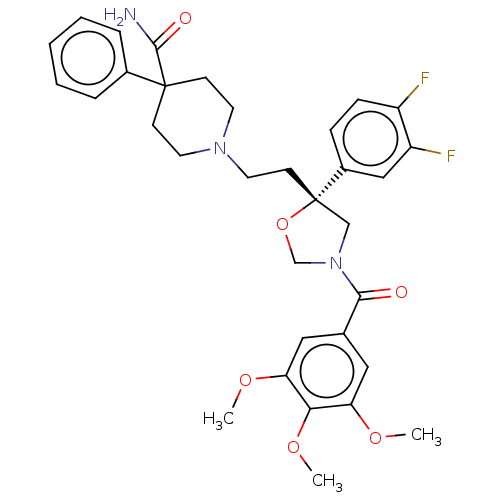 (CHEMBL170084) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription activator BRG1 (Homo sapiens (Human)) | BDBM50469325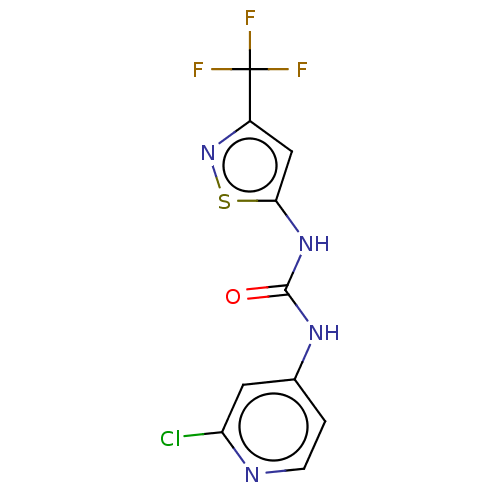 (CHEMBL4294655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged BRG1 ATPase-SnAC (658 to 1361 residues) (unknown origin) expressed in insect sf9 cells preincubated for 5 mins ... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469325 (CHEMBL4294655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120756 (US8703761, 1-28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50143818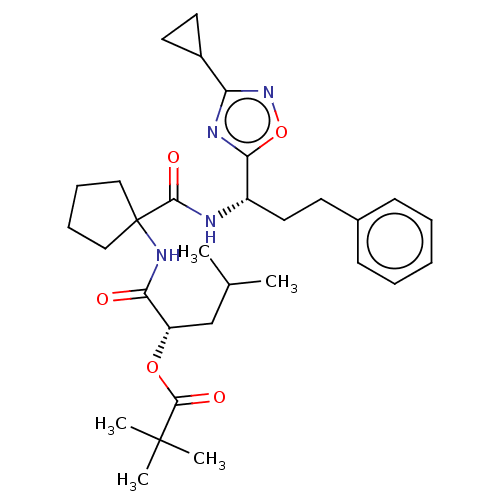 (CHEMBL3759363) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant 6His-tagged DAGT1 expressed in fall armyworm Sf9 cells using diolein and oleoyl-CoA incubated for 30 mins by LC/MS/MS... | Bioorg Med Chem Lett 26: 1245-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.025 BindingDB Entry DOI: 10.7270/Q2N29ZSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50057477 (4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Steroid 5-alpha-reductase type 2 in COS-1 cells was determined | Bioorg Med Chem Lett 8: 561-6 (1999) BindingDB Entry DOI: 10.7270/Q2571B5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50403790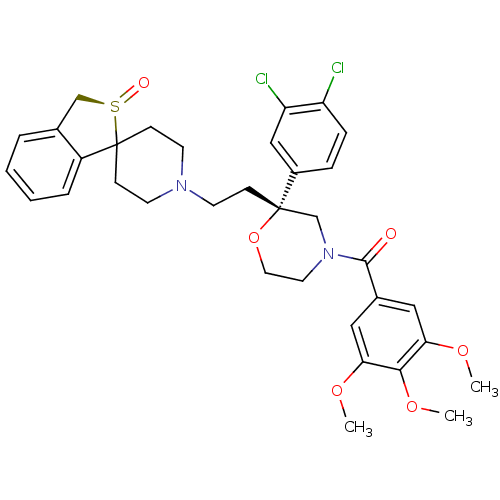 (CHEMBL2114963) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was evaluated in vitro for its ability to displace [3H]-SP from Tachykinin receptor 3 of male guinea pig lung membrane | Bioorg Med Chem Lett 10: 1665-8 (2000) BindingDB Entry DOI: 10.7270/Q2QZ297D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217528 (CHEMBL353596) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217519 (CHEMBL354709) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50217514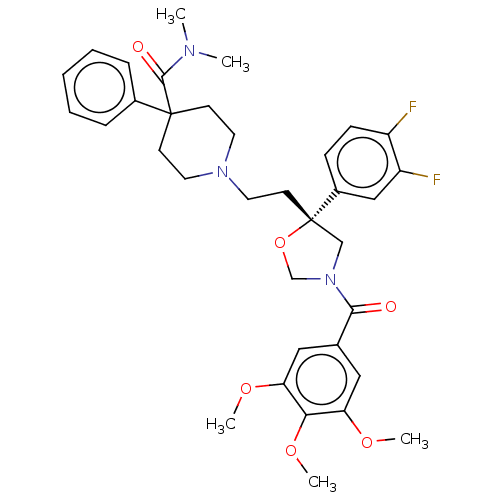 (CHEMBL369246) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239482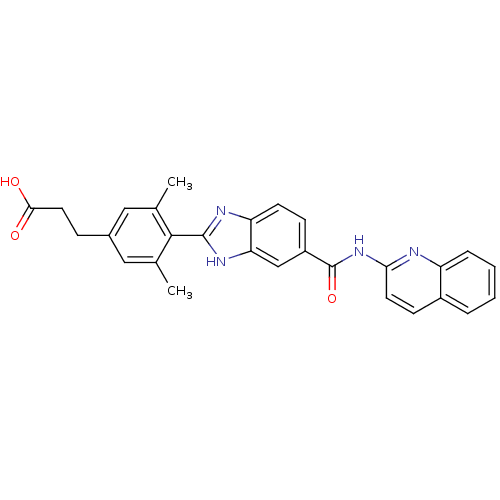 (CHEMBL1683001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217510 (CHEMBL355387) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50217512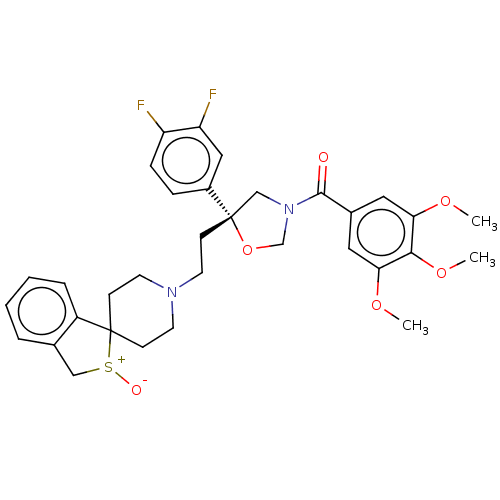 (CHEMBL169132) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung | Bioorg Med Chem Lett 9: 875-80 (1999) BindingDB Entry DOI: 10.7270/Q2PN97TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 398 total ) | Next | Last >> |