 Found 70 hits with Last Name = 'albaugh' and Initial = 'dr'
Found 70 hits with Last Name = 'albaugh' and Initial = 'dr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin G
(Homo sapiens (Human)) | BDBM50349174

(CHEMBL1807526)Show InChI InChI=1S/C20H18N2O3S/c1-13-5-4-8-17-19(13)14(12-26-17)11-22-16-7-3-2-6-15(16)21(20(22)25)10-9-18(23)24/h2-8,12H,9-11H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349176
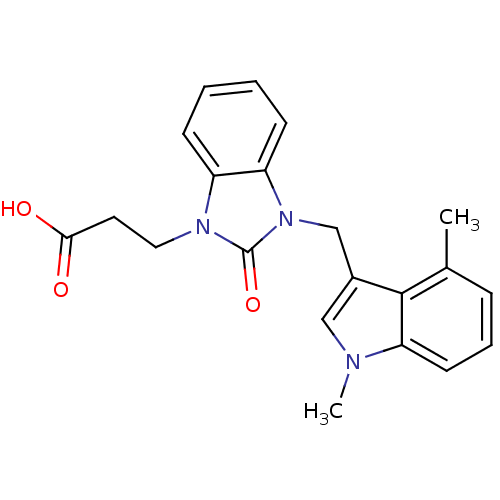
(CHEMBL1807531)Show SMILES Cc1cccc2n(C)cc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c12 Show InChI InChI=1S/C21H21N3O3/c1-14-6-5-9-18-20(14)15(12-22(18)2)13-24-17-8-4-3-7-16(17)23(21(24)27)11-10-19(25)26/h3-9,12H,10-11,13H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349173

(CHEMBL1807638)Show SMILES Cc1c(C)c2cc(C)cc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C23H25N3O3/c1-14-11-17(22-18(12-14)15(2)16(3)24(22)4)13-26-20-8-6-5-7-19(20)25(23(26)29)10-9-21(27)28/h5-8,11-12H,9-10,13H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349183
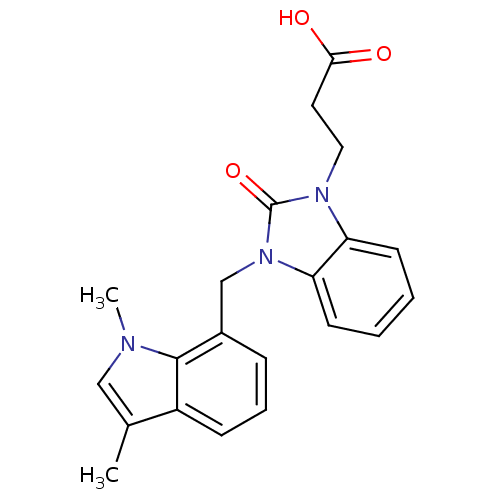
(CHEMBL1807538)Show SMILES Cc1cn(C)c2c(Cn3c4ccccc4n(CCC(O)=O)c3=O)cccc12 Show InChI InChI=1S/C21H21N3O3/c1-14-12-22(2)20-15(6-5-7-16(14)20)13-24-18-9-4-3-8-17(18)23(21(24)27)11-10-19(25)26/h3-9,12H,10-11,13H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349178
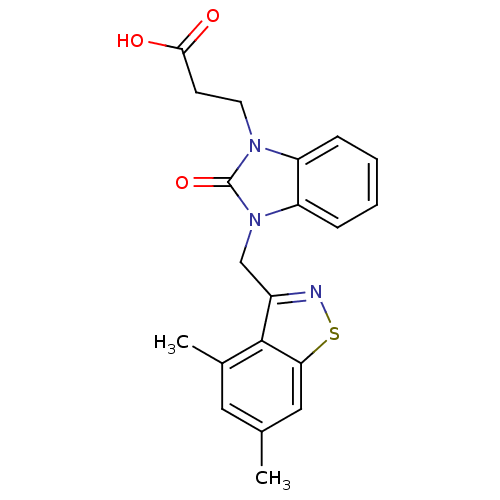
(CHEMBL1807535)Show SMILES Cc1cc(C)c2c(Cn3c4ccccc4n(CCC(O)=O)c3=O)nsc2c1 Show InChI InChI=1S/C20H19N3O3S/c1-12-9-13(2)19-14(21-27-17(19)10-12)11-23-16-6-4-3-5-15(16)22(20(23)26)8-7-18(24)25/h3-6,9-10H,7-8,11H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349182
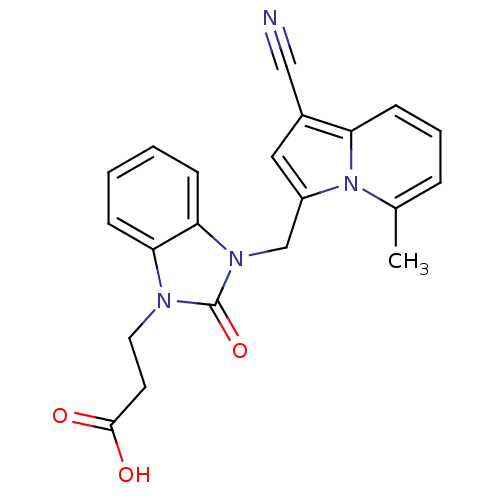
(CHEMBL1807534)Show SMILES Cc1cccc2c(cc(Cn3c4ccccc4n(CCC(O)=O)c3=O)n12)C#N Show InChI InChI=1S/C21H18N4O3/c1-14-5-4-8-17-15(12-22)11-16(25(14)17)13-24-19-7-3-2-6-18(19)23(21(24)28)10-9-20(26)27/h2-8,11H,9-10,13H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349181

(CHEMBL1807536)Show InChI InChI=1S/C20H19N3O3/c1-21-11-9-14-5-4-6-15(19(14)21)13-23-17-8-3-2-7-16(17)22(20(23)26)12-10-18(24)25/h2-9,11H,10,12-13H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349179
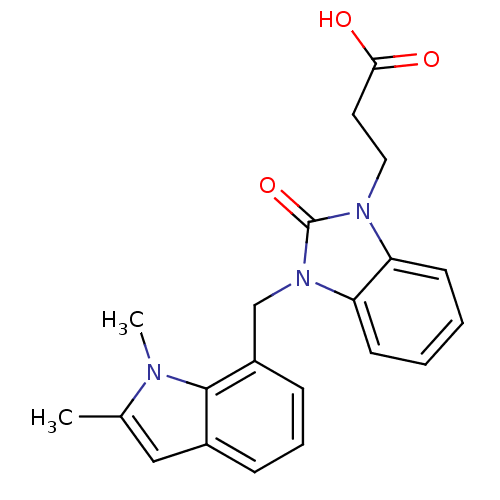
(CHEMBL1807537)Show SMILES Cc1cc2cccc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C21H21N3O3/c1-14-12-15-6-5-7-16(20(15)22(14)2)13-24-18-9-4-3-8-17(18)23(21(24)27)11-10-19(25)26/h3-9,12H,10-11,13H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349186

(CHEMBL1807539)Show SMILES Cc1cn(C)c2cccc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c12 Show InChI InChI=1S/C21H21N3O3/c1-14-12-22(2)18-9-5-6-15(20(14)18)13-24-17-8-4-3-7-16(17)23(21(24)27)11-10-19(25)26/h3-9,12H,10-11,13H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349175
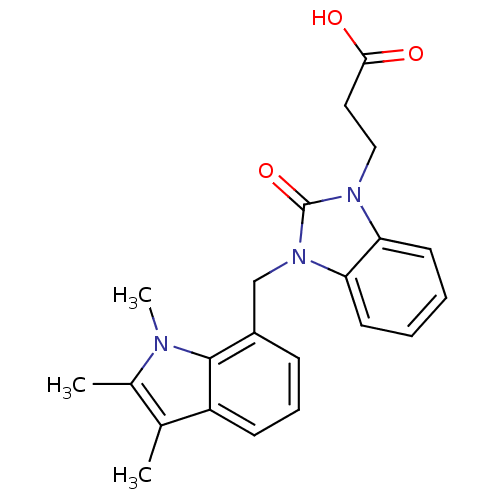
(CHEMBL1807637)Show SMILES Cc1c(C)c2cccc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C22H23N3O3/c1-14-15(2)23(3)21-16(7-6-8-17(14)21)13-25-19-10-5-4-9-18(19)24(22(25)28)12-11-20(26)27/h4-10H,11-13H2,1-3H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349177
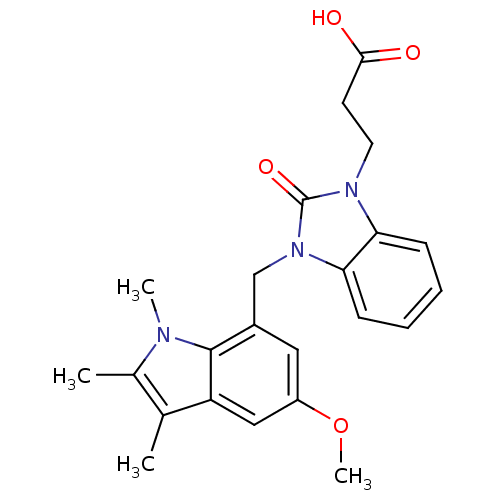
(CHEMBL1807639)Show SMILES COc1cc(Cn2c3ccccc3n(CCC(O)=O)c2=O)c2n(C)c(C)c(C)c2c1 Show InChI InChI=1S/C23H25N3O4/c1-14-15(2)24(3)22-16(11-17(30-4)12-18(14)22)13-26-20-8-6-5-7-19(20)25(23(26)29)10-9-21(27)28/h5-8,11-12H,9-10,13H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349190
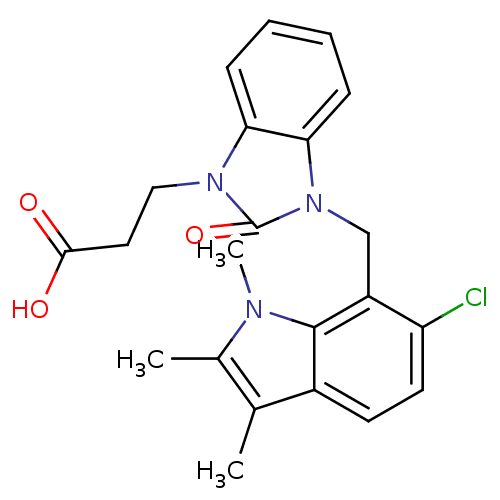
(CHEMBL1807641)Show SMILES Cc1c(C)c2ccc(Cl)c(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C22H22ClN3O3/c1-13-14(2)24(3)21-15(13)8-9-17(23)16(21)12-26-19-7-5-4-6-18(19)25(22(26)29)11-10-20(27)28/h4-9H,10-12H2,1-3H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349189
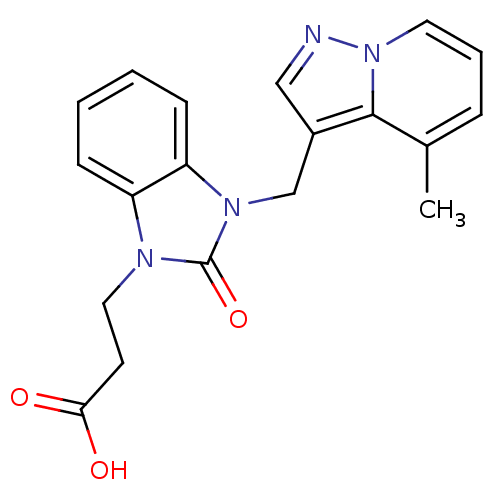
(CHEMBL1807533)Show InChI InChI=1S/C19H18N4O3/c1-13-5-4-9-23-18(13)14(11-20-23)12-22-16-7-3-2-6-15(16)21(19(22)26)10-8-17(24)25/h2-7,9,11H,8,10,12H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349188
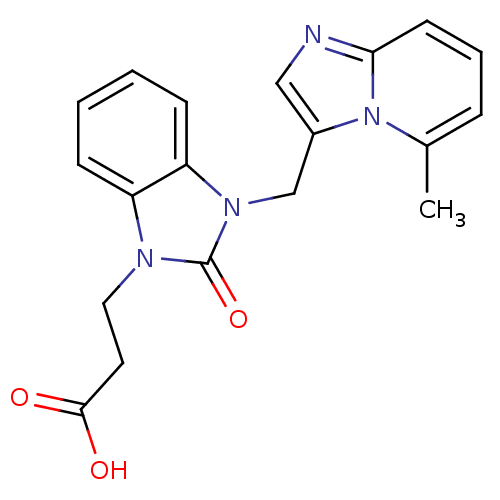
(CHEMBL1807532)Show InChI InChI=1S/C19H18N4O3/c1-13-5-4-8-17-20-11-14(23(13)17)12-22-16-7-3-2-6-15(16)21(19(22)26)10-9-18(24)25/h2-8,11H,9-10,12H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349180
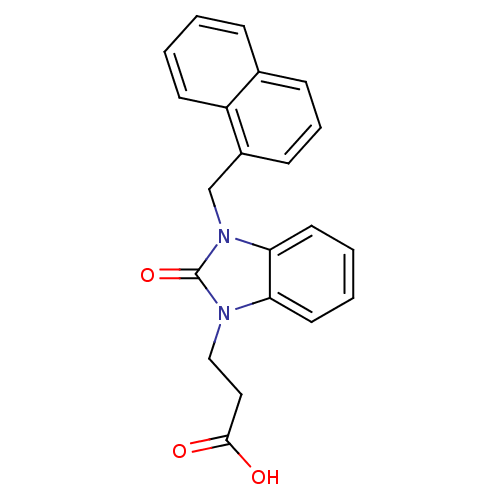
(CHEMBL1807530)Show InChI InChI=1S/C21H18N2O3/c24-20(25)12-13-22-18-10-3-4-11-19(18)23(21(22)26)14-16-8-5-7-15-6-1-2-9-17(15)16/h1-11H,12-14H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349185
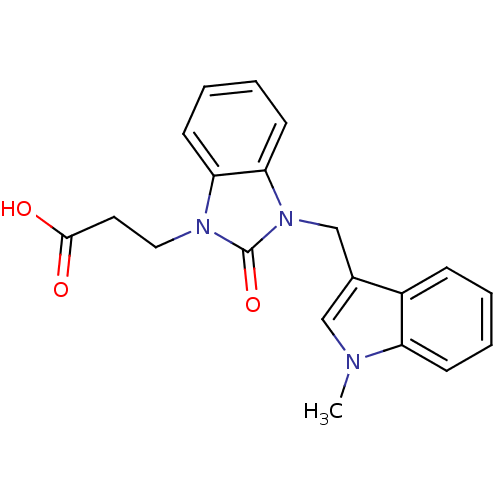
(CHEMBL1807529)Show InChI InChI=1S/C20H19N3O3/c1-21-12-14(15-6-2-3-7-16(15)21)13-23-18-9-5-4-8-17(18)22(20(23)26)11-10-19(24)25/h2-9,12H,10-11,13H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349184
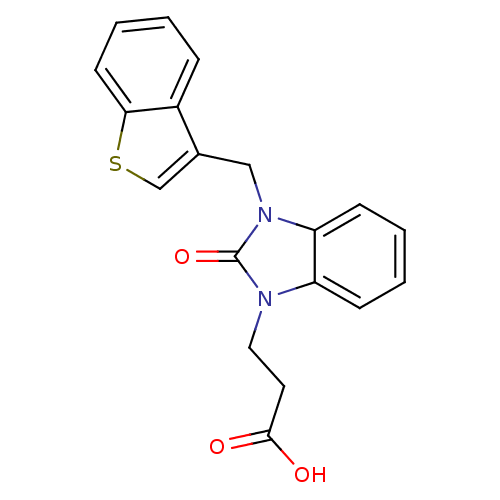
(CHEMBL1807527)Show InChI InChI=1S/C19H16N2O3S/c22-18(23)9-10-20-15-6-2-3-7-16(15)21(19(20)24)11-13-12-25-17-8-4-1-5-14(13)17/h1-8,12H,9-11H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349187
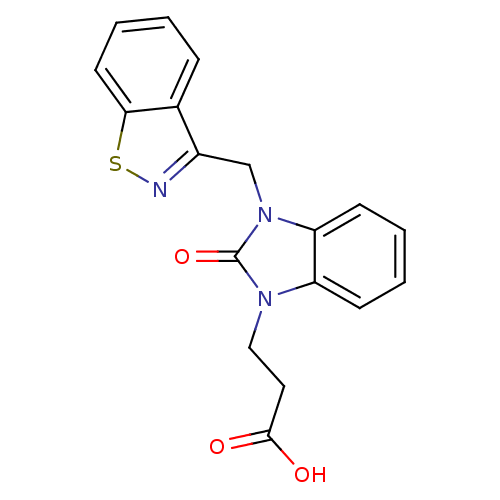
(CHEMBL1807528)Show InChI InChI=1S/C18H15N3O3S/c22-17(23)9-10-20-14-6-2-3-7-15(14)21(18(20)24)11-13-12-5-1-4-8-16(12)25-19-13/h1-8H,9-11H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349191

(CHEMBL1807642)Show InChI InChI=1S/C21H20N2O2S2/c1-14-6-4-9-18-20(14)15(13-27-18)12-23-17-8-3-2-7-16(17)22-21(23)26-11-5-10-19(24)25/h2-4,6-9,13H,5,10-12H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349172
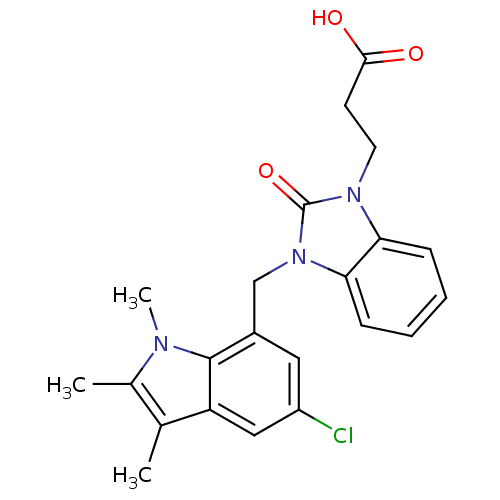
(CHEMBL1807640)Show SMILES Cc1c(C)c2cc(Cl)cc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C22H22ClN3O3/c1-13-14(2)24(3)21-15(10-16(23)11-17(13)21)12-26-19-7-5-4-6-18(19)25(22(26)29)9-8-20(27)28/h4-7,10-11H,8-9,12H2,1-3H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349172

(CHEMBL1807640)Show SMILES Cc1c(C)c2cc(Cl)cc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C22H22ClN3O3/c1-13-14(2)24(3)21-15(10-16(23)11-17(13)21)12-26-19-7-5-4-6-18(19)25(22(26)29)9-8-20(27)28/h4-7,10-11H,8-9,12H2,1-3H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50434109

(CHEMBL2381483)Show SMILES CCCC(CC(O)=O)n1c2ccccc2n(Cc2cc(Br)cc3NC(=O)Cc23)c1=O Show InChI InChI=1S/C22H22BrN3O4/c1-2-5-15(10-21(28)29)26-19-7-4-3-6-18(19)25(22(26)30)12-13-8-14(23)9-17-16(13)11-20(27)24-17/h3-4,6-9,15H,2,5,10-12H2,1H3,(H,24,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM50434124
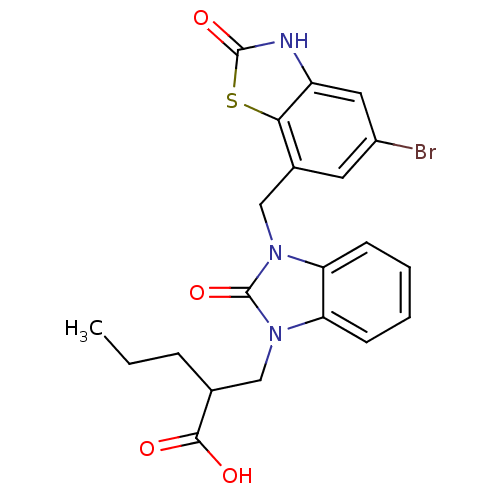
(CHEMBL2381480)Show SMILES CCCC(Cn1c2ccccc2n(Cc2cc(Br)cc3[nH]c(=O)sc23)c1=O)C(O)=O Show InChI InChI=1S/C21H20BrN3O4S/c1-2-5-12(19(26)27)10-24-16-6-3-4-7-17(16)25(21(24)29)11-13-8-14(22)9-15-18(13)30-20(28)23-15/h3-4,6-9,12H,2,5,10-11H2,1H3,(H,23,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349191

(CHEMBL1807642)Show InChI InChI=1S/C21H20N2O2S2/c1-14-6-4-9-18-20(14)15(13-27-18)12-23-17-8-3-2-7-16(17)22-21(23)26-11-5-10-19(24)25/h2-4,6-9,13H,5,10-12H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50434108
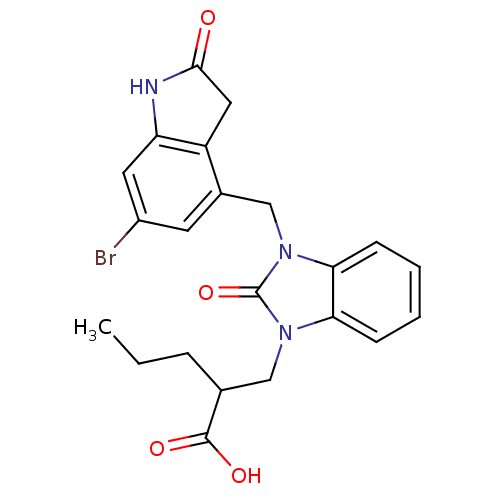
(CHEMBL2381482)Show SMILES CCCC(Cn1c2ccccc2n(Cc2cc(Br)cc3NC(=O)Cc23)c1=O)C(O)=O Show InChI InChI=1S/C22H22BrN3O4/c1-2-5-13(21(28)29)11-25-18-6-3-4-7-19(18)26(22(25)30)12-14-8-15(23)9-17-16(14)10-20(27)24-17/h3-4,6-9,13H,2,5,10-12H2,1H3,(H,24,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349173

(CHEMBL1807638)Show SMILES Cc1c(C)c2cc(C)cc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C23H25N3O3/c1-14-11-17(22-18(12-14)15(2)16(3)24(22)4)13-26-20-8-6-5-7-19(20)25(23(26)29)10-9-21(27)28/h5-8,11-12H,9-10,13H2,1-4H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349174

(CHEMBL1807526)Show InChI InChI=1S/C20H18N2O3S/c1-13-5-4-8-17-19(13)14(12-26-17)11-22-16-7-3-2-6-15(16)21(20(22)25)10-9-18(23)24/h2-8,12H,9-11H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349192
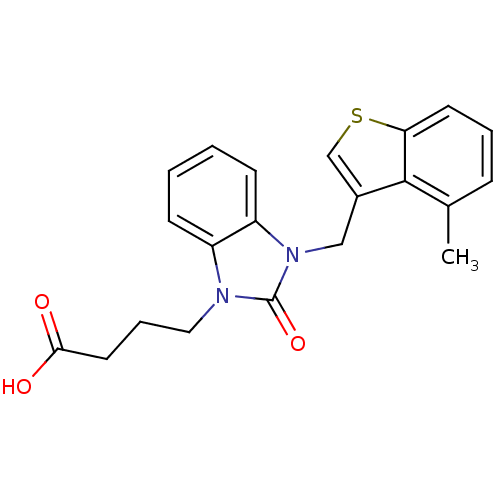
(CHEMBL1807643)Show SMILES Cc1cccc2scc(Cn3c4ccccc4n(CCCC(O)=O)c3=O)c12 Show InChI InChI=1S/C21H20N2O3S/c1-14-6-4-9-18-20(14)15(13-27-18)12-23-17-8-3-2-7-16(17)22(21(23)26)11-5-10-19(24)25/h2-4,6-9,13H,5,10-12H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM50349175

(CHEMBL1807637)Show SMILES Cc1c(C)c2cccc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C22H23N3O3/c1-14-15(2)23(3)21-16(7-6-8-17(14)21)13-25-19-10-5-4-9-18(19)24(22(25)28)12-11-20(26)27/h4-10H,11-13H2,1-3H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349176

(CHEMBL1807531)Show SMILES Cc1cccc2n(C)cc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c12 Show InChI InChI=1S/C21H21N3O3/c1-14-6-5-9-18-20(14)15(12-22(18)2)13-24-17-8-4-3-7-16(17)23(21(24)27)11-10-19(25)26/h3-9,12H,10-11,13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349192

(CHEMBL1807643)Show SMILES Cc1cccc2scc(Cn3c4ccccc4n(CCCC(O)=O)c3=O)c12 Show InChI InChI=1S/C21H20N2O3S/c1-14-6-4-9-18-20(14)15(13-27-18)12-23-17-8-3-2-7-16(17)22(21(23)26)11-5-10-19(24)25/h2-4,6-9,13H,5,10-12H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM50349177

(CHEMBL1807639)Show SMILES COc1cc(Cn2c3ccccc3n(CCC(O)=O)c2=O)c2n(C)c(C)c(C)c2c1 Show InChI InChI=1S/C23H25N3O4/c1-14-15(2)24(3)22-16(11-17(30-4)12-18(14)22)13-26-20-8-6-5-7-19(20)25(23(26)29)10-9-21(27)28/h5-8,11-12H,9-10,13H2,1-4H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50434110

(CHEMBL2381484)Show SMILES CCCC(CC(O)=O)n1c2ccccc2n(Cc2cc(Cl)cc3NC(=O)Cc23)c1=O Show InChI InChI=1S/C22H22ClN3O4/c1-2-5-15(10-21(28)29)26-19-7-4-3-6-18(19)25(22(26)30)12-13-8-14(23)9-17-16(13)11-20(27)24-17/h3-4,6-9,15H,2,5,10-12H2,1H3,(H,24,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349178

(CHEMBL1807535)Show SMILES Cc1cc(C)c2c(Cn3c4ccccc4n(CCC(O)=O)c3=O)nsc2c1 Show InChI InChI=1S/C20H19N3O3S/c1-12-9-13(2)19-14(21-27-17(19)10-12)11-23-16-6-4-3-5-15(16)22(20(23)26)8-7-18(24)25/h3-6,9-10H,7-8,11H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349179

(CHEMBL1807537)Show SMILES Cc1cc2cccc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c2n1C Show InChI InChI=1S/C21H21N3O3/c1-14-12-15-6-5-7-16(20(15)22(14)2)13-24-18-9-4-3-8-17(18)23(21(24)27)11-10-19(25)26/h3-9,12H,10-11,13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349180

(CHEMBL1807530)Show InChI InChI=1S/C21H18N2O3/c24-20(25)12-13-22-18-10-3-4-11-19(18)23(21(22)26)14-16-8-5-7-15-6-1-2-9-17(15)16/h1-11H,12-14H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349181

(CHEMBL1807536)Show InChI InChI=1S/C20H19N3O3/c1-21-11-9-14-5-4-6-15(19(14)21)13-23-17-8-3-2-7-16(17)22(20(23)26)12-10-18(24)25/h2-9,11H,10,12-13H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349182

(CHEMBL1807534)Show SMILES Cc1cccc2c(cc(Cn3c4ccccc4n(CCC(O)=O)c3=O)n12)C#N Show InChI InChI=1S/C21H18N4O3/c1-14-5-4-8-17-15(12-22)11-16(25(14)17)13-24-19-7-3-2-6-18(19)23(21(24)28)10-9-20(26)27/h2-8,11H,9-10,13H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349183

(CHEMBL1807538)Show SMILES Cc1cn(C)c2c(Cn3c4ccccc4n(CCC(O)=O)c3=O)cccc12 Show InChI InChI=1S/C21H21N3O3/c1-14-12-22(2)20-15(6-5-7-16(14)20)13-24-18-9-4-3-8-17(18)23(21(24)27)11-10-19(25)26/h3-9,12H,10-11,13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50434107

(CHEMBL2381481)Show SMILES CCCC(CC(O)=O)n1c2ccccc2n(Cc2cccc3NC(=O)Cc23)c1=O Show InChI InChI=1S/C22H23N3O4/c1-2-6-15(11-21(27)28)25-19-10-4-3-9-18(19)24(22(25)29)13-14-7-5-8-17-16(14)12-20(26)23-17/h3-5,7-10,15H,2,6,11-13H2,1H3,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50349192

(CHEMBL1807643)Show SMILES Cc1cccc2scc(Cn3c4ccccc4n(CCCC(O)=O)c3=O)c12 Show InChI InChI=1S/C21H20N2O3S/c1-14-6-4-9-18-20(14)15(13-27-18)12-23-17-8-3-2-7-16(17)22(21(23)26)11-5-10-19(24)25/h2-4,6-9,13H,5,10-12H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50434123

(CHEMBL2381479)Show SMILES OC(=O)CCCSc1nc2ccccc2n1Cc1cc(Cl)cc2NC(=O)Cc12 Show InChI InChI=1S/C20H18ClN3O3S/c21-13-8-12(14-10-18(25)22-16(14)9-13)11-24-17-5-2-1-4-15(17)23-20(24)28-7-3-6-19(26)27/h1-2,4-5,8-9H,3,6-7,10-11H2,(H,22,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349184

(CHEMBL1807527)Show InChI InChI=1S/C19H16N2O3S/c22-18(23)9-10-20-15-6-2-3-7-16(15)21(19(20)24)11-13-12-25-17-8-4-1-5-14(13)17/h1-8,12H,9-11H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50434111
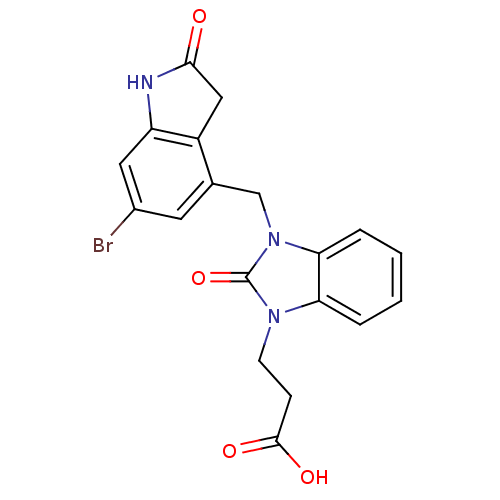
(CHEMBL2381485)Show SMILES OC(=O)CCn1c2ccccc2n(Cc2cc(Br)cc3NC(=O)Cc23)c1=O Show InChI InChI=1S/C19H16BrN3O4/c20-12-7-11(13-9-17(24)21-14(13)8-12)10-23-16-4-2-1-3-15(16)22(19(23)27)6-5-18(25)26/h1-4,7-8H,5-6,9-10H2,(H,21,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349185

(CHEMBL1807529)Show InChI InChI=1S/C20H19N3O3/c1-21-12-14(15-6-2-3-7-16(15)21)13-23-18-9-5-4-8-17(18)22(20(23)26)11-10-19(24)25/h2-9,12H,10-11,13H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349186

(CHEMBL1807539)Show SMILES Cc1cn(C)c2cccc(Cn3c4ccccc4n(CCC(O)=O)c3=O)c12 Show InChI InChI=1S/C21H21N3O3/c1-14-12-22(2)18-9-5-6-15(20(14)18)13-24-17-8-4-3-7-16(17)23(21(24)27)11-10-19(25)26/h3-9,12H,10-11,13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50434112

(CHEMBL2381487)Show SMILES OC(=O)CCn1c2ccccc2n(Cc2cc(Cl)cc3NC(=O)Cc23)c1=O Show InChI InChI=1S/C19H16ClN3O4/c20-12-7-11(13-9-17(24)21-14(13)8-12)10-23-16-4-2-1-3-15(16)22(19(23)27)6-5-18(25)26/h1-4,7-8H,5-6,9-10H2,(H,21,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50434123

(CHEMBL2381479)Show SMILES OC(=O)CCCSc1nc2ccccc2n1Cc1cc(Cl)cc2NC(=O)Cc12 Show InChI InChI=1S/C20H18ClN3O3S/c21-13-8-12(14-10-18(25)22-16(14)9-13)11-24-17-5-2-1-4-15(17)23-20(24)28-7-3-6-19(26)27/h1-2,4-5,8-9H,3,6-7,10-11H2,(H,22,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50349187

(CHEMBL1807528)Show InChI InChI=1S/C18H15N3O3S/c22-17(23)9-10-20-14-6-2-3-7-15(14)21(18(20)24)11-13-12-5-1-4-8-16(12)25-19-13/h1-8H,9-11H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human chymase after 1 hr by fluorometric assay |
Bioorg Med Chem Lett 21: 4533-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.126
BindingDB Entry DOI: 10.7270/Q23B60H0 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50434109

(CHEMBL2381483)Show SMILES CCCC(CC(O)=O)n1c2ccccc2n(Cc2cc(Br)cc3NC(=O)Cc23)c1=O Show InChI InChI=1S/C22H22BrN3O4/c1-2-5-15(10-21(28)29)26-19-7-4-3-6-18(19)25(22(26)30)12-13-8-14(23)9-17-16(13)11-20(27)24-17/h3-4,6-9,15H,2,5,10-12H2,1H3,(H,24,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using bis(succinoyl-L-alanyl-L-prolyl-L-phenylalanylamide) as substrate after 1 hr by fluorescence assay |
J Med Chem 56: 4465-81 (2013)
Article DOI: 10.1021/jm400138z
BindingDB Entry DOI: 10.7270/Q2C82BPN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data