 Found 3317 hits with Last Name = 'anthony' and Initial = 'n'
Found 3317 hits with Last Name = 'anthony' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347380

(CHEMBL1801350)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(Br)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H20BrClFN5O2/c1-14(30-24(35)25(8-9-25)31-23(34)15-10-16(26)13-29-12-15)18-7-6-17(11-20(18)28)33-21-5-3-2-4-19(21)22(27)32-33/h2-7,10-14H,8-9H2,1H3,(H,30,35)(H,31,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347351
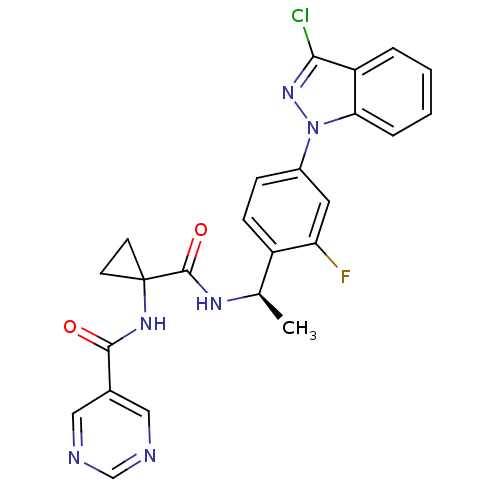
(CHEMBL1801352)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncnc1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C24H20ClFN6O2/c1-14(29-23(34)24(8-9-24)30-22(33)15-11-27-13-28-12-15)17-7-6-16(10-19(17)26)32-20-5-3-2-4-18(20)21(25)31-32/h2-7,10-14H,8-9H2,1H3,(H,29,34)(H,30,33)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347379
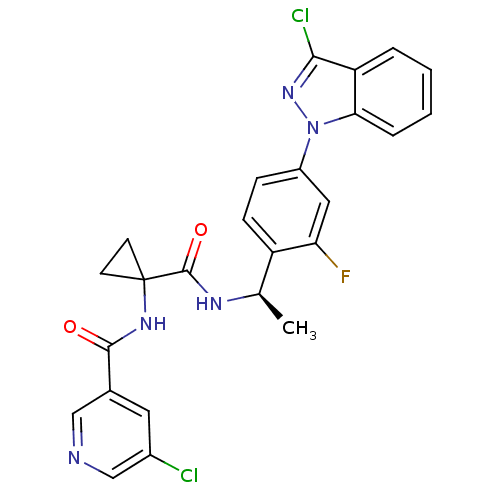
(CHEMBL1801349)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(Cl)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H20Cl2FN5O2/c1-14(30-24(35)25(8-9-25)31-23(34)15-10-16(26)13-29-12-15)18-7-6-17(11-20(18)28)33-21-5-3-2-4-19(21)22(27)32-33/h2-7,10-14H,8-9H2,1H3,(H,30,35)(H,31,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479471
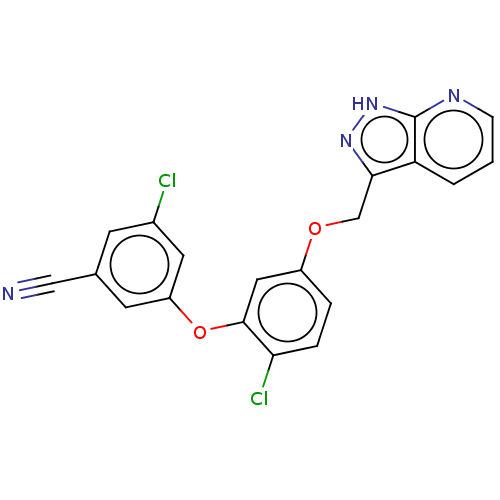
(CHEMBL491019 | MK-1107)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ncccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-6-12(10-23)7-15(8-13)28-19-9-14(3-4-17(19)22)27-11-18-16-2-1-5-24-20(16)26-25-18/h1-9H,11H2,(H,24,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co.
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay |
J Med Chem 54: 7920-33 (2011)
Article DOI: 10.1021/jm2010173
BindingDB Entry DOI: 10.7270/Q2W66PMC |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50243173
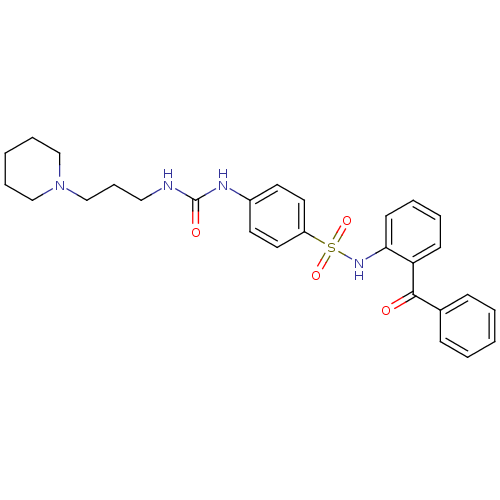
(CHEMBL487445 | N-(2-Benzoyl-phenyl)-4-[3-(3-piperi...)Show SMILES O=C(NCCCN1CCCCC1)Nc1ccc(cc1)S(=O)(=O)Nc1ccccc1C(=O)c1ccccc1 Show InChI InChI=1S/C28H32N4O4S/c33-27(22-10-3-1-4-11-22)25-12-5-6-13-26(25)31-37(35,36)24-16-14-23(15-17-24)30-28(34)29-18-9-21-32-19-7-2-8-20-32/h1,3-6,10-17,31H,2,7-9,18-21H2,(H2,29,30,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor |
J Med Chem 51: 3946-52 (2008)
Article DOI: 10.1021/jm800199h
BindingDB Entry DOI: 10.7270/Q2JH3N33 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347370

(CHEMBL1801087)Show SMILES COc1ncc(s1)C(=O)NC1(CC1)C(=O)N[C@H](C)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C24H21ClFN5O3S/c1-13(28-22(33)24(9-10-24)29-21(32)19-12-27-23(34-2)35-19)15-8-7-14(11-17(15)26)31-18-6-4-3-5-16(18)20(25)30-31/h3-8,11-13H,9-10H2,1-2H3,(H,28,33)(H,29,32)/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347376
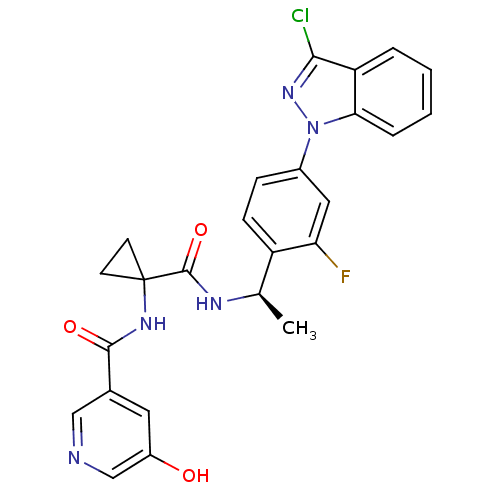
(CHEMBL1801346)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(O)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H21ClFN5O3/c1-14(29-24(35)25(8-9-25)30-23(34)15-10-17(33)13-28-12-15)18-7-6-16(11-20(18)27)32-21-5-3-2-4-19(21)22(26)31-32/h2-7,10-14,33H,8-9H2,1H3,(H,29,35)(H,30,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484029
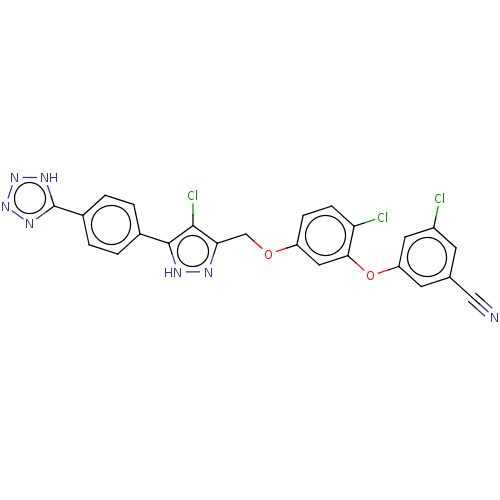
(CHEMBL1801258)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C24H14Cl3N7O2/c25-16-7-13(11-28)8-18(9-16)36-21-10-17(5-6-19(21)26)35-12-20-22(27)23(30-29-20)14-1-3-15(4-2-14)24-31-33-34-32-24/h1-10H,12H2,(H,29,30)(H,31,32,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484039
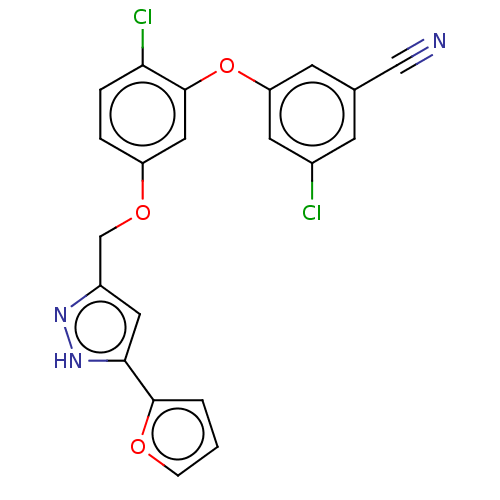
(CHEMBL1800087)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccco3)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C21H13Cl2N3O3/c22-14-6-13(11-24)7-17(8-14)29-21-10-16(3-4-18(21)23)28-12-15-9-19(26-25-15)20-2-1-5-27-20/h1-10H,12H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347378

(CHEMBL1801348)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(F)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H20ClF2N5O2/c1-14(30-24(35)25(8-9-25)31-23(34)15-10-16(27)13-29-12-15)18-7-6-17(11-20(18)28)33-21-5-3-2-4-19(21)22(26)32-33/h2-7,10-14H,8-9H2,1H3,(H,30,35)(H,31,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347373
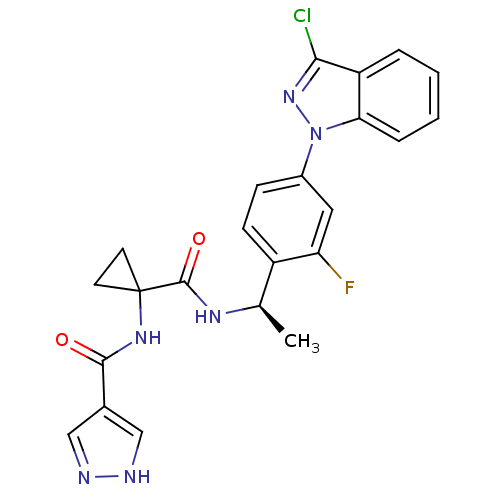
(CHEMBL1801341)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cn[nH]c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C23H20ClFN6O2/c1-13(28-22(33)23(8-9-23)29-21(32)14-11-26-27-12-14)16-7-6-15(10-18(16)25)31-19-5-3-2-4-17(19)20(24)30-31/h2-7,10-13H,8-9H2,1H3,(H,26,27)(H,28,33)(H,29,32)/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479470

(CHEMBL489586 | MK-4965)Show SMILES Nc1ccc2c(COc3ccc(Cl)c(Oc4cc(Cl)cc(c4)C#N)c3)n[nH]c2n1 Show InChI InChI=1S/C20H13Cl2N5O2/c21-12-5-11(9-23)6-14(7-12)29-18-8-13(1-3-16(18)22)28-10-17-15-2-4-19(24)25-20(15)27-26-17/h1-8H,10H2,(H3,24,25,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co.
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay |
J Med Chem 54: 7920-33 (2011)
Article DOI: 10.1021/jm2010173
BindingDB Entry DOI: 10.7270/Q2W66PMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479470

(CHEMBL489586 | MK-4965)Show SMILES Nc1ccc2c(COc3ccc(Cl)c(Oc4cc(Cl)cc(c4)C#N)c3)n[nH]c2n1 Show InChI InChI=1S/C20H13Cl2N5O2/c21-12-5-11(9-23)6-14(7-12)29-18-8-13(1-3-16(18)22)28-10-17-15-2-4-19(24)25-20(15)27-26-17/h1-8H,10H2,(H3,24,25,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs.
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 21: 7344-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.027
BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484030

(CHEMBL1801256)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccccn1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-14-7-13(11-26)8-16(9-14)31-20-10-15(4-5-17(20)24)30-12-19-21(25)22(29-28-19)18-3-1-2-6-27-18/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484635
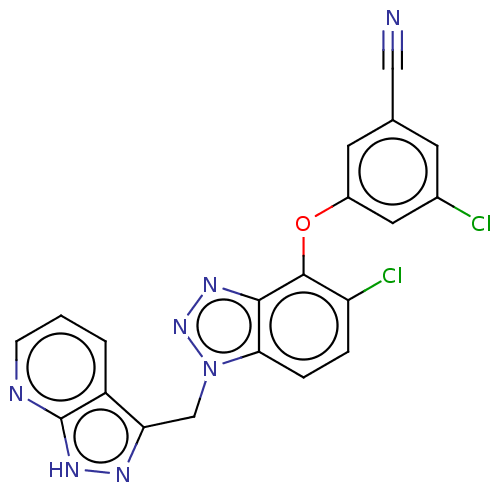
(CHEMBL1939500)Show SMILES Clc1cc(Oc2c(Cl)ccc3n(Cc4n[nH]c5ncccc45)nnc23)cc(c1)C#N Show InChI InChI=1S/C20H11Cl2N7O/c21-12-6-11(9-23)7-13(8-12)30-19-15(22)3-4-17-18(19)26-28-29(17)10-16-14-2-1-5-24-20(14)27-25-16/h1-8H,10H2,(H,24,25,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co.
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay |
J Med Chem 54: 7920-33 (2011)
Article DOI: 10.1021/jm2010173
BindingDB Entry DOI: 10.7270/Q2W66PMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347375

(CHEMBL1801345)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(C)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C26H23ClFN5O2/c1-15-11-17(14-29-13-15)24(34)31-26(9-10-26)25(35)30-16(2)19-8-7-18(12-21(19)28)33-22-6-4-3-5-20(22)23(27)32-33/h3-8,11-14,16H,9-10H2,1-2H3,(H,30,35)(H,31,34)/t16-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484496

(CHEMBL1928648)Show SMILES Clc1cc(Oc2cc(Cc3n[nH]c4ncccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O/c21-14-6-13(11-23)7-15(10-14)27-19-9-12(3-4-17(19)22)8-18-16-2-1-5-24-20(16)26-25-18/h1-7,9-10H,8H2,(H,24,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs.
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 21: 7344-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.027
BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347383

(CHEMBL1801344)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1ccc(Br)nc1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H20BrClFN5O2/c1-14(30-24(35)25(10-11-25)31-23(34)15-6-9-21(26)29-13-15)17-8-7-16(12-19(17)28)33-20-5-3-2-4-18(20)22(27)32-33/h2-9,12-14H,10-11H2,1H3,(H,30,35)(H,31,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484045
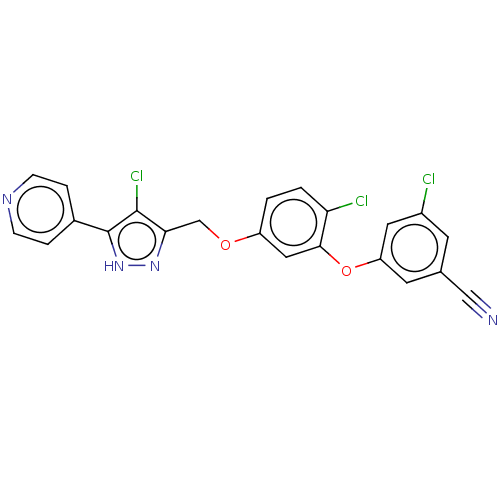
(CHEMBL1801257)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccncc1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-15-7-13(11-26)8-17(9-15)31-20-10-16(1-2-18(20)24)30-12-19-21(25)22(29-28-19)14-3-5-27-6-4-14/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484030

(CHEMBL1801256)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccccn1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-14-7-13(11-26)8-16(9-14)31-20-10-15(4-5-17(20)24)30-12-19-21(25)22(29-28-19)18-3-1-2-6-27-18/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484045

(CHEMBL1801257)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccncc1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-15-7-13(11-26)8-17(9-15)31-20-10-16(1-2-18(20)24)30-12-19-21(25)22(29-28-19)14-3-5-27-6-4-14/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347377

(CHEMBL1801347)Show SMILES COc1cncc(c1)C(=O)NC1(CC1)C(=O)N[C@H](C)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C26H23ClFN5O3/c1-15(30-25(35)26(9-10-26)31-24(34)16-11-18(36-2)14-29-13-16)19-8-7-17(12-21(19)28)33-22-6-4-3-5-20(22)23(27)32-33/h3-8,11-15H,9-10H2,1-2H3,(H,30,35)(H,31,34)/t15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347369
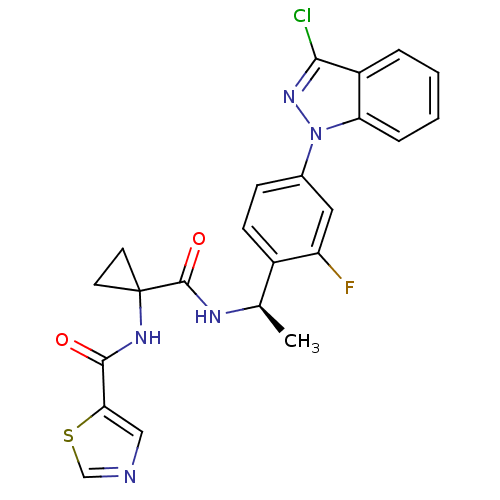
(CHEMBL1801086)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncs1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C23H19ClFN5O2S/c1-13(27-22(32)23(8-9-23)28-21(31)19-11-26-12-33-19)15-7-6-14(10-17(15)25)30-18-5-3-2-4-16(18)20(24)29-30/h2-7,10-13H,8-9H2,1H3,(H,27,32)(H,28,31)/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484632

(Mk-6186 | Mk6186)Show SMILES Clc1cc(Oc2c(Cl)ccc3n(Cc4n[nH]c5ncccc45)ncc23)cc(c1)C#N Show InChI InChI=1S/C21H12Cl2N6O/c22-13-6-12(9-24)7-14(8-13)30-20-16-10-26-29(19(16)4-3-17(20)23)11-18-15-2-1-5-25-21(15)28-27-18/h1-8,10H,11H2,(H,25,27,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co.
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay |
J Med Chem 54: 7920-33 (2011)
Article DOI: 10.1021/jm2010173
BindingDB Entry DOI: 10.7270/Q2W66PMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484030

(CHEMBL1801256)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccccn1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-14-7-13(11-26)8-16(9-14)31-20-10-15(4-5-17(20)24)30-12-19-21(25)22(29-28-19)18-3-1-2-6-27-18/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484029

(CHEMBL1801258)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C24H14Cl3N7O2/c25-16-7-13(11-28)8-18(9-16)36-21-10-17(5-6-19(21)26)35-12-20-22(27)23(30-29-20)14-1-3-15(4-2-14)24-31-33-34-32-24/h1-10H,12H2,(H,29,30)(H,31,32,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347381
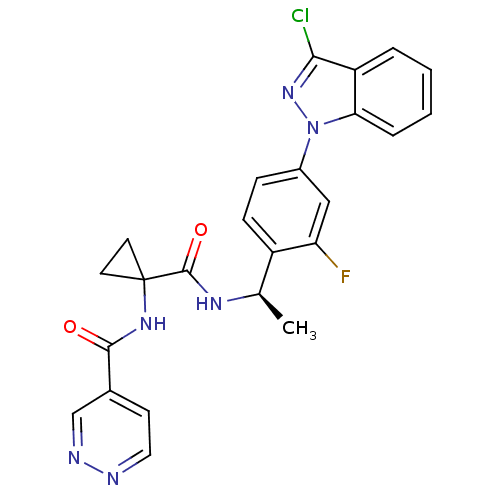
(CHEMBL1801351)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1ccnnc1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C24H20ClFN6O2/c1-14(29-23(34)24(9-10-24)30-22(33)15-8-11-27-28-13-15)17-7-6-16(12-19(17)26)32-20-5-3-2-4-18(20)21(25)31-32/h2-8,11-14H,9-10H2,1H3,(H,29,34)(H,30,33)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347363

(CHEMBL1801085)Show SMILES COc1cc(on1)C(=O)NC1(CC1)C(=O)N[C@H](C)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C24H21ClFN5O4/c1-13(27-23(33)24(9-10-24)28-22(32)19-12-20(34-2)30-35-19)15-8-7-14(11-17(15)26)31-18-6-4-3-5-16(18)21(25)29-31/h3-8,11-13H,9-10H2,1-2H3,(H,27,33)(H,28,32)/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484031
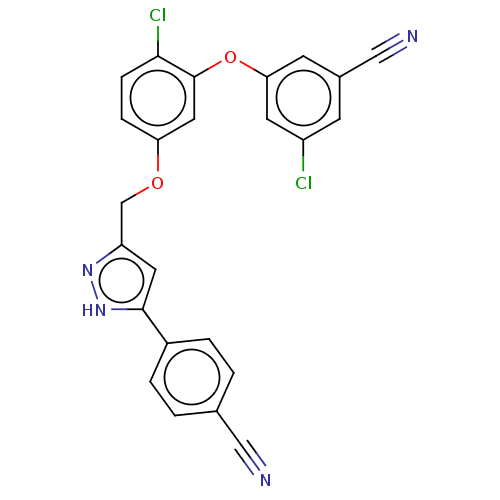
(CHEMBL1801255)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccc(cc3)C#N)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C24H14Cl2N4O2/c25-18-7-16(13-28)8-21(9-18)32-24-11-20(5-6-22(24)26)31-14-19-10-23(30-29-19)17-3-1-15(12-27)2-4-17/h1-11H,14H2,(H,29,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484032
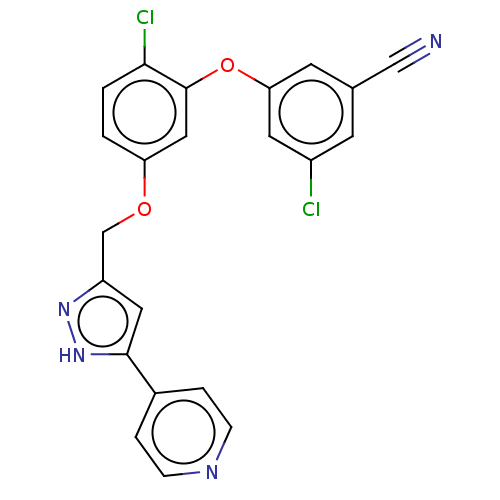
(CHEMBL1801231)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccncc3)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C22H14Cl2N4O2/c23-16-7-14(12-25)8-19(9-16)30-22-11-18(1-2-20(22)24)29-13-17-10-21(28-27-17)15-3-5-26-6-4-15/h1-11H,13H2,(H,27,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484029

(CHEMBL1801258)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C24H14Cl3N7O2/c25-16-7-13(11-28)8-18(9-16)36-21-10-17(5-6-19(21)26)35-12-20-22(27)23(30-29-20)14-1-3-15(4-2-14)24-31-33-34-32-24/h1-10H,12H2,(H,29,30)(H,31,32,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484045

(CHEMBL1801257)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccncc1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-15-7-13(11-26)8-17(9-15)31-20-10-16(1-2-18(20)24)30-12-19-21(25)22(29-28-19)14-3-5-27-6-4-14/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484040
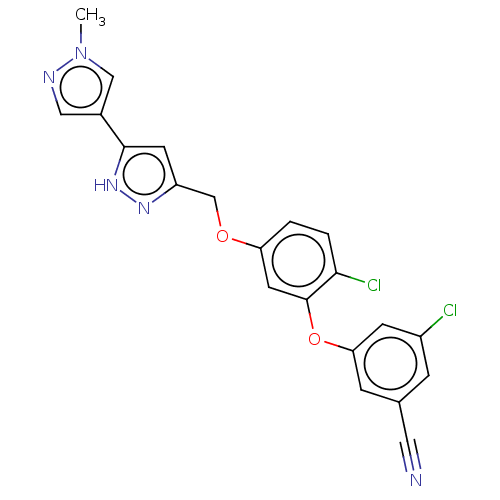
(CHEMBL1801228)Show SMILES Cn1cc(cn1)-c1cc(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]1 Show InChI InChI=1S/C21H15Cl2N5O2/c1-28-11-14(10-25-28)20-7-16(26-27-20)12-29-17-2-3-19(23)21(8-17)30-18-5-13(9-24)4-15(22)6-18/h2-8,10-11H,12H2,1H3,(H,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50243301
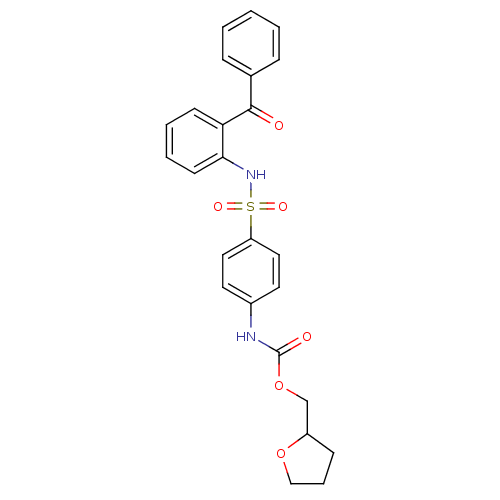
(CHEMBL454085 | Tetrahydrofuran-2-ylmethyl 4-{[(2-b...)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)Nc1ccccc1C(=O)c1ccccc1)OCC1CCCO1 Show InChI InChI=1S/C25H24N2O6S/c28-24(18-7-2-1-3-8-18)22-10-4-5-11-23(22)27-34(30,31)21-14-12-19(13-15-21)26-25(29)33-17-20-9-6-16-32-20/h1-5,7-8,10-15,20,27H,6,9,16-17H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor |
J Med Chem 51: 3946-52 (2008)
Article DOI: 10.1021/jm800199h
BindingDB Entry DOI: 10.7270/Q2JH3N33 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347372
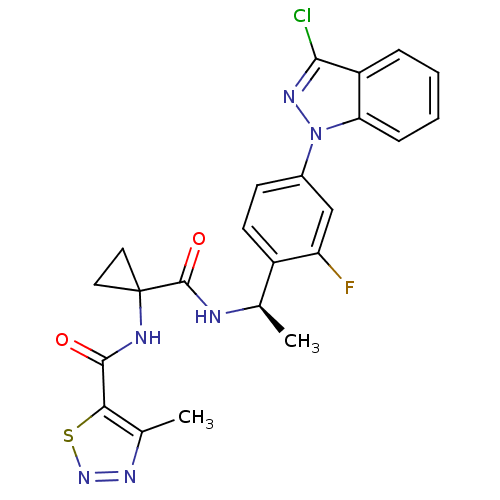
(CHEMBL1801340)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1snnc1C)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C23H20ClFN6O2S/c1-12(26-22(33)23(9-10-23)27-21(32)19-13(2)28-30-34-19)15-8-7-14(11-17(15)25)31-18-6-4-3-5-16(18)20(24)29-31/h3-8,11-12H,9-10H2,1-2H3,(H,26,33)(H,27,32)/t12-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347359
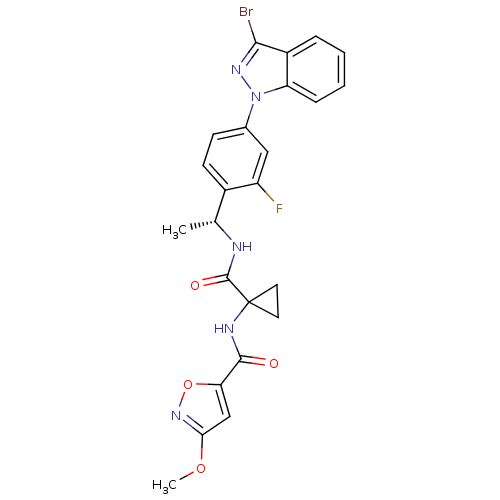
(CHEMBL1801076)Show SMILES COc1cc(on1)C(=O)NC1(CC1)C(=O)N[C@H](C)c1ccc(cc1F)-n1nc(Br)c2ccccc12 |r| Show InChI InChI=1S/C24H21BrFN5O4/c1-13(27-23(33)24(9-10-24)28-22(32)19-12-20(34-2)30-35-19)15-8-7-14(11-17(15)26)31-18-6-4-3-5-16(18)21(25)29-31/h3-8,11-13H,9-10H2,1-2H3,(H,27,33)(H,28,32)/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484022
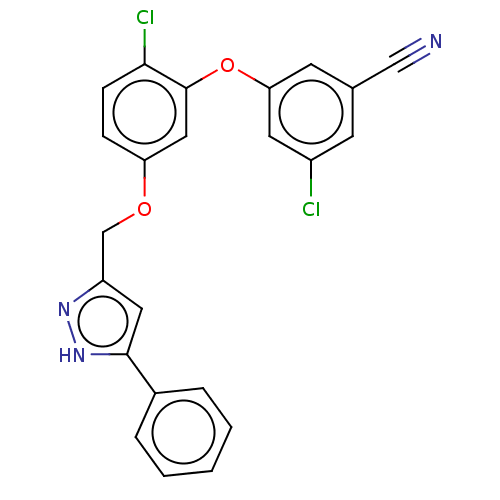
(CHEMBL1801223)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccccc3)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C23H15Cl2N3O2/c24-17-8-15(13-26)9-20(10-17)30-23-12-19(6-7-21(23)25)29-14-18-11-22(28-27-18)16-4-2-1-3-5-16/h1-12H,14H2,(H,27,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484629
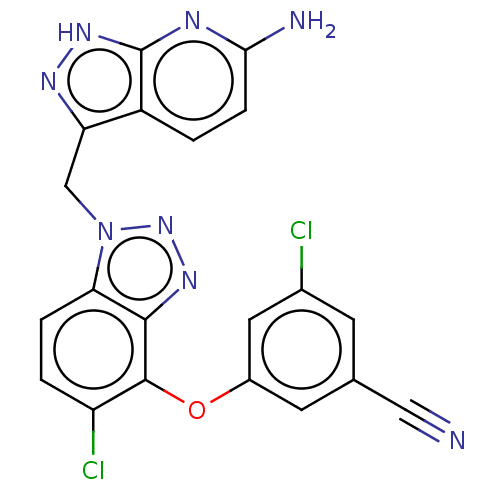
(CHEMBL1939503)Show SMILES Nc1ccc2c(Cn3nnc4c(Oc5cc(Cl)cc(c5)C#N)c(Cl)ccc34)n[nH]c2n1 Show InChI InChI=1S/C20H12Cl2N8O/c21-11-5-10(8-23)6-12(7-11)31-19-14(22)2-3-16-18(19)27-29-30(16)9-15-13-1-4-17(24)25-20(13)28-26-15/h1-7H,9H2,(H3,24,25,26,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co.
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay |
J Med Chem 54: 7920-33 (2011)
Article DOI: 10.1021/jm2010173
BindingDB Entry DOI: 10.7270/Q2W66PMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484023
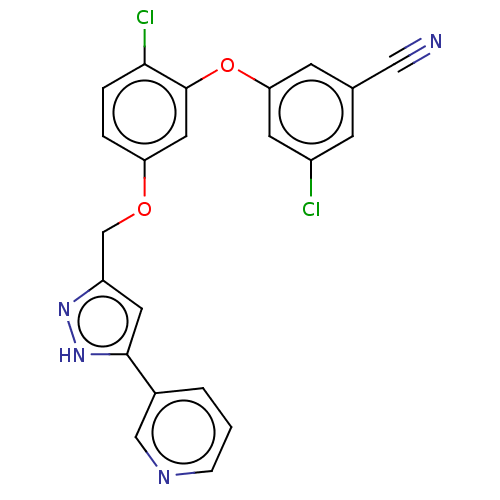
(CHEMBL1801230)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3cccnc3)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C22H14Cl2N4O2/c23-16-6-14(11-25)7-19(8-16)30-22-10-18(3-4-20(22)24)29-13-17-9-21(28-27-17)15-2-1-5-26-12-15/h1-10,12H,13H2,(H,27,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347353
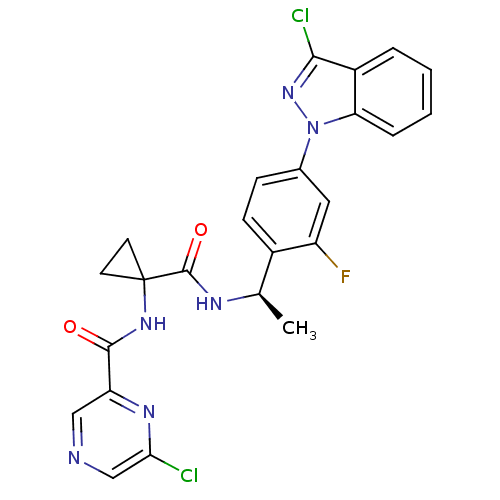
(CHEMBL1801354)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(Cl)n1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C24H19Cl2FN6O2/c1-13(29-23(35)24(8-9-24)31-22(34)18-11-28-12-20(25)30-18)15-7-6-14(10-17(15)27)33-19-5-3-2-4-16(19)21(26)32-33/h2-7,10-13H,8-9H2,1H3,(H,29,35)(H,31,34)/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50243174
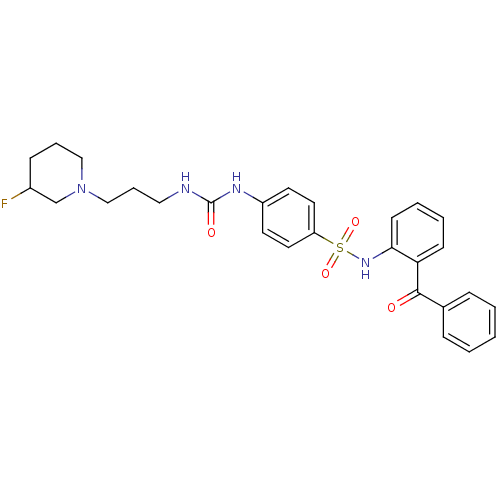
(CHEMBL488662 | N-(2-Benzoylphenyl)-4-[({[3-(3-fluo...)Show SMILES FC1CCCN(CCCNC(=O)Nc2ccc(cc2)S(=O)(=O)Nc2ccccc2C(=O)c2ccccc2)C1 Show InChI InChI=1S/C28H31FN4O4S/c29-22-10-6-18-33(20-22)19-7-17-30-28(35)31-23-13-15-24(16-14-23)38(36,37)32-26-12-5-4-11-25(26)27(34)21-8-2-1-3-9-21/h1-5,8-9,11-16,22,32H,6-7,10,17-20H2,(H2,30,31,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor |
J Med Chem 51: 3946-52 (2008)
Article DOI: 10.1021/jm800199h
BindingDB Entry DOI: 10.7270/Q2JH3N33 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347355
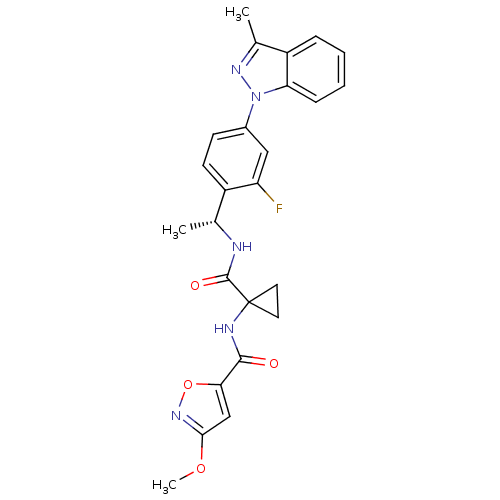
(CHEMBL1801072)Show SMILES COc1cc(on1)C(=O)NC1(CC1)C(=O)N[C@H](C)c1ccc(cc1F)-n1nc(C)c2ccccc12 |r| Show InChI InChI=1S/C25H24FN5O4/c1-14(27-24(33)25(10-11-25)28-23(32)21-13-22(34-3)30-35-21)17-9-8-16(12-19(17)26)31-20-7-5-4-6-18(20)15(2)29-31/h4-9,12-14H,10-11H2,1-3H3,(H,27,33)(H,28,32)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50243302

(CHEMBL454086 | Tetrahydrofuran-2-ylmethyl[4-({[2-(...)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)Nc1ccccc1C(=O)c1ccccn1)OCC1CCCO1 Show InChI InChI=1S/C24H23N3O6S/c28-23(22-9-3-4-14-25-22)20-7-1-2-8-21(20)27-34(30,31)19-12-10-17(11-13-19)26-24(29)33-16-18-6-5-15-32-18/h1-4,7-14,18,27H,5-6,15-16H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor |
J Med Chem 51: 3946-52 (2008)
Article DOI: 10.1021/jm800199h
BindingDB Entry DOI: 10.7270/Q2JH3N33 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50243302

(CHEMBL454086 | Tetrahydrofuran-2-ylmethyl[4-({[2-(...)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)Nc1ccccc1C(=O)c1ccccn1)OCC1CCCO1 Show InChI InChI=1S/C24H23N3O6S/c28-23(22-9-3-4-14-25-22)20-7-1-2-8-21(20)27-34(30,31)19-12-10-17(11-13-19)26-24(29)33-16-18-6-5-15-32-18/h1-4,7-14,18,27H,5-6,15-16H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor |
J Med Chem 51: 3946-52 (2008)
Article DOI: 10.1021/jm800199h
BindingDB Entry DOI: 10.7270/Q2JH3N33 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347382
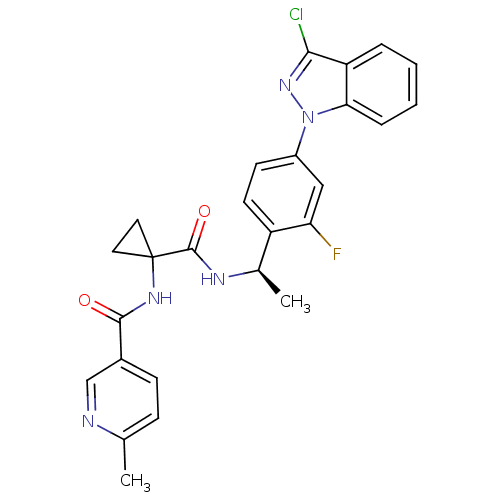
(CHEMBL1801343)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1ccc(C)nc1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C26H23ClFN5O2/c1-15-7-8-17(14-29-15)24(34)31-26(11-12-26)25(35)30-16(2)19-10-9-18(13-21(19)28)33-22-6-4-3-5-20(22)23(27)32-33/h3-10,13-14,16H,11-12H2,1-2H3,(H,30,35)(H,31,34)/t16-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347374
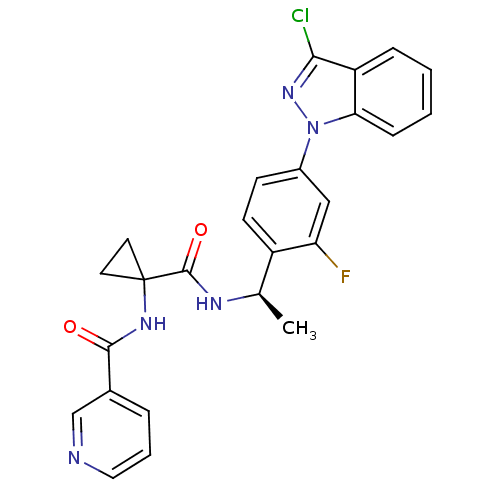
(CHEMBL1801342)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cccnc1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H21ClFN5O2/c1-15(29-24(34)25(10-11-25)30-23(33)16-5-4-12-28-14-16)18-9-8-17(13-20(18)27)32-21-7-3-2-6-19(21)22(26)31-32/h2-9,12-15H,10-11H2,1H3,(H,29,34)(H,30,33)/t15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484495
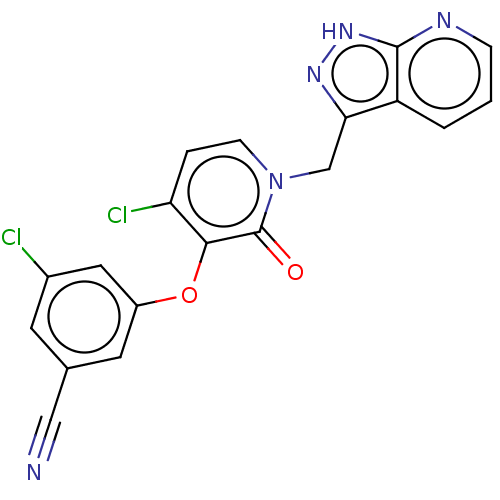
(CHEMBL1928645)Show SMILES Clc1cc(Oc2c(Cl)ccn(Cc3n[nH]c4ncccc34)c2=O)cc(c1)C#N Show InChI InChI=1S/C19H11Cl2N5O2/c20-12-6-11(9-22)7-13(8-12)28-17-15(21)3-5-26(19(17)27)10-16-14-2-1-4-23-18(14)25-24-16/h1-8H,10H2,(H,23,24,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs.
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 21: 7344-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.027
BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484039

(CHEMBL1800087)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccco3)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C21H13Cl2N3O3/c22-14-6-13(11-24)7-17(8-14)29-21-10-16(3-4-18(21)23)28-12-15-9-19(26-25-15)20-2-1-5-27-20/h1-10H,12H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data