

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060725 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM85212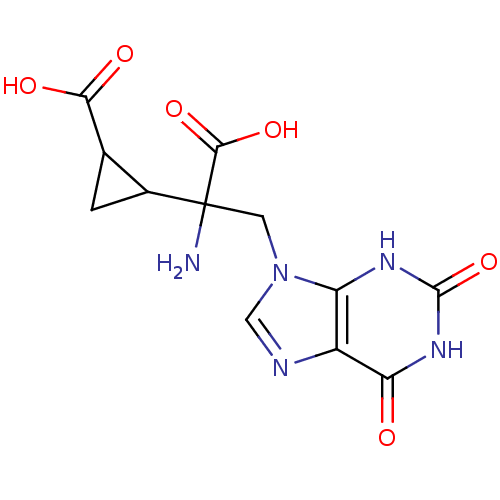 (CAS_5311260 | LY341495 | NSC_5311260) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 453-60 (2001) BindingDB Entry DOI: 10.7270/Q2V986M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065468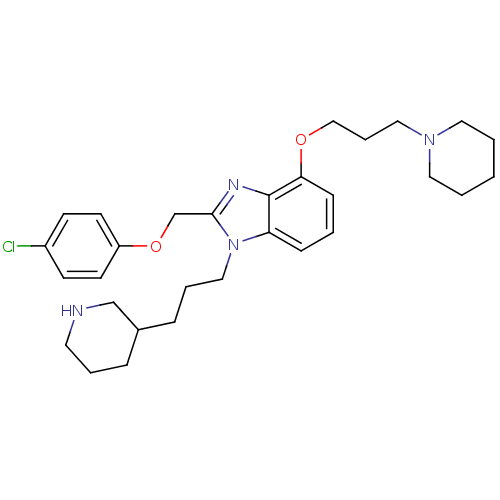 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065469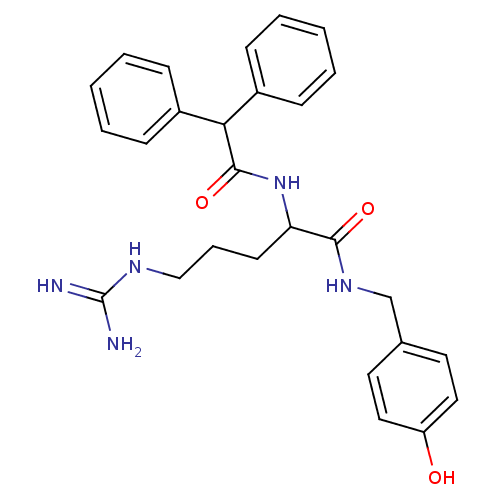 (2-Diphenylacetylamino-5-guanidino-pentanoic acid 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177564 (CHEMBL380540 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065463 (2-(4-Chloro-phenoxymethyl)-4-((S)-3-piperidin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177560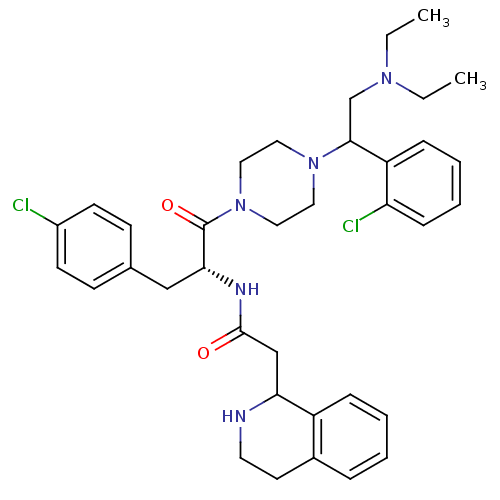 (CHEMBL206042 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177560 (CHEMBL206042 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50074749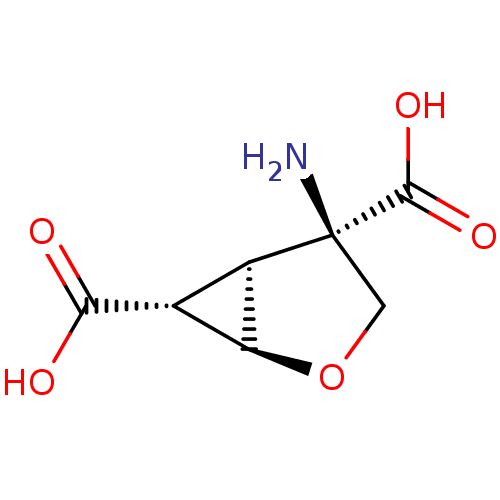 ((1R,4R,5S,6R)-4-Amino-2-oxa-bicyclo[3.1.0]hexane-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 453-60 (2001) BindingDB Entry DOI: 10.7270/Q2V986M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065460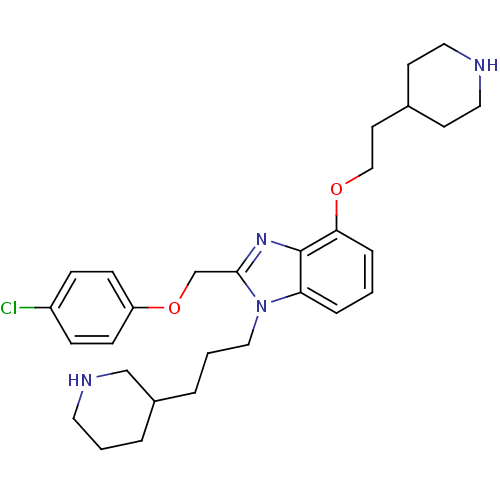 (2-(4-Chloro-phenoxymethyl)-4-(2-piperidin-4-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177563 (CHEMBL380855 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177557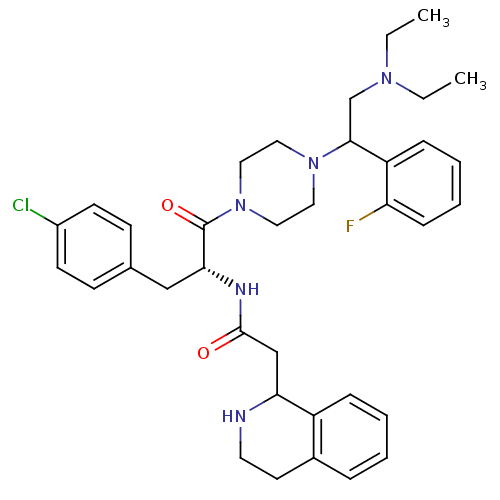 (CHEMBL380365 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065477 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-2-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065467 (2-(4-Chloro-phenoxymethyl)-4-((S)-3-piperidin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177565 (CHEMBL380858 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065466 (2-(4-Chloro-phenoxymethyl)-4-(2-piperidin-3-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065459 (2-(4-Chloro-phenoxymethyl)-4-((R)-3-piperidin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065479 (2-(4-Chloro-phenoxymethyl)-4-(2-piperidin-2-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065461 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-3-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177561 ((R)-N-((R)-3-(4-chlorophenyl)-1-(4-(2-(diethylamin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065462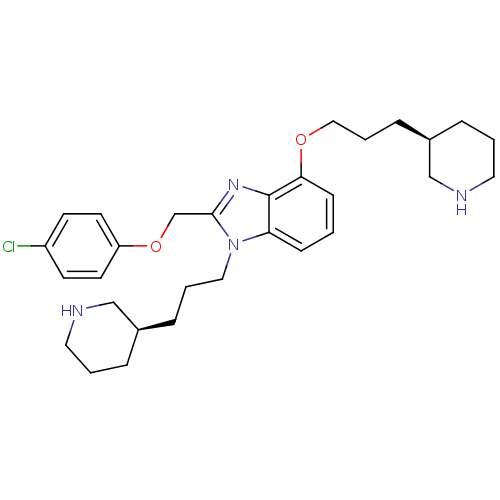 (2-(4-Chloro-phenoxymethyl)-4-((R)-3-piperidin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065475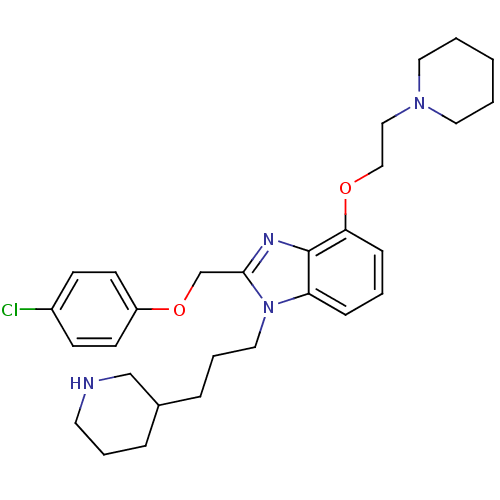 (2-(4-Chloro-phenoxymethyl)-4-(2-piperidin-1-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50034503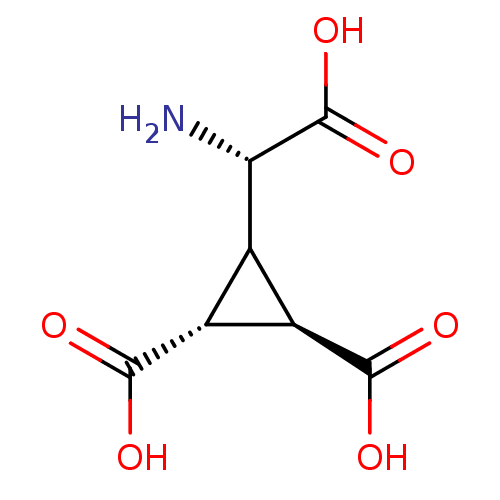 ((1R,2R)-3-((S)-Amino-carboxy-methyl)-cyclopropane-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 453-60 (2001) BindingDB Entry DOI: 10.7270/Q2V986M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177558 (CHEMBL438432 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50013055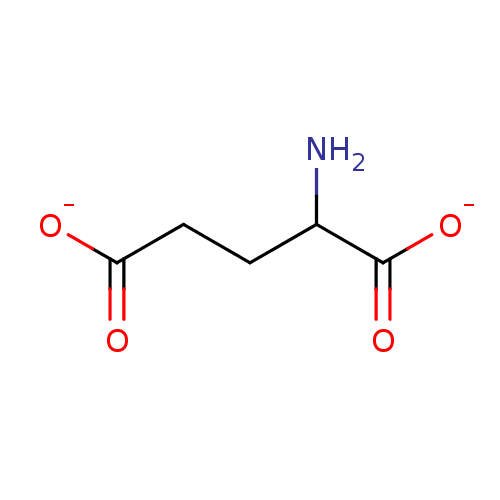 (2-aminopentanedioateglutamate | L-Glutamate | glut...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 453-60 (2001) BindingDB Entry DOI: 10.7270/Q2V986M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177562 (CHEMBL206840 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065465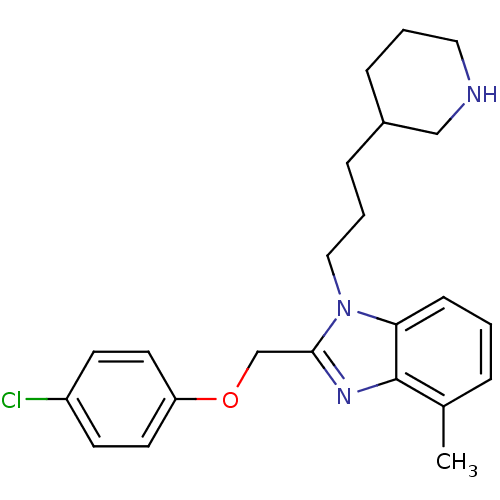 (2-(4-Chloro-phenoxymethyl)-4-methyl-1-(3-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177559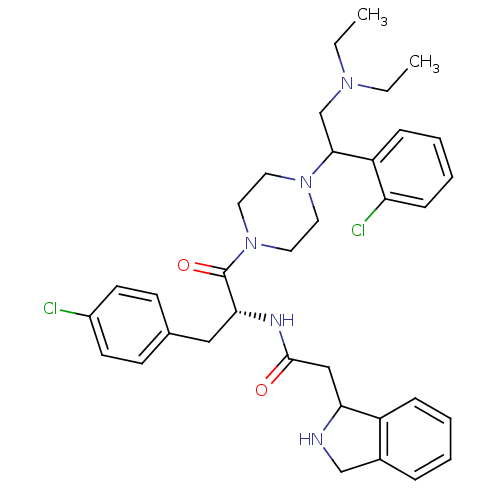 (CHEMBL379352 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50177559 (CHEMBL379352 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065476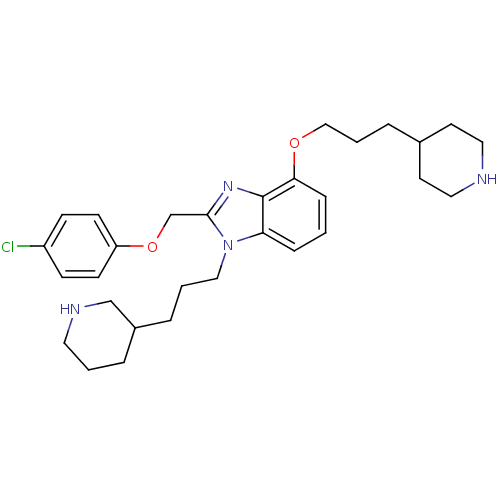 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-4-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50004899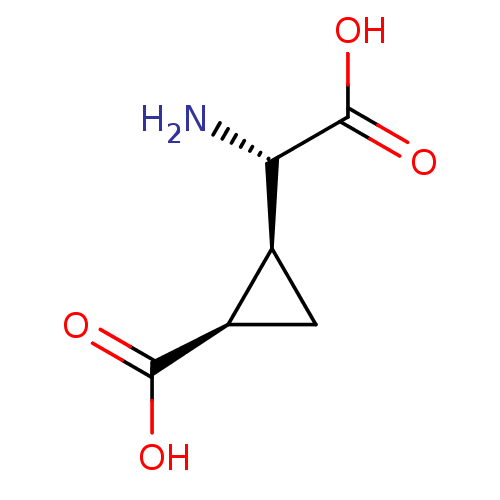 ((1R,2S)-2-((S)-Amino-carboxy-methyl)-cyclopropanec...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 334 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 453-60 (2001) BindingDB Entry DOI: 10.7270/Q2V986M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065474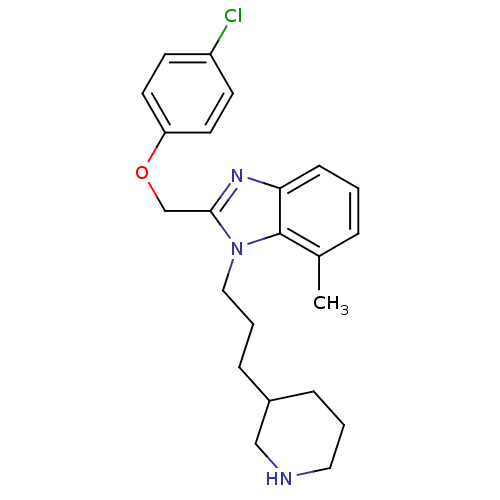 (2-(4-Chloro-phenoxymethyl)-7-methyl-1-(3-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50177564 (CHEMBL380540 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC5R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065481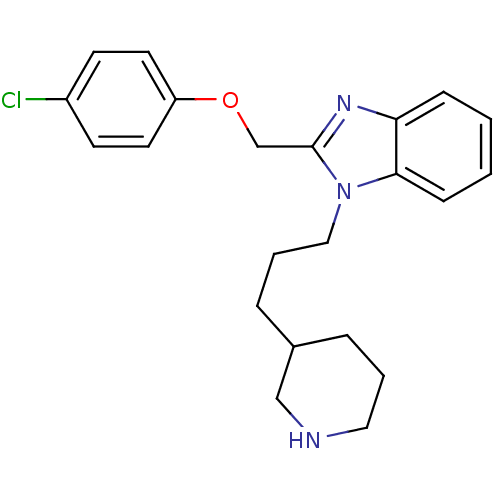 (2-(4-Chloro-phenoxymethyl)-1-(3-piperidin-3-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50177557 (CHEMBL380365 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC5R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50177562 (CHEMBL206840 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC5R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065471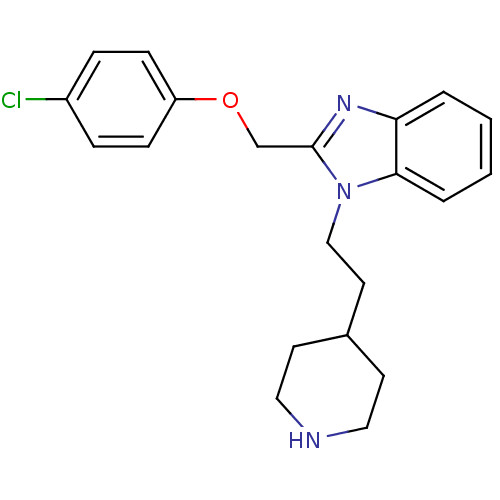 (2-(4-Chloro-phenoxymethyl)-1-(2-piperidin-4-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50177561 ((R)-N-((R)-3-(4-chlorophenyl)-1-(4-(2-(diethylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC5R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065472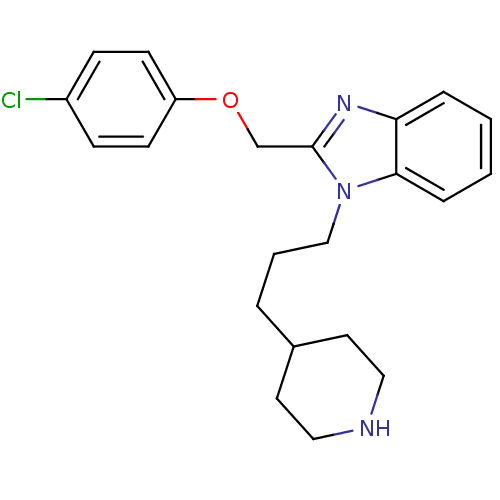 (2-(4-Chloro-phenoxymethyl)-1-(3-piperidin-4-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50177563 (CHEMBL380855 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC5R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065483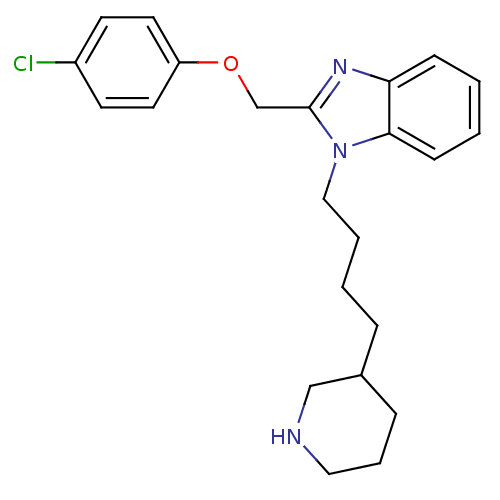 (2-(4-Chloro-phenoxymethyl)-1-(4-piperidin-3-yl-but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065473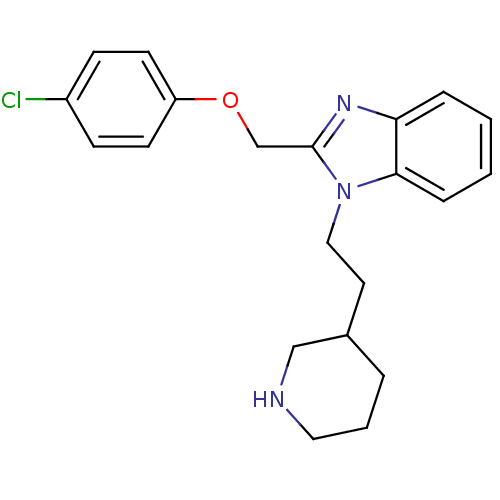 (2-(4-Chloro-phenoxymethyl)-1-(2-piperidin-3-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065464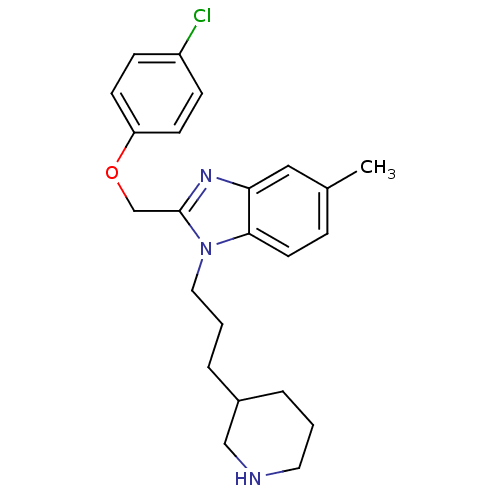 (2-(4-Chloro-phenoxymethyl)-5-methyl-1-(3-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50177558 (CHEMBL438432 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC5R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50177562 (CHEMBL206840 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC3R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50177564 (CHEMBL380540 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC3R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50177565 (CHEMBL380858 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]NDP-alpha-MSH from cloned human MC5R expressed in HEK293 cell | Bioorg Med Chem Lett 16: 2341-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.103 BindingDB Entry DOI: 10.7270/Q2NS0TF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50049747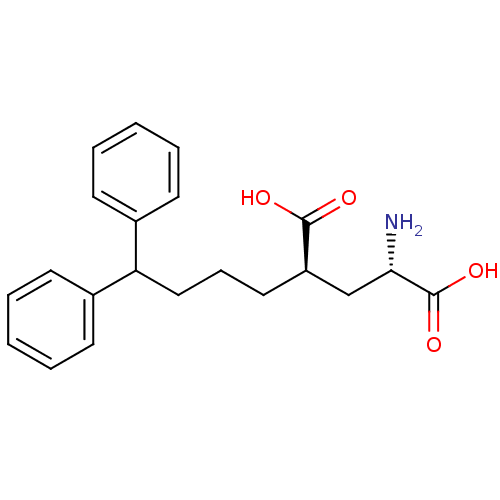 ((2S,4R)-2-Amino-4-(4,4-diphenyl-butyl)-pentanedioi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 453-60 (2001) BindingDB Entry DOI: 10.7270/Q2V986M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060727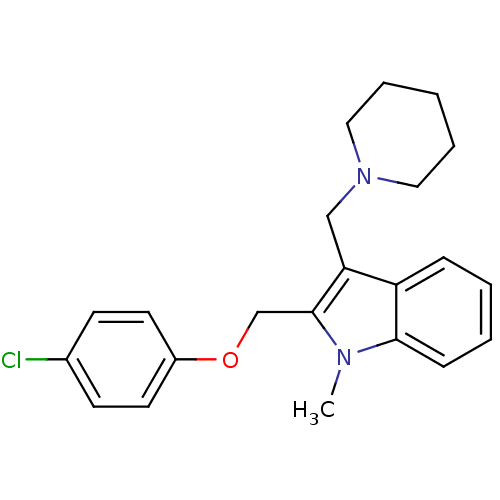 (2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50052398 ((2R,4R)-4-aminopyrrolidine-2,4-dicarboxylic acid |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 453-60 (2001) BindingDB Entry DOI: 10.7270/Q2V986M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 165 total ) | Next | Last >> |