

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50072528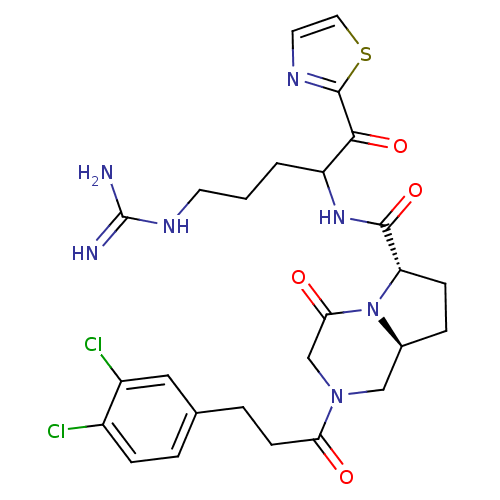 ((6S,8aS)-2-[3-(3,4-Dichloro-phenyl)-propionyl]-4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072536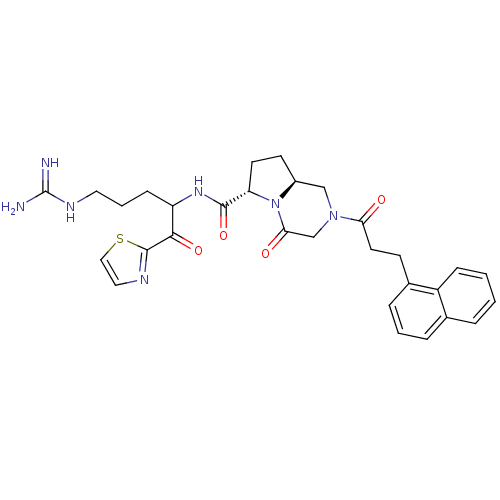 ((6S,8aS)-2-(3-Naphthalen-1-yl-propionyl)-4-oxo-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072524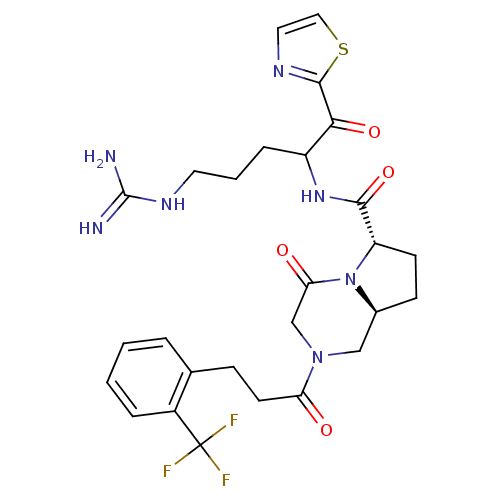 ((6S,8aS)-4-Oxo-2-[3-(2-trifluoromethyl-phenyl)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072527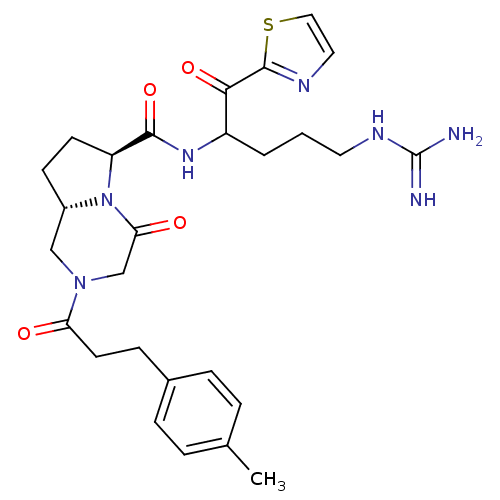 ((6S,8aS)-4-Oxo-2-(3-p-tolyl-propionyl)-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072537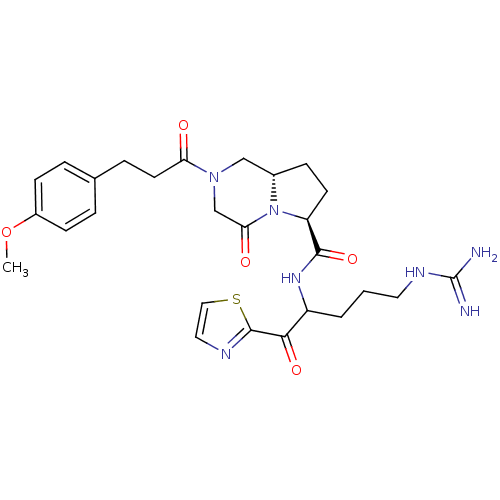 ((6S,8aS)-2-[3-(4-Methoxy-phenyl)-propionyl]-4-oxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072534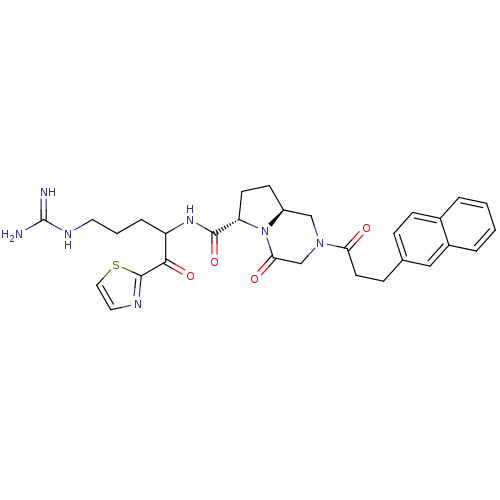 ((6S,8aS)-2-(3-Naphthalen-2-yl-propionyl)-4-oxo-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072532 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072520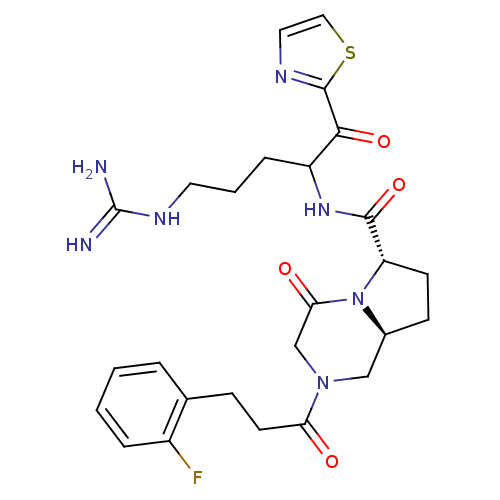 ((6S,8aS)-2-[3-(2-Fluoro-phenyl)-propionyl]-4-oxo-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50426284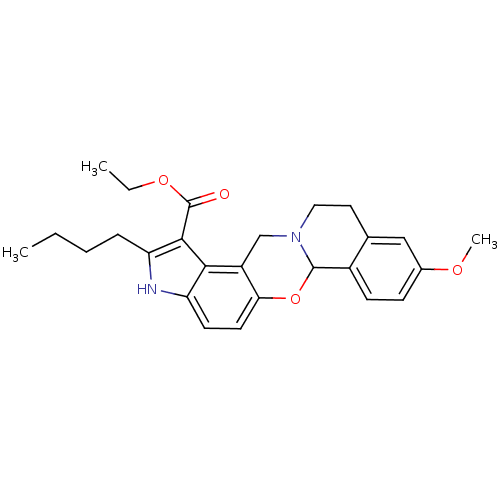 (CHEMBL326450) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473815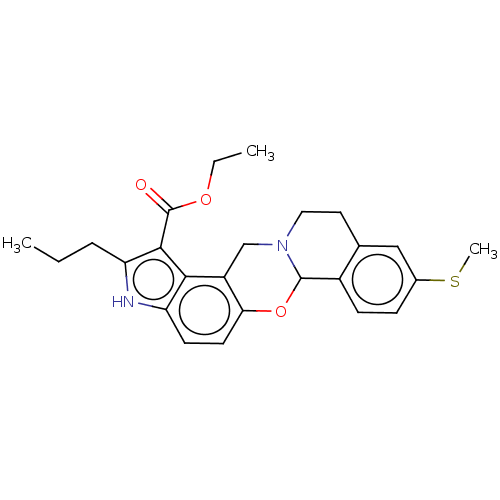 (CHEMBL104693) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072533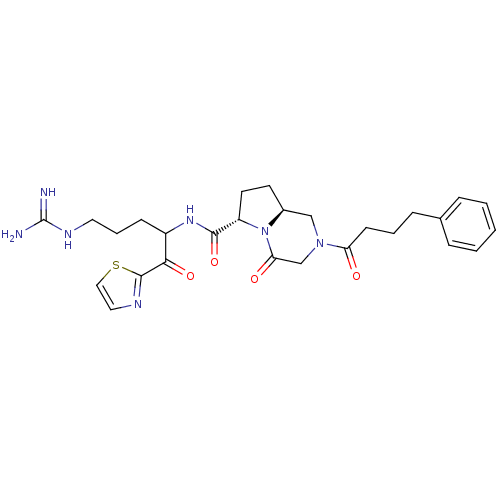 ((6S,8aS)-4-Oxo-2-(4-phenyl-butyryl)-octahydro-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080924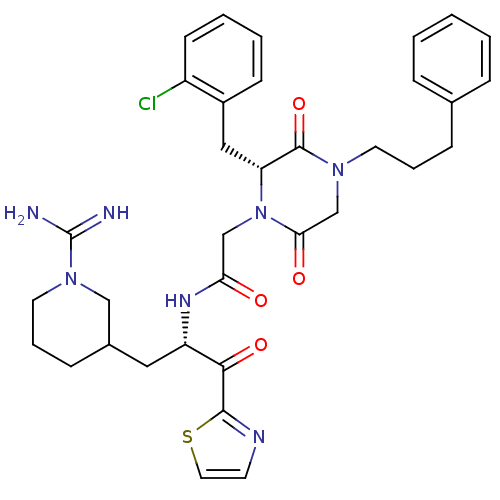 (CHEMBL83260 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 2503-8 (1999) BindingDB Entry DOI: 10.7270/Q2028QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473807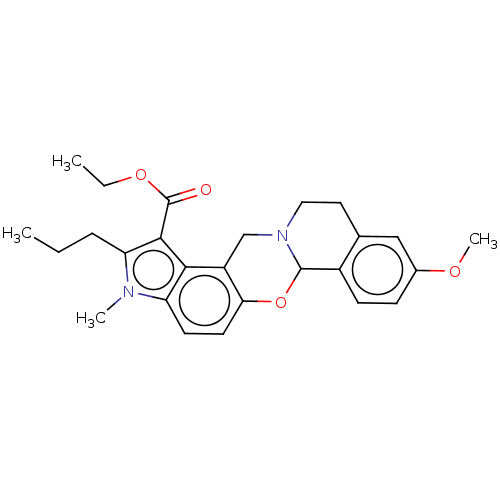 (CHEMBL106224) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473806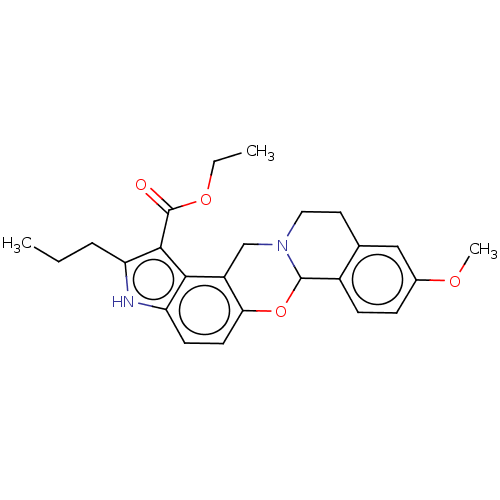 (CHEMBL320465) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473810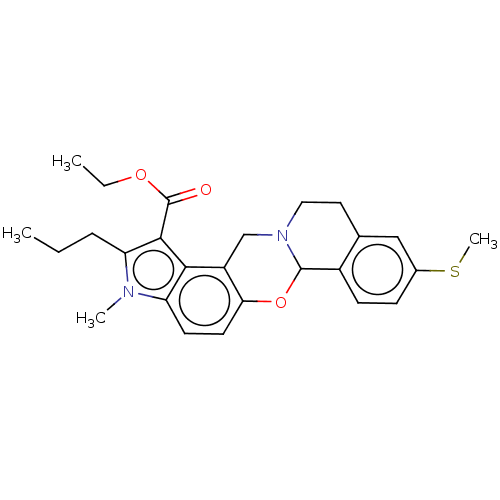 (CHEMBL280220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072530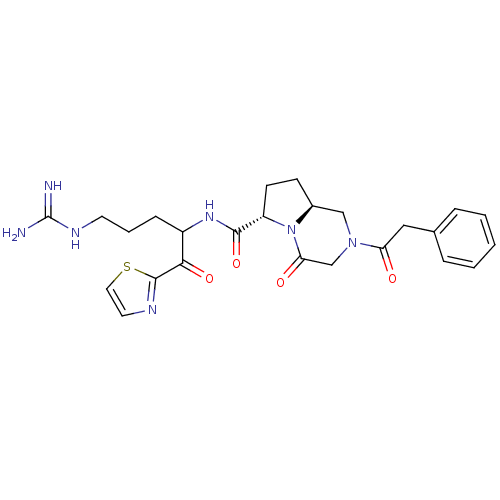 ((6S,8aS)-4-Oxo-2-phenylacetyl-octahydro-pyrrolo[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473811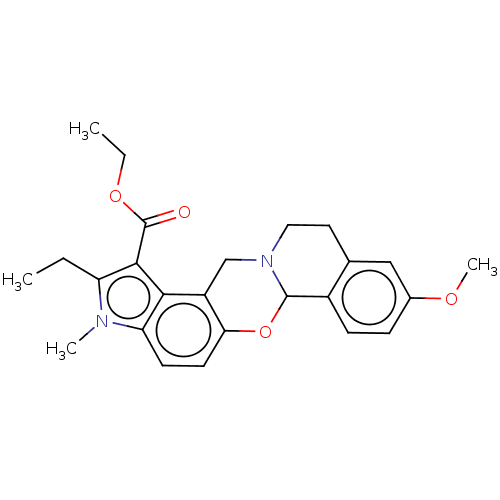 (CHEMBL106218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072519 ((6S,8aS)-2-[(S)-2-Amino-3-(4-fluoro-phenyl)-propio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473808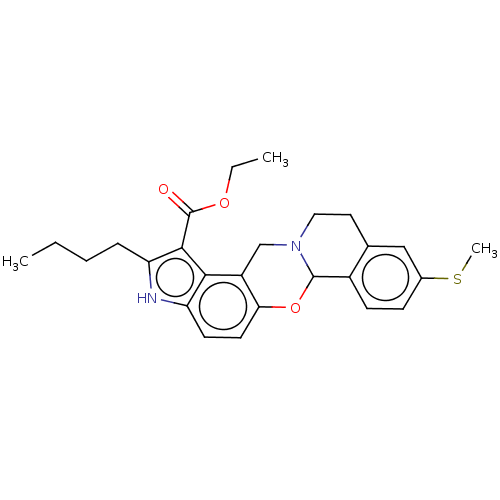 (CHEMBL318995) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072541 ((6S,8aS)-4-Oxo-2-(3-pyridin-2-yl-propionyl)-octahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080911 (2-((S)-4-Benzyl-2-naphthalen-2-ylmethyl-3,6-dioxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against thrombin was determined | Bioorg Med Chem Lett 9: 2503-8 (1999) BindingDB Entry DOI: 10.7270/Q2028QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473798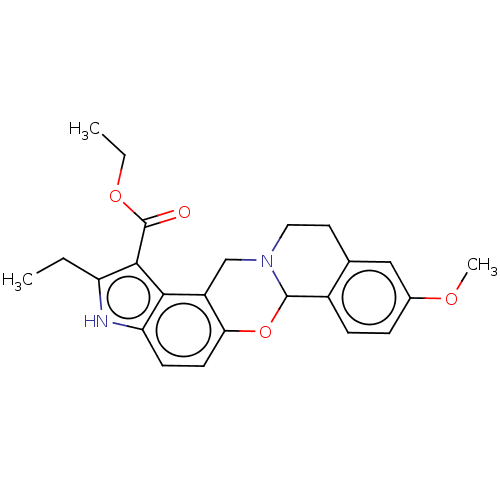 (CHEMBL105148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072529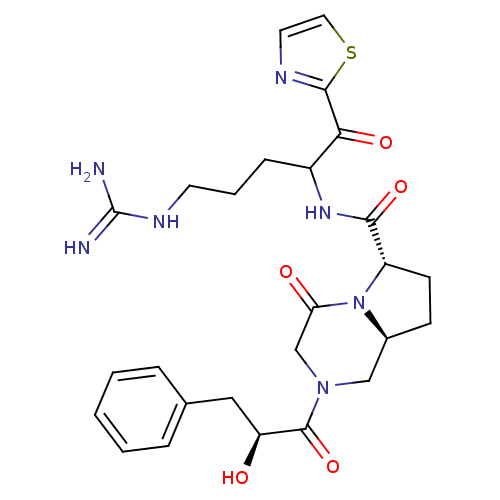 ((6S,8aS)-2-((S)-2-Hydroxy-3-phenyl-propionyl)-4-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072535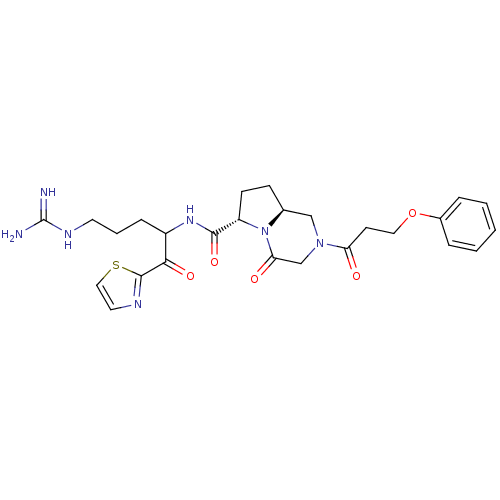 ((6S,8aS)-4-Oxo-2-(3-phenoxy-propionyl)-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50084137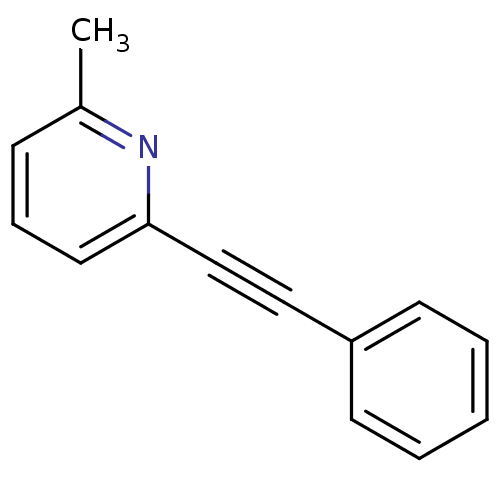 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from mGlu5 receptor in rat brain | Bioorg Med Chem Lett 17: 4415-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.030 BindingDB Entry DOI: 10.7270/Q28G8KDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50071171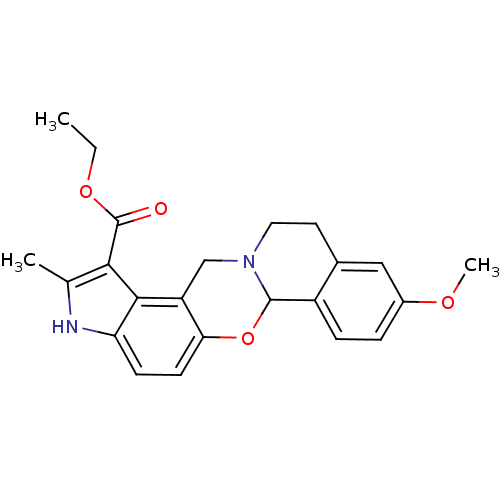 (9-Methoxy-2-methyl-11,12-dihydro-3H,6aH,13H-6-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473796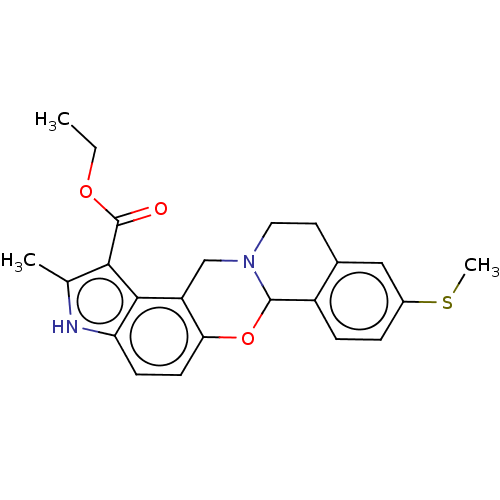 (CHEMBL105122) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080920 (CHEMBL313769 | N-[(S)-1-(1-Carbamimidoyl-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 2503-8 (1999) BindingDB Entry DOI: 10.7270/Q2028QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473817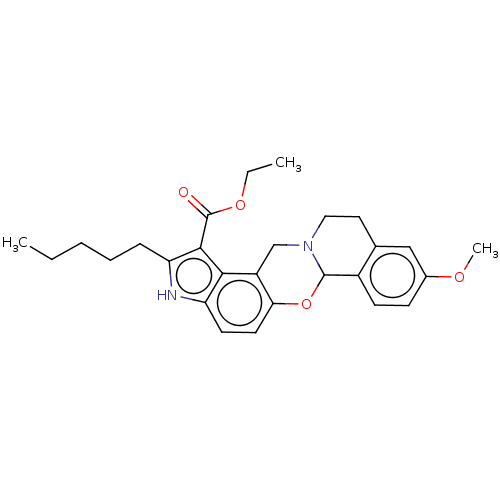 (CHEMBL104210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473812 (CHEMBL105160) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473805 (CHEMBL107444) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50473813 (CHEMBL420662) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M4 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072521 ((6S,8aS)-4-Oxo-2-(2-phenoxy-acetyl)-octahydro-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072538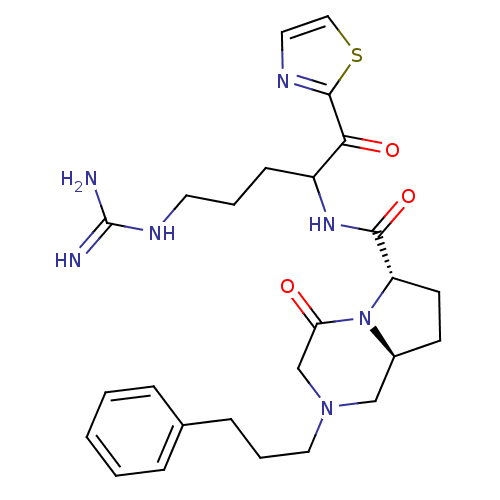 ((6S,8aS)-4-Oxo-2-(3-phenyl-propyl)-octahydro-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072526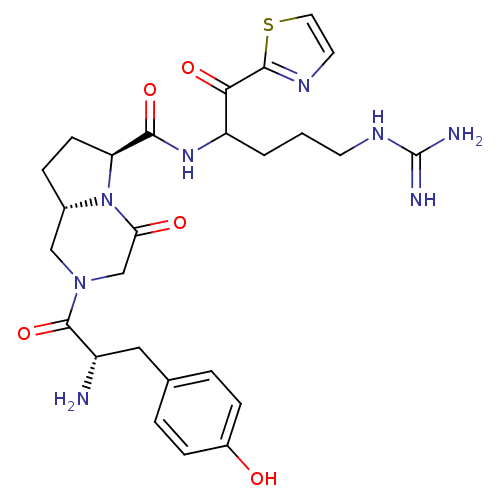 ((6S,8aS)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072526 ((6S,8aS)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080916 (CHEMBL84084 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 2503-8 (1999) BindingDB Entry DOI: 10.7270/Q2028QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50216746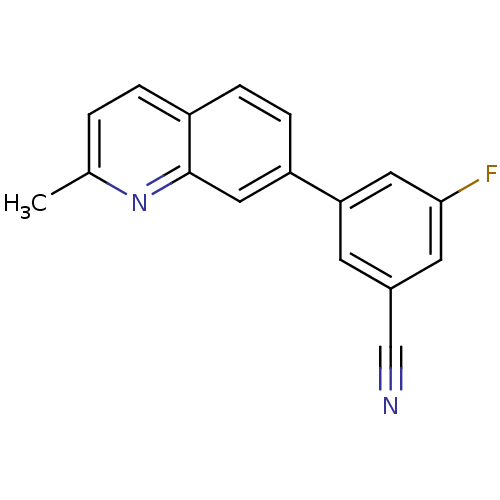 (3-fluoro-5-(2-methylquinolin-7-yl)benzonitrile | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from mGlu5 receptor in rat brain | Bioorg Med Chem Lett 17: 4415-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.030 BindingDB Entry DOI: 10.7270/Q28G8KDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072539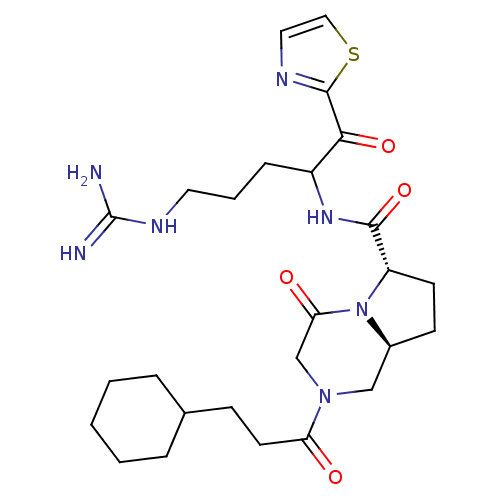 ((6S,8aS)-2-(3-Cyclohexyl-propionyl)-4-oxo-octahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072540 ((6S,8aS)-2-Benzoyl-4-oxo-octahydro-pyrrolo[1,2-a]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072525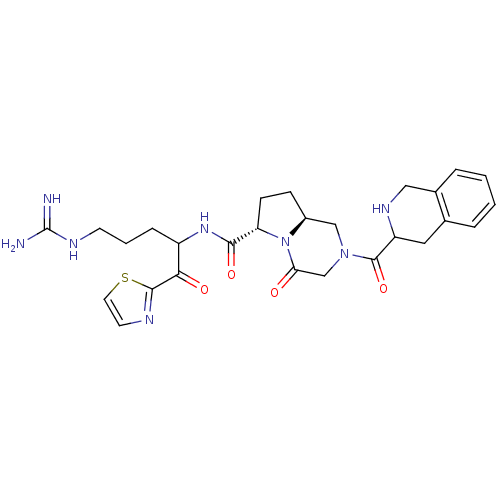 ((6S,8aS)-4-Oxo-2-(1,2,3,4-tetrahydro-isoquinoline-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080915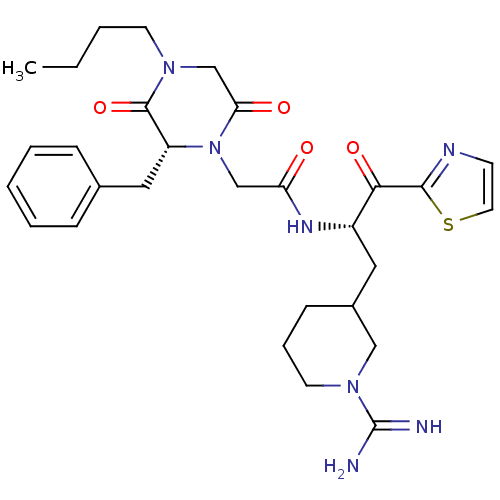 (2-((R)-2-Benzyl-4-butyl-3,6-dioxo-piperazin-1-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 2503-8 (1999) BindingDB Entry DOI: 10.7270/Q2028QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM23346 (1-{4,6-dimethoxy-12-oxo-11,17-diazatetracyclo[11.3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -41.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 15 |
Pfizer | Assay Description PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... | J Med Chem 49: 1202-6 (2006) Article DOI: 10.1021/jm049161u BindingDB Entry DOI: 10.7270/Q2BR8QG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM23345 (1-(3,4-dichlorophenyl)-2-{4,6-dimethoxy-12-oxo-11,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -41.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 15 |
Pfizer | Assay Description PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... | J Med Chem 49: 1202-6 (2006) Article DOI: 10.1021/jm049161u BindingDB Entry DOI: 10.7270/Q2BR8QG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM23347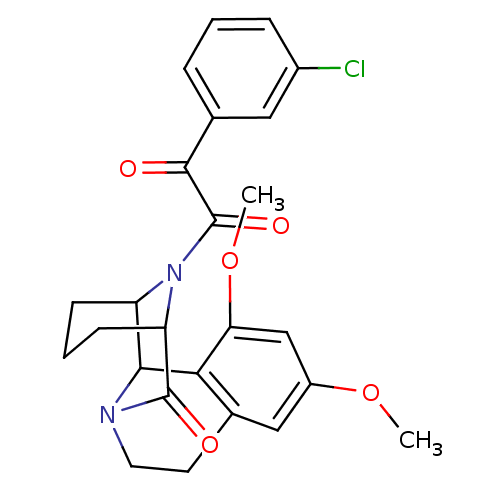 (1-(3-chlorophenyl)-2-{4,6-dimethoxy-12-oxo-11,17-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | -40.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 15 |
Pfizer | Assay Description PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... | J Med Chem 49: 1202-6 (2006) Article DOI: 10.1021/jm049161u BindingDB Entry DOI: 10.7270/Q2BR8QG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50473816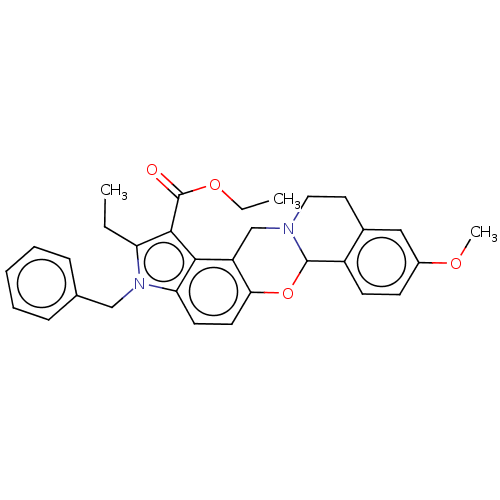 (CHEMBL106219) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human muscarinic acetylcholine receptor M3 in transfected CHO cells. | J Med Chem 45: 3094-102 (2002) Article DOI: 10.1021/jm011116o BindingDB Entry DOI: 10.7270/Q2BZ68S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072518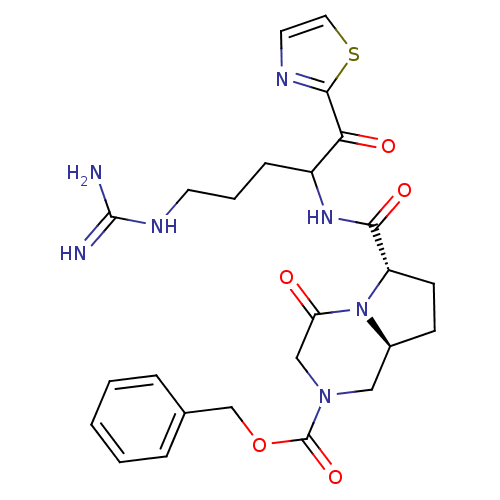 ((6S,8aS)-6-[4-Guanidino-1-(thiazole-2-carbonyl)-bu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Bos taurus (bovine)) | BDBM23397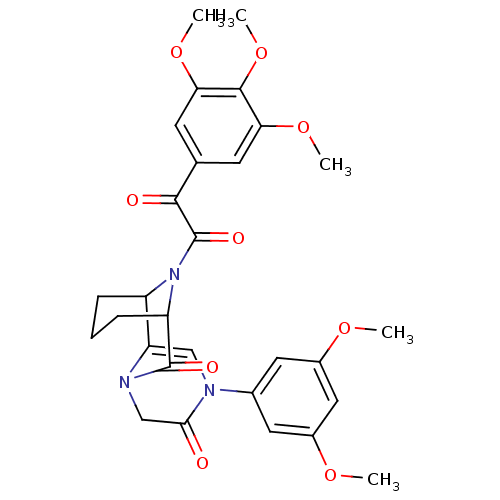 (4-(3,5-dimethoxyphenyl)-13-[2-oxo-2-(3,4,5-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... | J Med Chem 49: 1202-6 (2006) Article DOI: 10.1021/jm049161u BindingDB Entry DOI: 10.7270/Q2BR8QG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Bos taurus (bovine)) | BDBM23372 (17-[(3-bromobenzene)sulfonyl]-4,6-dimethoxy-11,17-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observat... | J Med Chem 49: 1202-6 (2006) Article DOI: 10.1021/jm049161u BindingDB Entry DOI: 10.7270/Q2BR8QG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080930 (2-[(R)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 2503-8 (1999) BindingDB Entry DOI: 10.7270/Q2028QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1133 total ) | Next | Last >> |