 Found 1112 hits with Last Name = 'awad' and Initial = 'l'
Found 1112 hits with Last Name = 'awad' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50432208
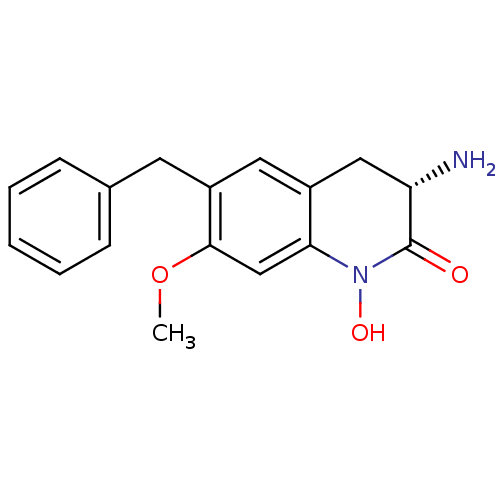
(CHEMBL2347110)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Cc1ccccc1 |r| Show InChI InChI=1S/C17H18N2O3/c1-22-16-10-15-12(9-14(18)17(20)19(15)21)8-13(16)7-11-5-3-2-4-6-11/h2-6,8,10,14,21H,7,9,18H2,1H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426340
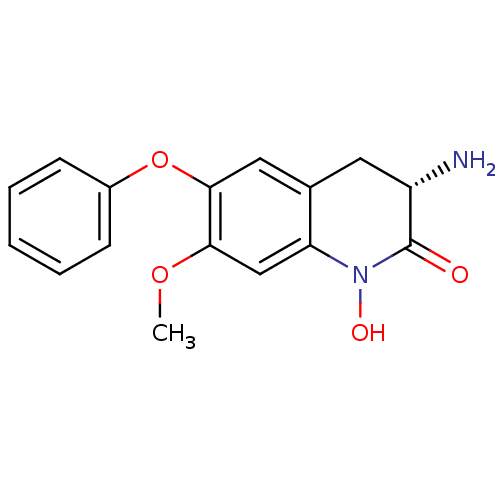
(CHEMBL2321943)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Oc1ccccc1 |r| Show InChI InChI=1S/C16H16N2O4/c1-21-14-9-13-10(7-12(17)16(19)18(13)20)8-15(14)22-11-5-3-2-4-6-11/h2-6,8-9,12,20H,7,17H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107730
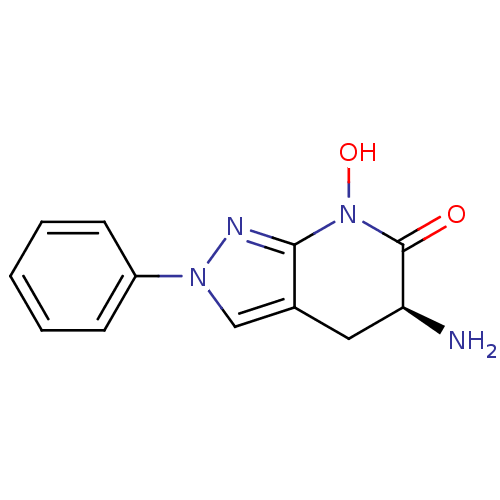
(CHEMBL2347108 | US8933095, 14)Show InChI InChI=1S/C12H12N4O2/c13-10-6-8-7-15(9-4-2-1-3-5-9)14-11(8)16(18)12(10)17/h1-5,7,10,18H,6,13H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426341
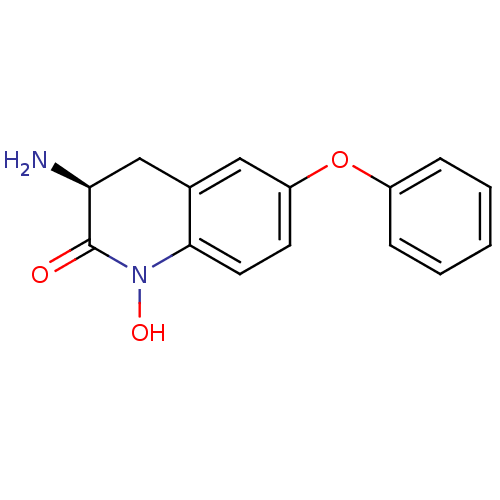
(CHEMBL2321944)Show InChI InChI=1S/C15H14N2O3/c16-13-9-10-8-12(20-11-4-2-1-3-5-11)6-7-14(10)17(19)15(13)18/h1-8,13,19H,9,16H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310
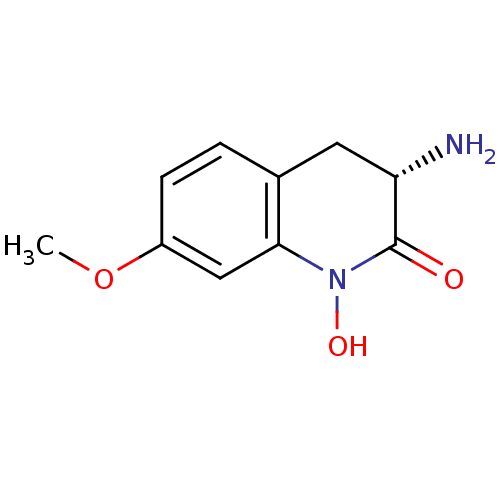
(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107747
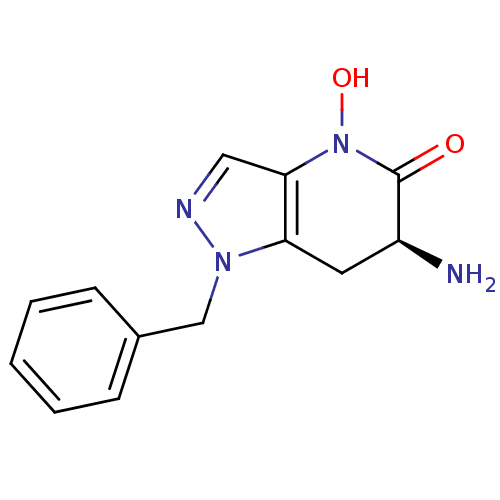
(CHEMBL2347115 | US8933095, 4)Show InChI InChI=1S/C13H14N4O2/c14-10-6-11-12(17(19)13(10)18)7-15-16(11)8-9-4-2-1-3-5-9/h1-5,7,10,19H,6,8,14H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292
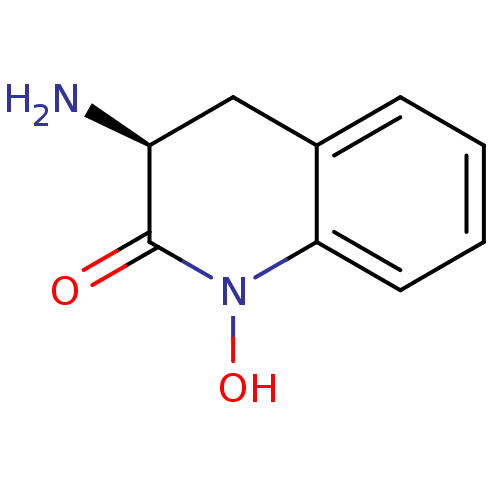
(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107738
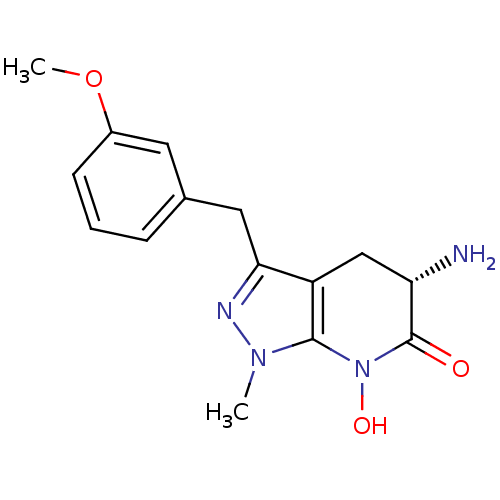
(CHEMBL2347113 | US8933095, 22)Show SMILES COc1cccc(Cc2nn(C)c3N(O)C(=O)[C@@H](N)Cc23)c1 |r| Show InChI InChI=1S/C15H18N4O3/c1-18-14-11(8-12(16)15(20)19(14)21)13(17-18)7-9-4-3-5-10(6-9)22-2/h3-6,12,21H,7-8,16H2,1-2H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107746
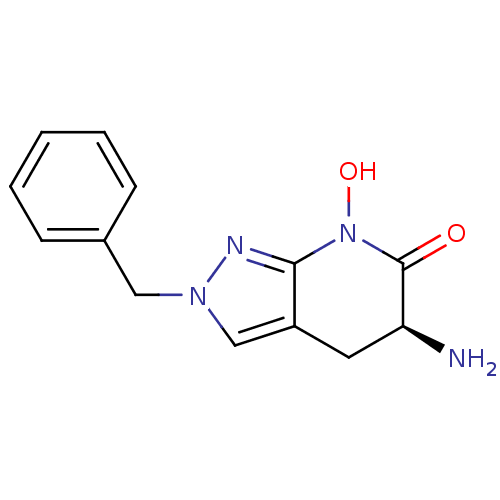
(CHEMBL2347107 | US8933095, 1)Show InChI InChI=1S/C13H14N4O2/c14-11-6-10-8-16(7-9-4-2-1-3-5-9)15-12(10)17(19)13(11)18/h1-5,8,11,19H,6-7,14H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107720
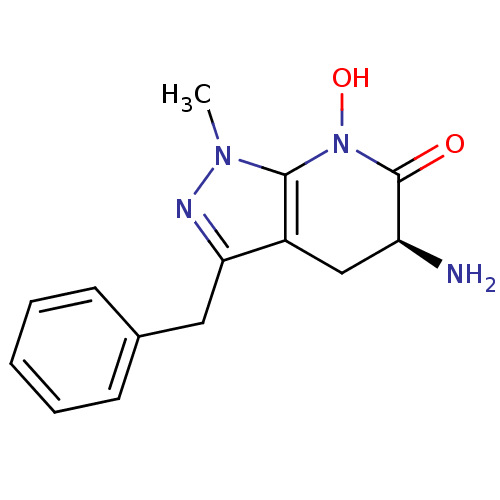
(CHEMBL2347112 | US8598200, 2)Show SMILES Cn1nc(Cc2ccccc2)c2C[C@H](N)C(=O)N(O)c12 |r| Show InChI InChI=1S/C14H16N4O2/c1-17-13-10(8-11(15)14(19)18(13)20)12(16-17)7-9-5-3-2-4-6-9/h2-6,11,20H,7-8,15H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
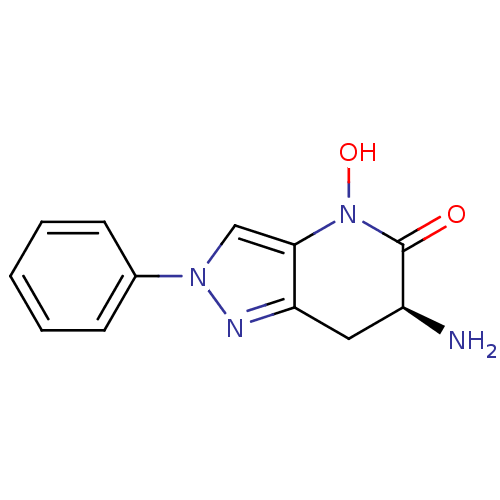
(Homo sapiens (Human)) | BDBM50432200
(CHEMBL2347109 | US8933095, 16)Show InChI InChI=1S/C12H12N4O2/c13-9-6-10-11(16(18)12(9)17)7-15(14-10)8-4-2-1-3-5-8/h1-5,7,9,18H,6,13H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107722
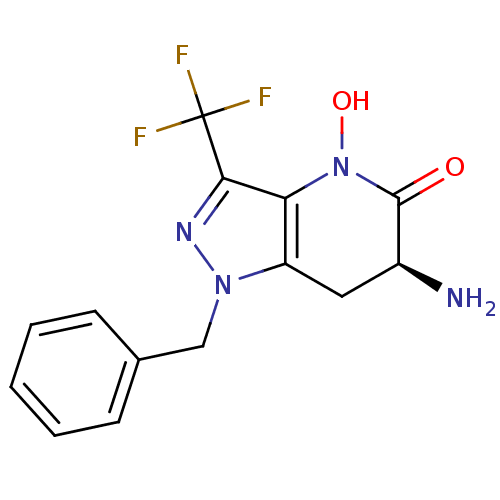
(CHEMBL2347114 | US8933095, 5)Show SMILES N[C@H]1Cc2c(N(O)C1=O)c(nn2Cc1ccccc1)C(F)(F)F |r| Show InChI InChI=1S/C14H13F3N4O2/c15-14(16,17)12-11-10(6-9(18)13(22)21(11)23)20(19-12)7-8-4-2-1-3-5-8/h1-5,9,23H,6-7,18H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50241727
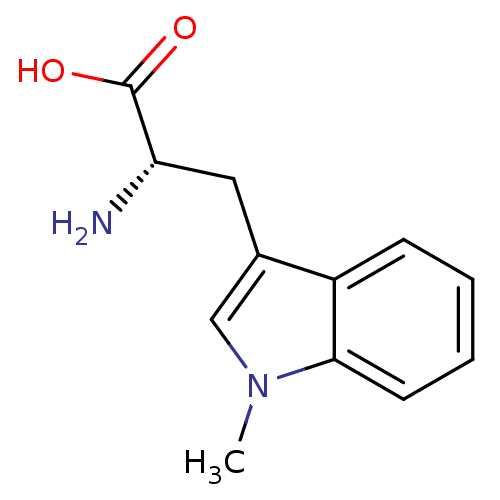
((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of IDO by cell-free assay |
J Med Chem 53: 1172-89 (2010)
Article DOI: 10.1021/jm9014718
BindingDB Entry DOI: 10.7270/Q2BC40G9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50572694

(CHEMBL4854379)Show SMILES CNC(=O)C(Cc1ccccc1)NC(=O)C(CC(C)C)SC1CCCC1S | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MMP9 (unknown origin) by colorimetric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50327224
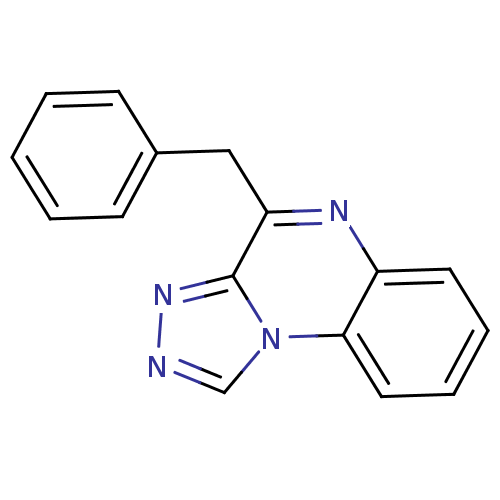
(4-Benzyl-[1,2,4]triazolo[4,3-a]quinoxaline | CHEMB...)Show InChI InChI=1S/C16H12N4/c1-2-6-12(7-3-1)10-14-16-19-17-11-20(16)15-9-5-4-8-13(15)18-14/h1-9,11H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-A (unknown origin) by multi-well spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503407

(CHEMBL4440829)Show SMILES CCOc1ncc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C23H26N4O4/c1-3-5-8-27-15-19(17-6-7-24-20(17)23(27)29)16-13-18(21(25-14-16)31-4-2)22(28)26-9-11-30-12-10-26/h3,6-7,13-15,24H,1,4-5,8-12H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50572691
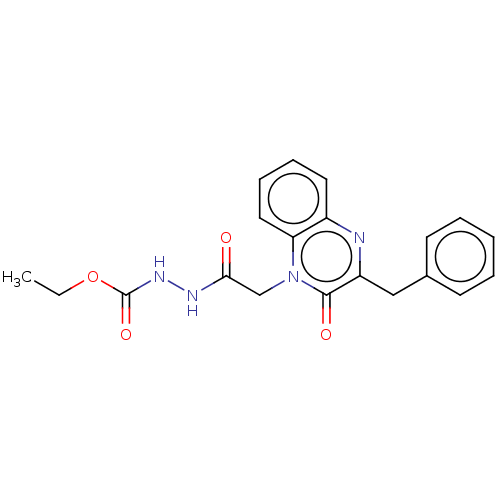
(CHEMBL4862110)Show SMILES CCOC(=O)NNC(=O)Cn1c2ccccc2nc(Cc2ccccc2)c1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MMP9 (unknown origin) by colorimetric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50059889
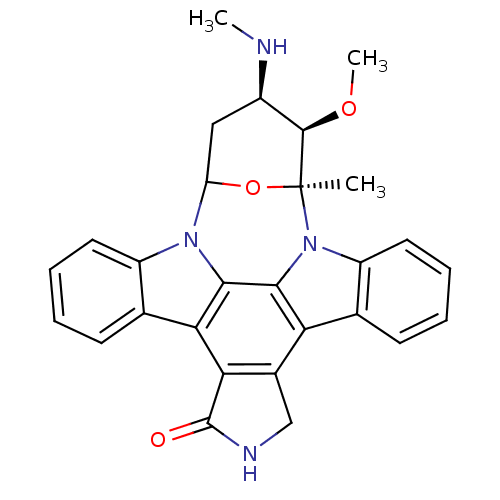
((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Universitaire
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 45: 3389-93 (2010)
Article DOI: 10.1016/j.ejmech.2010.04.026
BindingDB Entry DOI: 10.7270/Q2TH8MWC |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM321424
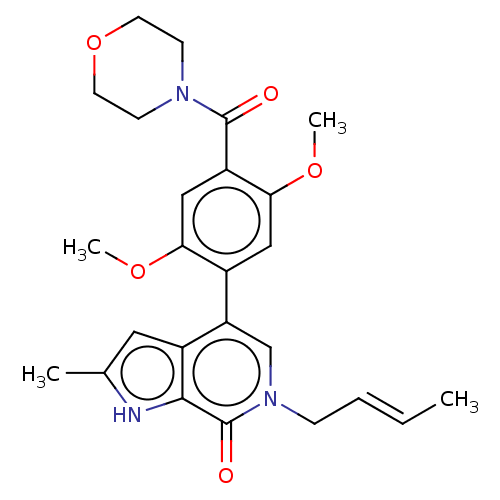
(6-[(E)-but-2-enyl]-4-[2,5-dimethoxy-4-(morpholine-...)Show SMILES COc1cc(c(OC)cc1C(=O)N1CCOCC1)-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12 Show InChI InChI=1S/C25H29N3O5/c1-5-6-7-28-15-20(18-12-16(2)26-23(18)25(28)30)17-13-22(32-4)19(14-21(17)31-3)24(29)27-8-10-33-11-9-27/h5-6,12-15,26H,7-11H2,1-4H3/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50572689
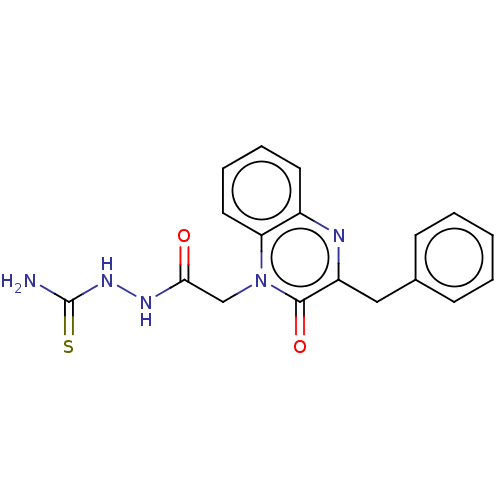
(CHEMBL4871376) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MMP9 (unknown origin) by colorimetric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50073514

(CHEMBL3408913)Show SMILES Cc1ccc(cc1)C(=O)NNC(=O)Cn1c2ccccc2nc(Cc2ccccc2)c1=O Show InChI InChI=1S/C25H22N4O3/c1-17-11-13-19(14-12-17)24(31)28-27-23(30)16-29-22-10-6-5-9-20(22)26-21(25(29)32)15-18-7-3-2-4-8-18/h2-14H,15-16H2,1H3,(H,27,30)(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-A (unknown origin) by multi-well spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50572690
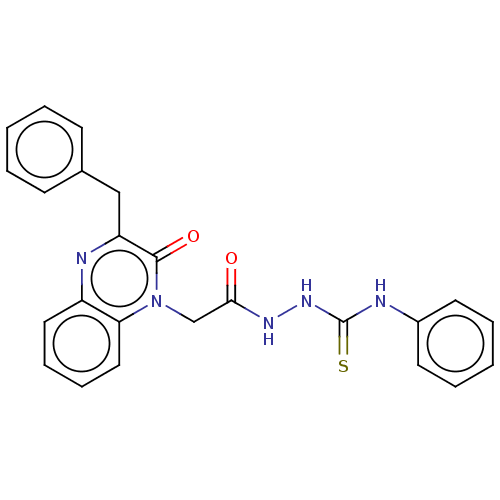
(CHEMBL4852596)Show SMILES O=C(Cn1c2ccccc2nc(Cc2ccccc2)c1=O)NNC(=S)Nc1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-A (unknown origin) by multi-well spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321463

(6-(but-3-en-1-yl)-4-(3-(difluoromethoxy)-5-(morpho...)Show SMILES FC(F)Oc1cc(cc(c1)-c1cn(CCC=C)c(=O)c2[nH]ccc12)C(=O)N1CCOCC1 Show InChI InChI=1S/C23H23F2N3O4/c1-2-3-6-28-14-19(18-4-5-26-20(18)22(28)30)15-11-16(13-17(12-15)32-23(24)25)21(29)27-7-9-31-10-8-27/h2,4-5,11-14,23,26H,1,3,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM321411

(6-[(E)-but-2-enyl]-4-[3-methoxy-4-(morpholine-4-ca...)Show SMILES COc1cc(ccc1C(=O)N1CCOCC1)-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12 Show InChI InChI=1S/C24H27N3O4/c1-4-5-8-27-15-20(19-13-16(2)25-22(19)24(27)29)17-6-7-18(21(14-17)30-3)23(28)26-9-11-31-12-10-26/h4-7,13-15,25H,8-12H2,1-3H3/b5-4+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321467
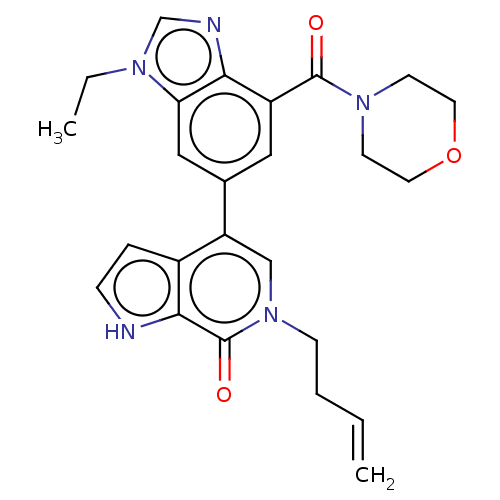
(6-but-3-enyl-4-[3-ethyl-7-(morpholine-4-carbonyl)b...)Show SMILES CCn1cnc2c(cc(cc12)-c1cn(CCC=C)c(=O)c2[nH]ccc12)C(=O)N1CCOCC1 Show InChI InChI=1S/C25H27N5O3/c1-3-5-8-30-15-20(18-6-7-26-23(18)25(30)32)17-13-19(24(31)29-9-11-33-12-10-29)22-21(14-17)28(4-2)16-27-22/h3,6-7,13-16,26H,1,4-5,8-12H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503399

(CHEMBL4441257)Show SMILES Clc1ccc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H22ClN3O3/c1-2-3-8-26-14-18(16-6-7-24-20(16)22(26)28)15-4-5-19(23)17(13-15)21(27)25-9-11-29-12-10-25/h2,4-7,13-14,24H,1,3,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50572690

(CHEMBL4852596)Show SMILES O=C(Cn1c2ccccc2nc(Cc2ccccc2)c1=O)NNC(=S)Nc1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-B (unknown origin) by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503413

(CHEMBL4566599)Show SMILES COc1ncc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H24N4O4/c1-3-4-7-26-14-18(16-5-6-23-19(16)22(26)28)15-12-17(20(29-2)24-13-15)21(27)25-8-10-30-11-9-25/h3,5-6,12-14,23H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM321412
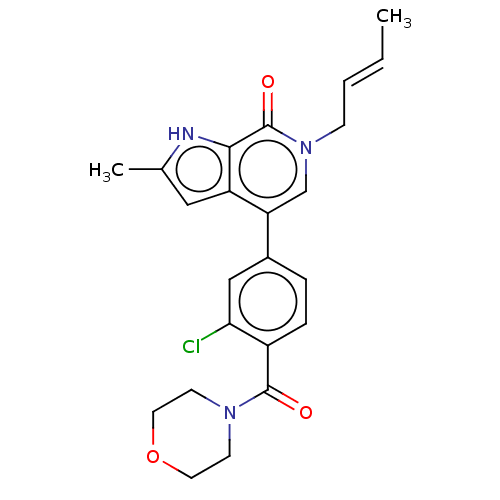
(6-[(E)-but-2-enyl]-4-[3-chloro-4-(morpholine-4-car...)Show SMILES C\C=C\Cn1cc(-c2ccc(C(=O)N3CCOCC3)c(Cl)c2)c2cc(C)[nH]c2c1=O Show InChI InChI=1S/C23H24ClN3O3/c1-3-4-7-27-14-19(18-12-15(2)25-21(18)23(27)29)16-5-6-17(20(24)13-16)22(28)26-8-10-30-11-9-26/h3-6,12-14,25H,7-11H2,1-2H3/b4-3+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50572689

(CHEMBL4871376) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-B (unknown origin) by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM321437
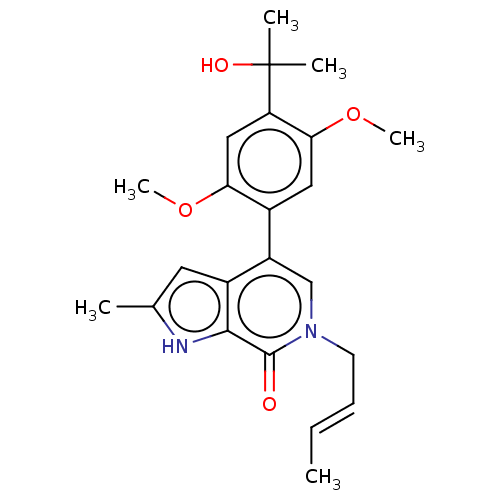
((E)-6-(but-2-en-1-yl)-4-(4-(2-hydroxypropan-2-yl)-...)Show SMILES COc1cc(c(OC)cc1-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12)C(C)(C)O Show InChI InChI=1S/C23H28N2O4/c1-7-8-9-25-13-17(16-10-14(2)24-21(16)22(25)26)15-11-20(29-6)18(23(3,4)27)12-19(15)28-5/h7-8,10-13,24,27H,9H2,1-6H3/b8-7+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM321422

(5-[6-[(E)-but-2-enyl]-2-methyl-7-oxo-1H-pyrrolo[2,...)Show SMILES C\C=C\Cn1cc(-c2ccc(C(=O)N3CCOCC3)c(c2)C#N)c2cc(C)[nH]c2c1=O Show InChI InChI=1S/C24H24N4O3/c1-3-4-7-28-15-21(20-12-16(2)26-22(20)24(28)30)17-5-6-19(18(13-17)14-25)23(29)27-8-10-31-11-9-27/h3-6,12-13,15,26H,7-11H2,1-2H3/b4-3+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50572690

(CHEMBL4852596)Show SMILES O=C(Cn1c2ccccc2nc(Cc2ccccc2)c1=O)NNC(=S)Nc1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MMP9 (unknown origin) by colorimetric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Protein polybromo-1
(Homo sapiens (Human)) | BDBM394583
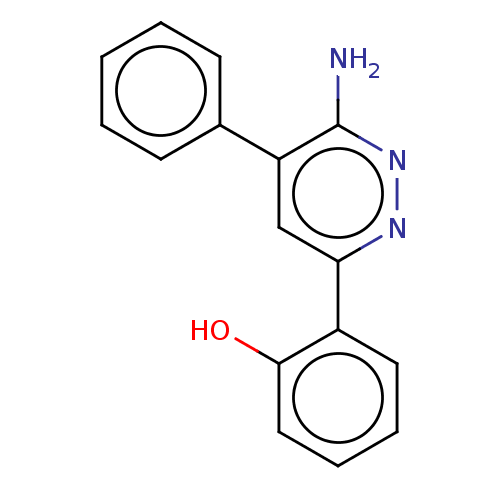
(2-(6-amino-5-phenylpyridazin-3-yl)phenol | US10308...)Show InChI InChI=1S/C16H13N3O/c17-16-13(11-6-2-1-3-7-11)10-14(18-19-16)12-8-4-5-9-15(12)20/h1-10,20H,(H2,17,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00662
BindingDB Entry DOI: 10.7270/Q2TB1BX4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM321415

(6-[(E)-but-2-enyl]-2-methyl-4-[3-methyl-4-(morphol...)Show SMILES C\C=C\Cn1cc(-c2ccc(C(=O)N3CCOCC3)c(C)c2)c2cc(C)[nH]c2c1=O Show InChI InChI=1S/C24H27N3O3/c1-4-5-8-27-15-21(20-14-17(3)25-22(20)24(27)29)18-6-7-19(16(2)13-18)23(28)26-9-11-30-12-10-26/h4-7,13-15,25H,8-12H2,1-3H3/b5-4+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50572692
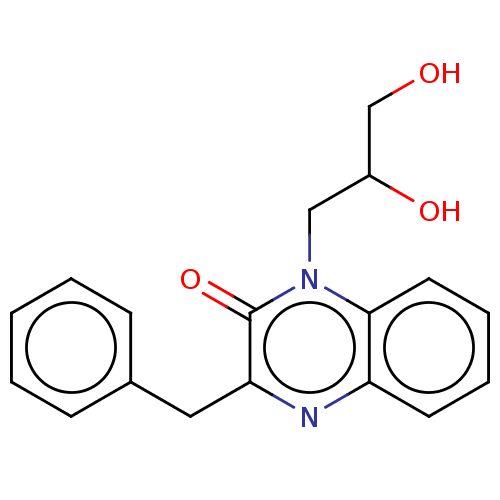
(CHEMBL4878125) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MMP9 (unknown origin) by colorimetric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50200375
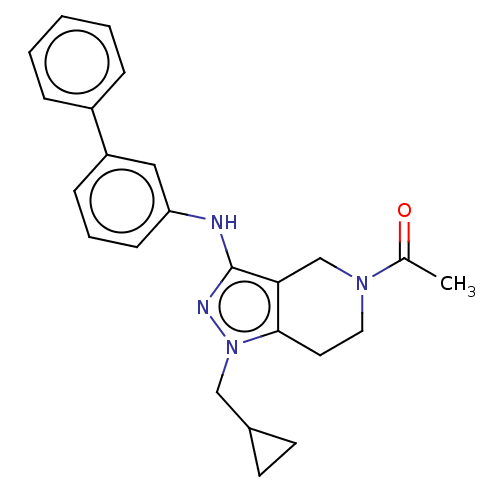
(CHEMBL3902514)Show SMILES CC(=O)N1CCc2c(C1)c(Nc1cccc(c1)-c1ccccc1)nn2CC1CC1 Show InChI InChI=1S/C24H26N4O/c1-17(29)27-13-12-23-22(16-27)24(26-28(23)15-18-10-11-18)25-21-9-5-8-20(14-21)19-6-3-2-4-7-19/h2-9,14,18H,10-13,15-16H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of biotinylated ligand from recombinant His-tagged CBP/EP300 (unknown origin) measured after 10 mins by TR-FRET assay |
J Med Chem 59: 10549-10563 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01022
BindingDB Entry DOI: 10.7270/Q27083DF |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321462

(6-but-3-enyl-4-[4-fluoro-3-(morpholine-4-carbonyl)...)Show SMILES Fc1ccc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H22FN3O3/c1-2-3-8-26-14-18(16-6-7-24-20(16)22(26)28)15-4-5-19(23)17(13-15)21(27)25-9-11-29-12-10-25/h2,4-7,13-14,24H,1,3,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50553677
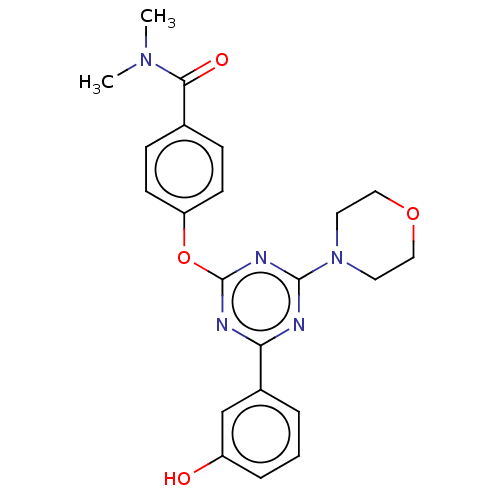
(CHEMBL4797516)Show SMILES CN(C)C(=O)c1ccc(Oc2nc(nc(n2)-c2cccc(O)c2)N2CCOCC2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110 alpha/p85 alpha expressed in baculovirus expression system using PIP2 as subst... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01061
BindingDB Entry DOI: 10.7270/Q2WD4474 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503406
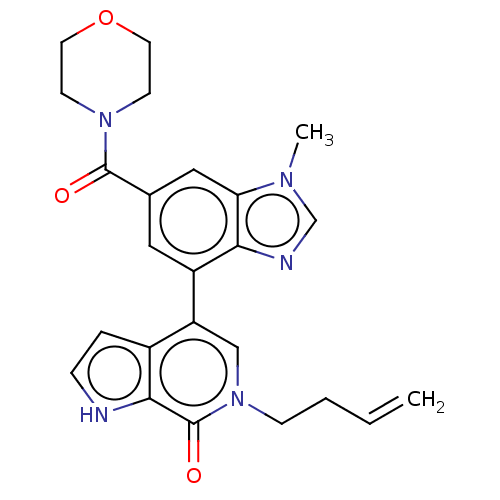
(CHEMBL4463538)Show SMILES Cn1cnc2c(cc(cc12)C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C24H25N5O3/c1-3-4-7-29-14-19(17-5-6-25-22(17)24(29)31)18-12-16(13-20-21(18)26-15-27(20)2)23(30)28-8-10-32-11-9-28/h3,5-6,12-15,25H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50572689

(CHEMBL4871376) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-A (unknown origin) by multi-well spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503404

(CHEMBL4455513)Show SMILES Cn1cnc2cc(cc(C(=O)N3CCOCC3)c12)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C24H25N5O3/c1-3-4-7-29-14-19(17-5-6-25-21(17)24(29)31)16-12-18(22-20(13-16)26-15-27(22)2)23(30)28-8-10-32-11-9-28/h3,5-6,12-15,25H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50553686
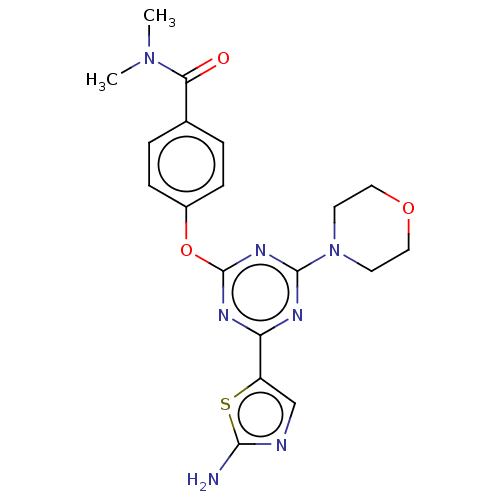
(CHEMBL4782133)Show SMILES CN(C)C(=O)c1ccc(Oc2nc(nc(n2)-c2cnc(N)s2)N2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01061
BindingDB Entry DOI: 10.7270/Q2WD4474 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50572691

(CHEMBL4862110)Show SMILES CCOC(=O)NNC(=O)Cn1c2ccccc2nc(Cc2ccccc2)c1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-B (unknown origin) by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM321458

(6-but-3-enyl-4-[3,4-difluoro-5-(morpholine-4-carbo...)Show SMILES Fc1cc(cc(C(=O)N2CCOCC2)c1F)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H21F2N3O3/c1-2-3-6-27-13-17(15-4-5-25-20(15)22(27)29)14-11-16(19(24)18(23)12-14)21(28)26-7-9-30-10-8-26/h2,4-5,11-13,25H,1,3,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50572692

(CHEMBL4878125) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-B (unknown origin) by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50572691

(CHEMBL4862110)Show SMILES CCOC(=O)NNC(=O)Cn1c2ccccc2nc(Cc2ccccc2)c1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAO-A (unknown origin) by multi-well spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113558
BindingDB Entry DOI: 10.7270/Q21R6V95 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM321407
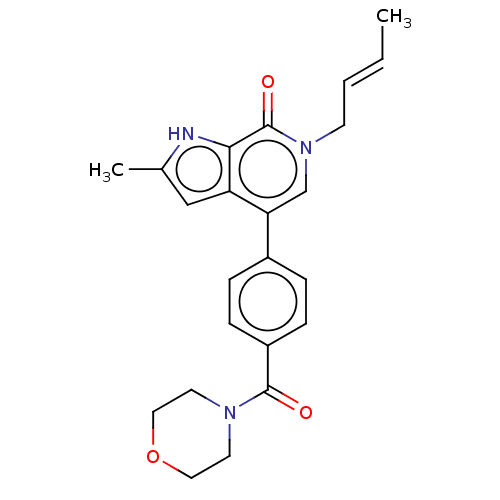
(6-[(E)-but-2-enyl]-2-methyl-4-[4-(morpholine-4-car...)Show SMILES C\C=C\Cn1cc(-c2ccc(cc2)C(=O)N2CCOCC2)c2cc(C)[nH]c2c1=O Show InChI InChI=1S/C23H25N3O3/c1-3-4-9-26-15-20(19-14-16(2)24-21(19)23(26)28)17-5-7-18(8-6-17)22(27)25-10-12-29-13-11-25/h3-8,14-15,24H,9-13H2,1-2H3/b4-3+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503408

(CHEMBL4459660)Show SMILES Cc1ncc(cc1C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H24N4O3/c1-3-4-7-26-14-19(17-5-6-23-20(17)22(26)28)16-12-18(15(2)24-13-16)21(27)25-8-10-29-11-9-25/h3,5-6,12-14,23H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data