 Found 33 hits with Last Name = 'badger' and Initial = 'a'
Found 33 hits with Last Name = 'badger' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1B
(Homo sapiens (Human)) | BDBM50029191
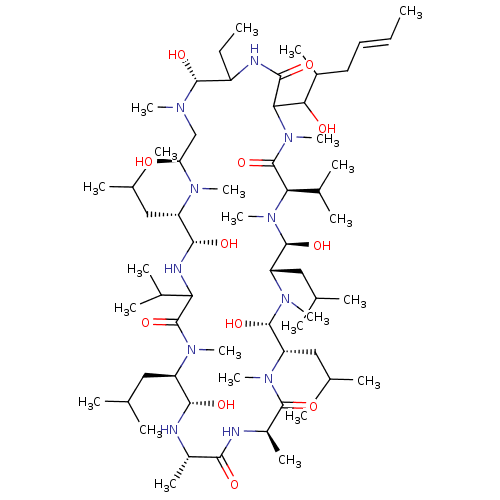
(15-Benzyl-30-ethyl-12-hydroxymethyl-33-(1-hydroxy-...)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)[C@@H](C(C)C)N(C)[C@@H](O)[C@H](CC(C)C)N(C)[C@@H](O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)N[C@@H](O)[C@@H](CC(C)C)N(C)C(=O)C(N[C@@H](O)[C@H](CC(C)C)N(C)[C@@H](O)CN(C)[C@@H]1O)C(C)C Show InChI InChI=1S/C62H123N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-52,54-55,58-60,63,66,74-75,77-78,81-83H,26,28-33H2,1-24H3,(H,64,76)(H,65,79)/b27-25+/t40?,41-,42+,43?,44-,45+,46-,47-,48-,49?,50+,51?,52?,54-,55-,58+,59-,60-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). |
J Med Chem 38: 4164-70 (1995)
BindingDB Entry DOI: 10.7270/Q2X34WGK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403336
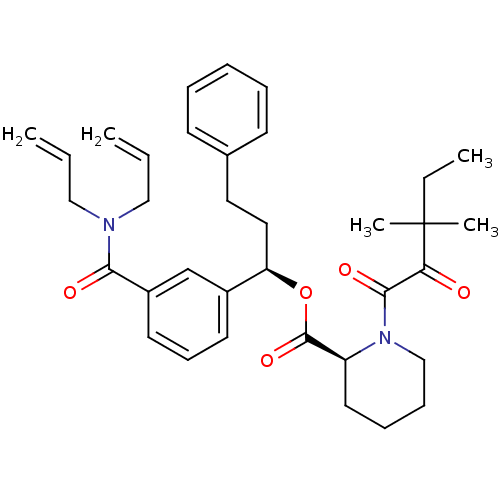
(CHEMBL326881)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1cccc(c1)C(=O)N(CC=C)CC=C Show InChI InChI=1S/C35H44N2O5/c1-6-22-36(23-7-2)32(39)28-18-14-17-27(25-28)30(21-20-26-15-10-9-11-16-26)42-34(41)29-19-12-13-24-37(29)33(40)31(38)35(4,5)8-3/h6-7,9-11,14-18,25,29-30H,1-2,8,12-13,19-24H2,3-5H3/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403339
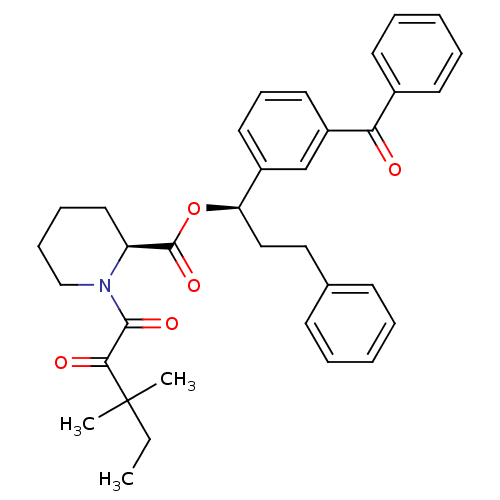
(CHEMBL109950)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1cccc(c1)C(=O)c1ccccc1 Show InChI InChI=1S/C35H39NO5/c1-4-35(2,3)32(38)33(39)36-23-12-11-20-29(36)34(40)41-30(22-21-25-14-7-5-8-15-25)27-18-13-19-28(24-27)31(37)26-16-9-6-10-17-26/h5-10,13-19,24,29-30H,4,11-12,20-23H2,1-3H3/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1B
(Homo sapiens (Human)) | BDBM50029192

(15-Benzyl-30-ethyl-12-hydroxymethyl-33-(1-hydroxy-...)Show SMILES [H][C@]1(C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](CO)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)C(C)C\C=C\C)N(C)C1=O)C(C)C Show InChI InChI=1S/C68H115N11O13/c1-23-25-29-45(15)58(82)57-62(86)69-47(24-2)63(87)73(16)37-54(81)74(17)50(32-39(3)4)61(85)72-55(43(11)12)67(91)75(18)51(33-40(5)6)60(84)70-48(36-46-30-27-26-28-31-46)59(83)71-49(38-80)64(88)76(19)52(34-41(7)8)65(89)77(20)53(35-42(9)10)66(90)78(21)56(44(13)14)68(92)79(57)22/h23,25-28,30-31,39-45,47-53,55-58,80,82H,24,29,32-38H2,1-22H3,(H,69,86)(H,70,84)(H,71,83)(H,72,85)/b25-23+/t45?,47-,48+,49-,50-,51+,52+,53-,55-,56+,57-,58+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). |
J Med Chem 38: 4164-70 (1995)
BindingDB Entry DOI: 10.7270/Q2X34WGK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1B
(Homo sapiens (Human)) | BDBM50029190

(15-Benzyl-30-ethyl-33-(1-hydroxy-2-methyl-hex-4-en...)Show SMILES [H][C@]1(C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@H](C(O)C(C)C\C=C\C)N(C)C1=O)C(C)C Show InChI InChI=1S/C69H117N11O12/c1-25-27-31-45(15)58(81)57-62(85)71-49(26-2)65(88)74(18)47(17)64(87)75(19)52(35-40(5)6)61(84)73-55(43(11)12)68(91)76(20)51(34-39(3)4)60(83)72-50(38-48-32-29-28-30-33-48)59(82)70-46(16)63(86)77(21)53(36-41(7)8)66(89)78(22)54(37-42(9)10)67(90)79(23)56(44(13)14)69(92)80(57)24/h25,27-30,32-33,39-47,49-58,81H,26,31,34-38H2,1-24H3,(H,70,82)(H,71,85)(H,72,83)(H,73,84)/b27-25+/t45?,46-,47-,49+,50-,51-,52+,53-,54+,55+,56-,57+,58?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). |
J Med Chem 38: 4164-70 (1995)
BindingDB Entry DOI: 10.7270/Q2X34WGK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1B
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). |
J Med Chem 38: 4164-70 (1995)
BindingDB Entry DOI: 10.7270/Q2X34WGK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403332
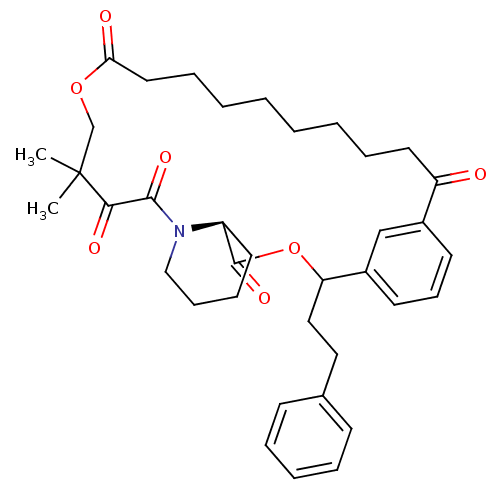
(CHEMBL323633)Show SMILES CC1(C)COC(=O)CCCCCCCCC(=O)c2cccc(c2)C(CCc2ccccc2)OC(=O)[C@@H]2CCCCN2C(=O)C1=O Show InChI InChI=1S/C37H47NO7/c1-37(2)26-44-33(40)21-11-6-4-3-5-10-20-31(39)28-17-14-18-29(25-28)32(23-22-27-15-8-7-9-16-27)45-36(43)30-19-12-13-24-38(30)35(42)34(37)41/h7-9,14-18,25,30,32H,3-6,10-13,19-24,26H2,1-2H3/t30-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403335
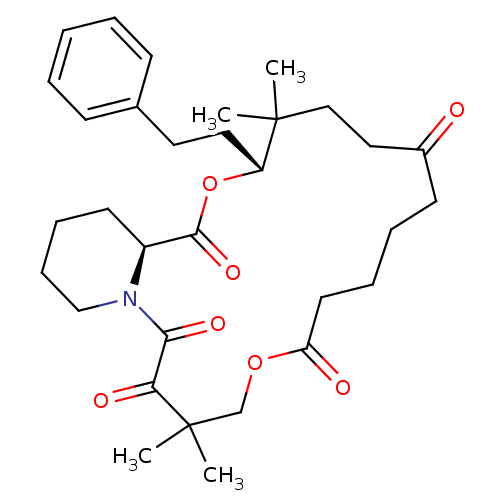
(CHEMBL325145)Show SMILES CC1(C)CCC(=O)CCCCC(=O)OCC(C)(C)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]1CCc1ccccc1 Show InChI InChI=1S/C32H45NO7/c1-31(2)20-19-24(34)14-8-9-16-27(35)39-22-32(3,4)28(36)29(37)33-21-11-10-15-25(33)30(38)40-26(31)18-17-23-12-6-5-7-13-23/h5-7,12-13,25-26H,8-11,14-22H2,1-4H3/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1B
(Homo sapiens (Human)) | BDBM50029194
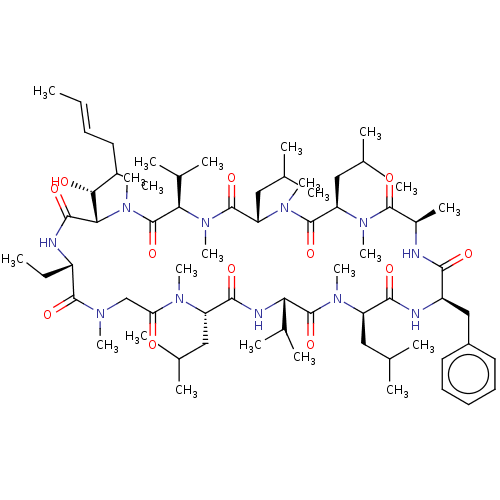
(15-Benzyl-30-ethyl-33-(1-hydroxy-2-methyl-hex-4-en...)Show SMILES [H][C@]1(C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)C(C)C\C=C\C)N(C)C1=O)C(C)C Show InChI InChI=1S/C68H115N11O12/c1-24-26-30-45(15)58(81)57-62(85)70-48(25-2)64(87)73(17)38-54(80)74(18)50(33-39(3)4)61(84)72-55(43(11)12)67(90)75(19)51(34-40(5)6)60(83)71-49(37-47-31-28-27-29-32-47)59(82)69-46(16)63(86)76(20)52(35-41(7)8)65(88)77(21)53(36-42(9)10)66(89)78(22)56(44(13)14)68(91)79(57)23/h24,26-29,31-32,39-46,48-53,55-58,81H,25,30,33-38H2,1-23H3,(H,69,82)(H,70,85)(H,71,83)(H,72,84)/b26-24+/t45?,46-,48+,49-,50+,51-,52-,53+,55+,56-,57+,58-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). |
J Med Chem 38: 4164-70 (1995)
BindingDB Entry DOI: 10.7270/Q2X34WGK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403331

(CHEMBL419576)Show SMILES C[C@H]1C\C(C)=C\[C@@H](CC=C)C(=O)OCCCOC(=O)[C@@H]2CCCCN2C(=O)C(=O)C(C)(C)COC1=O |t:4| Show InChI InChI=1S/C27H39NO8/c1-6-10-20-16-18(2)15-19(3)24(31)36-17-27(4,5)22(29)23(30)28-12-8-7-11-21(28)26(33)35-14-9-13-34-25(20)32/h6,16,19-21H,1,7-15,17H2,2-5H3/b18-16+/t19-,20+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403337
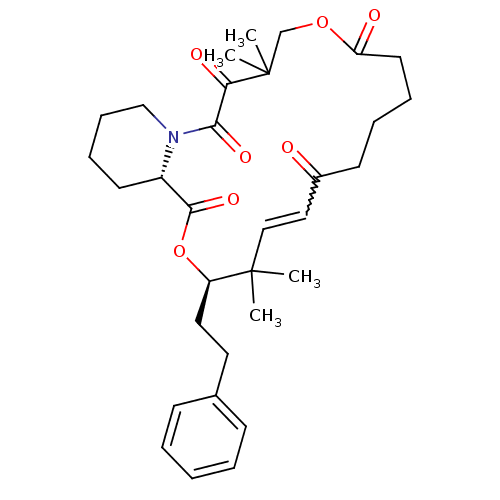
(CHEMBL324539)Show SMILES CC1(C)COC(=O)CCCCC(=O)C=CC(C)(C)[C@@H](CCc2ccccc2)OC(=O)[C@@H]2CCCCN2C(=O)C1=O |w:13.12| Show InChI InChI=1S/C32H43NO7/c1-31(2)20-19-24(34)14-8-9-16-27(35)39-22-32(3,4)28(36)29(37)33-21-11-10-15-25(33)30(38)40-26(31)18-17-23-12-6-5-7-13-23/h5-7,12-13,19-20,25-26H,8-11,14-18,21-22H2,1-4H3/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403333
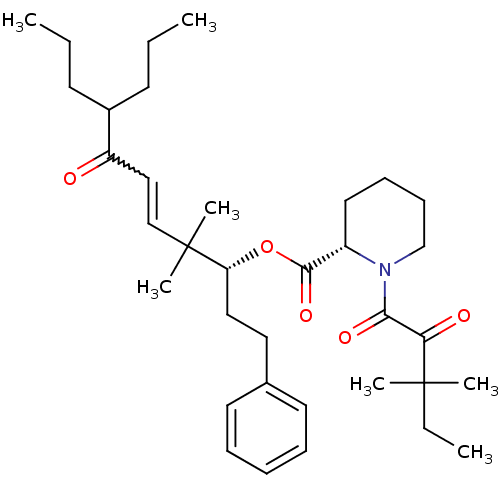
(CHEMBL111266)Show SMILES CCCC(CCC)C(=O)C=CC(C)(C)[C@@H](CCc1ccccc1)OC(=O)[C@@H]1CCCCN1C(=O)C(=O)C(C)(C)CC |w:9.8| Show InChI InChI=1S/C35H53NO5/c1-8-16-27(17-9-2)29(37)23-24-35(6,7)30(22-21-26-18-12-11-13-19-26)41-33(40)28-20-14-15-25-36(28)32(39)31(38)34(4,5)10-3/h11-13,18-19,23-24,27-28,30H,8-10,14-17,20-22,25H2,1-7H3/t28-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12); Value ranges from 190-900 nM |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403340
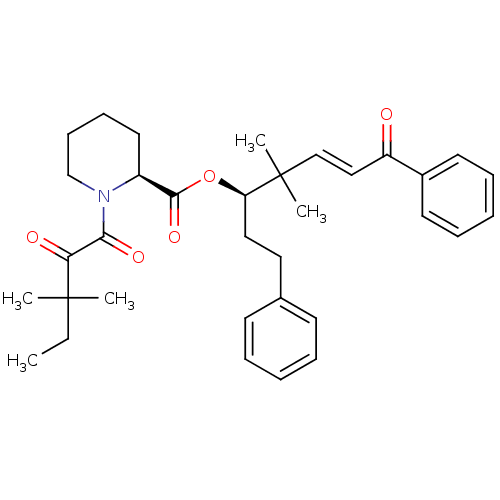
(CHEMBL110010)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)C(C)(C)\C=C\C(=O)c1ccccc1 Show InChI InChI=1S/C34H43NO5/c1-6-33(2,3)30(37)31(38)35-24-14-13-19-27(35)32(39)40-29(21-20-25-15-9-7-10-16-25)34(4,5)23-22-28(36)26-17-11-8-12-18-26/h7-12,15-18,22-23,27,29H,6,13-14,19-21,24H2,1-5H3/b23-22+/t27-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12); Value ranges from 190-900 nM |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403334
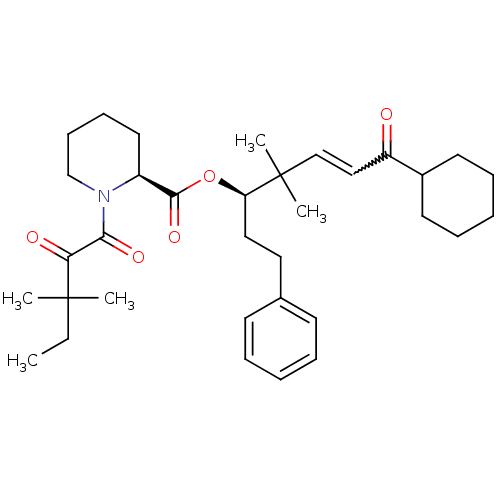
(CHEMBL321670)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)C(C)(C)C=CC(=O)C1CCCCC1 |w:31.33| Show InChI InChI=1S/C34H49NO5/c1-6-33(2,3)30(37)31(38)35-24-14-13-19-27(35)32(39)40-29(21-20-25-15-9-7-10-16-25)34(4,5)23-22-28(36)26-17-11-8-12-18-26/h7,9-10,15-16,22-23,26-27,29H,6,8,11-14,17-21,24H2,1-5H3/t27-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403330
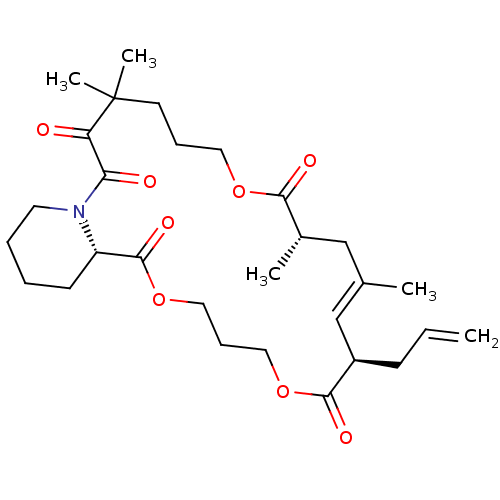
(CHEMBL116062)Show SMILES C[C@H]1C\C(C)=C\[C@@H](CC=C)C(=O)OCCCOC(=O)[C@@H]2CCCCN2C(=O)C(=O)C(C)(C)CCCOC1=O |t:4| Show InChI InChI=1S/C29H43NO8/c1-6-11-22-19-20(2)18-21(3)26(33)36-15-9-13-29(4,5)24(31)25(32)30-14-8-7-12-23(30)28(35)38-17-10-16-37-27(22)34/h6,19,21-23H,1,7-18H2,2-5H3/b20-19+/t21-,22+,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403341
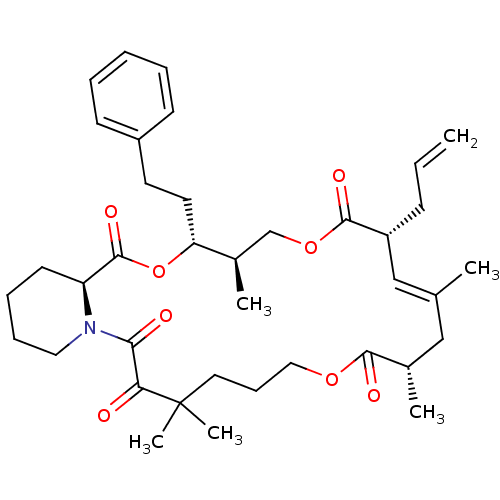
(CHEMBL443019)Show SMILES C[C@@H]1COC(=O)[C@H](CC=C)\C=C(C)\C[C@H](C)C(=O)OCCCC(C)(C)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]1CCc1ccccc1 |c:10| Show InChI InChI=1S/C38H53NO8/c1-7-14-30-24-26(2)23-27(3)35(42)45-22-13-20-38(5,6)33(40)34(41)39-21-12-11-17-31(39)37(44)47-32(28(4)25-46-36(30)43)19-18-29-15-9-8-10-16-29/h7-10,15-16,24,27-28,30-32H,1,11-14,17-23,25H2,2-6H3/b26-24+/t27-,28+,30+,31-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403338
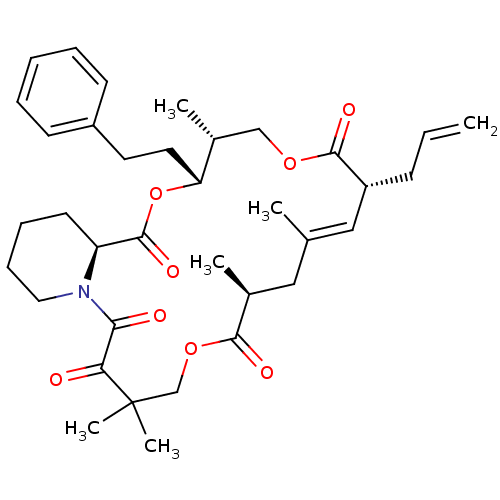
(CHEMBL321246)Show SMILES C[C@@H]1COC(=O)[C@H](CC=C)\C=C(C)\C[C@H](C)C(=O)OCC(C)(C)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]1CCc1ccccc1 |c:10| Show InChI InChI=1S/C36H49NO8/c1-7-13-28-21-24(2)20-25(3)33(40)44-23-36(5,6)31(38)32(39)37-19-12-11-16-29(37)35(42)45-30(26(4)22-43-34(28)41)18-17-27-14-9-8-10-15-27/h7-10,14-15,21,25-26,28-30H,1,11-13,16-20,22-23H2,2-6H3/b24-21+/t25-,26+,28+,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50053432

(2-(4-Methylsulfanyl-phenyl)-3-pyridin-4-yl-6,7-dih...)Show InChI InChI=1S/C18H17N3S/c1-22-15-6-4-13(5-7-15)17-18(14-8-10-19-11-9-14)21-12-2-3-16(21)20-17/h4-11H,2-3,12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in rat RBL-1 cells |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in rat RBL-1 cells |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM14832
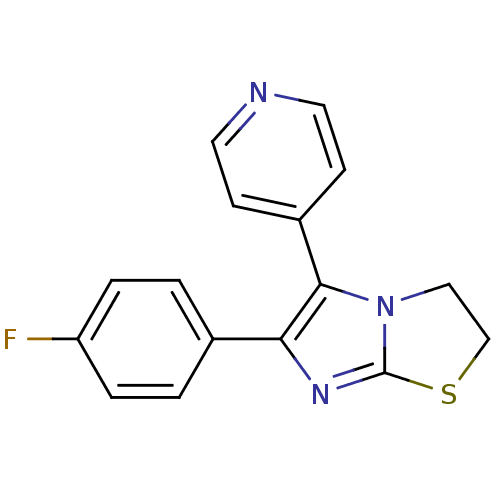
(3-(4-fluorophenyl)-2-pyridin-4-yl-6-thia-1,4-diaza...)Show InChI InChI=1S/C16H12FN3S/c17-13-3-1-11(2-4-13)14-15(12-5-7-18-8-6-12)20-9-10-21-16(20)19-14/h1-8H,9-10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin H2 synthase 1 (PGHS-1) from ram seminal vesicle |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM14832

(3-(4-fluorophenyl)-2-pyridin-4-yl-6-thia-1,4-diaza...)Show InChI InChI=1S/C16H12FN3S/c17-13-3-1-11(2-4-13)14-15(12-5-7-18-8-6-12)20-9-10-21-16(20)19-14/h1-8H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in rat RBL-1 cells |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50053430

(4-[3-Cyclopropyl-5-(4-fluoro-phenyl)-3H-imidazol-4...)Show InChI InChI=1S/C17H14FN3/c18-14-3-1-12(2-4-14)16-17(13-7-9-19-10-8-13)21(11-20-16)15-5-6-15/h1-4,7-11,15H,5-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin H2 synthase 1 (PGHS-1) from ram seminal vesicle |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50339185
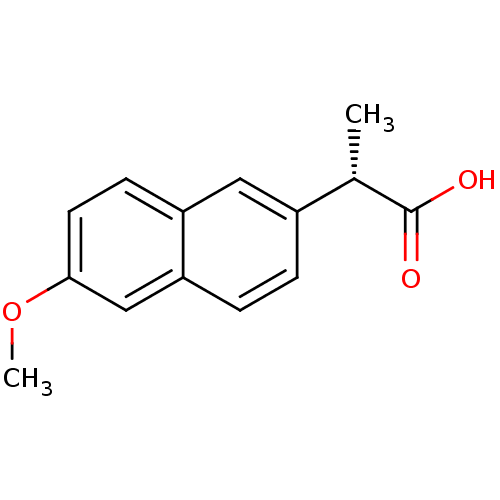
((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin H2 synthase 1 (PGHS-1) from ram seminal vesicle |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM15237

(4-[1-(cyclopropylmethyl)-4-(4-fluorophenyl)-1H-imi...)Show InChI InChI=1S/C18H16FN3/c19-16-5-3-14(4-6-16)17-18(15-7-9-20-10-8-15)22(12-21-17)11-13-1-2-13/h3-10,12-13H,1-2,11H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin H2 synthase 1 (PGHS-1) from ram seminal vesicle |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50053430

(4-[3-Cyclopropyl-5-(4-fluoro-phenyl)-3H-imidazol-4...)Show InChI InChI=1S/C17H14FN3/c18-14-3-1-12(2-4-14)16-17(13-7-9-19-10-8-13)21(11-20-16)15-5-6-15/h1-4,7-11,15H,5-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in rat RBL-1 cells |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50053432

(2-(4-Methylsulfanyl-phenyl)-3-pyridin-4-yl-6,7-dih...)Show InChI InChI=1S/C18H17N3S/c1-22-15-6-4-13(5-7-15)17-18(14-8-10-19-11-9-14)21-12-2-3-16(21)20-17/h4-11H,2-3,12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin H2 synthase 1 (PGHS-1) from ram seminal vesicle |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50053424

(4-{3-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-imidazol-...)Show InChI InChI=1S/C21H23FN4O/c22-19-4-2-17(3-5-19)20-21(18-6-8-23-9-7-18)26(16-24-20)11-1-10-25-12-14-27-15-13-25/h2-9,16H,1,10-15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in rat RBL-1 cells |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50053410
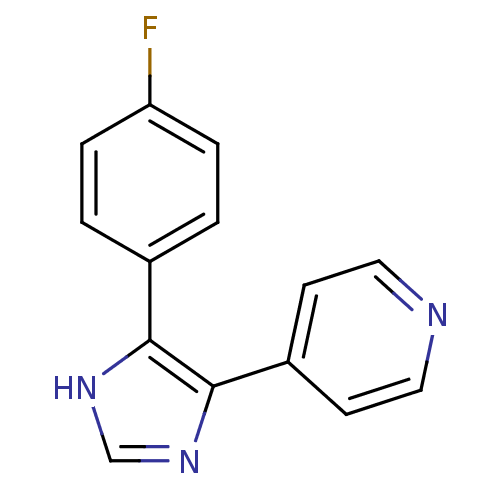
(4-(4-Fluorophenyl)-5-(pyridin-4-yl)-1H-imidazole |...)Show InChI InChI=1S/C14H10FN3/c15-12-3-1-10(2-4-12)13-14(18-9-17-13)11-5-7-16-8-6-11/h1-9H,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin H2 synthase 1 (PGHS-1) from ram seminal vesicle |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50053410

(4-(4-Fluorophenyl)-5-(pyridin-4-yl)-1H-imidazole |...)Show InChI InChI=1S/C14H10FN3/c15-12-3-1-10(2-4-12)13-14(18-9-17-13)11-5-7-16-8-6-11/h1-9H,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in rat RBL-1 cells |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50053424

(4-{3-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-imidazol-...)Show InChI InChI=1S/C21H23FN4O/c22-19-4-2-17(3-5-19)20-21(18-6-8-23-9-7-18)26(16-24-20)11-1-10-25-12-14-27-15-13-25/h2-9,16H,1,10-15H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin H2 synthase 1 (PGHS-1) from ram seminal vesicle |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50053412

(4-{3-[4-(3,4-Dichloro-phenyl)-5-pyridin-4-yl-imida...)Show SMILES Clc1ccc(cc1Cl)-c1ncn(CCCN2CCOCC2)c1-c1ccncc1 Show InChI InChI=1S/C21H22Cl2N4O/c22-18-3-2-17(14-19(18)23)20-21(16-4-6-24-7-5-16)27(15-25-20)9-1-8-26-10-12-28-13-11-26/h2-7,14-15H,1,8-13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin H2 synthase 1 (PGHS-1) from ram seminal vesicle |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM50053412

(4-{3-[4-(3,4-Dichloro-phenyl)-5-pyridin-4-yl-imida...)Show SMILES Clc1ccc(cc1Cl)-c1ncn(CCCN2CCOCC2)c1-c1ccncc1 Show InChI InChI=1S/C21H22Cl2N4O/c22-18-3-2-17(14-19(18)23)20-21(16-4-6-24-7-5-16)27(15-25-20)9-1-8-26-10-12-28-13-11-26/h2-7,14-15H,1,8-13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in rat RBL-1 cells |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Rattus norvegicus) | BDBM15237

(4-[1-(cyclopropylmethyl)-4-(4-fluorophenyl)-1H-imi...)Show InChI InChI=1S/C18H16FN3/c19-16-5-3-14(4-6-16)17-18(15-7-9-20-10-8-15)22(12-21-17)11-13-1-2-13/h3-10,12-13H,1-2,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in rat RBL-1 cells |
J Med Chem 39: 3929-37 (1996)
Article DOI: 10.1021/jm960415o
BindingDB Entry DOI: 10.7270/Q29W0G4Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data