

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50200340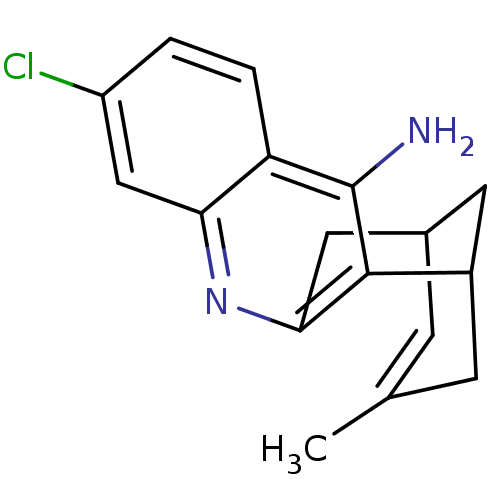 ((+/-)-huprine Y-HCl | (+/-)-huprineY hydrochloride...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Binding affinity to human AChE | J Med Chem 49: 6833-40 (2006) Article DOI: 10.1021/jm060945c BindingDB Entry DOI: 10.7270/Q29K4C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379268 (CHEMBL3216556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AChE by Lineweaver-Burk plot analysis | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271142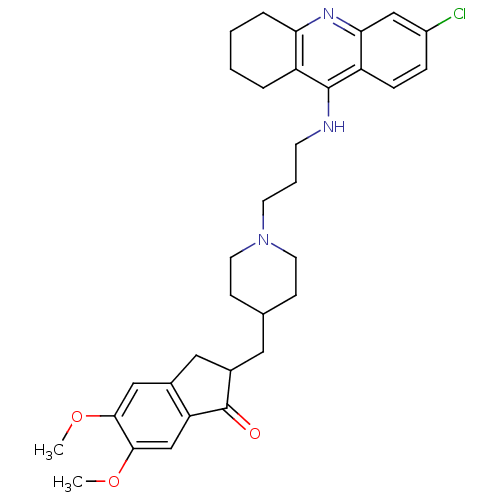 (6-Chloro-9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50369748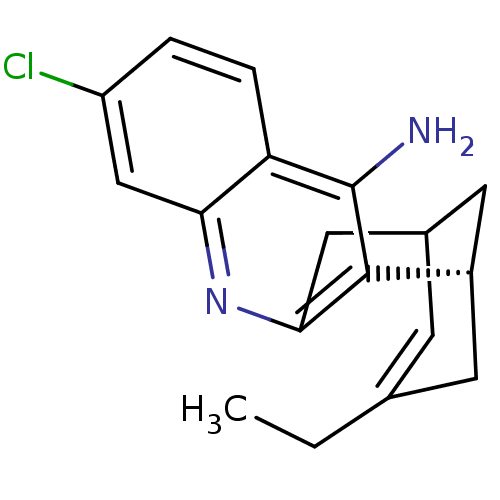 (CHEMBL208599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50369748 (CHEMBL208599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271142 (6-Chloro-9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10590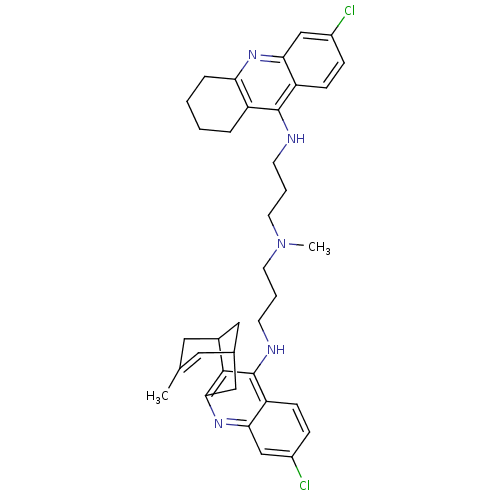 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271140 (9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)methyl]pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10590 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592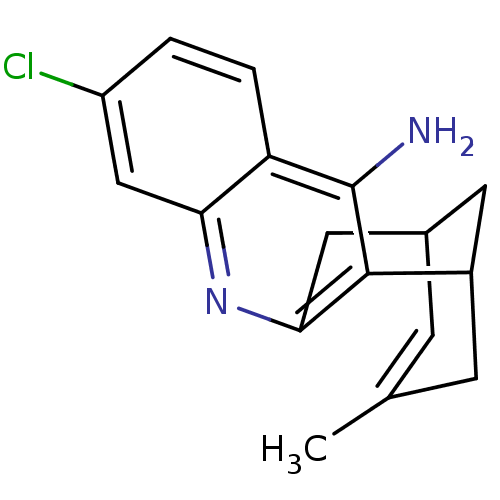 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10586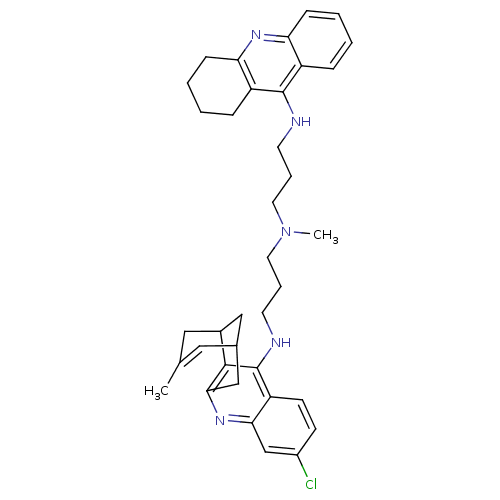 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10585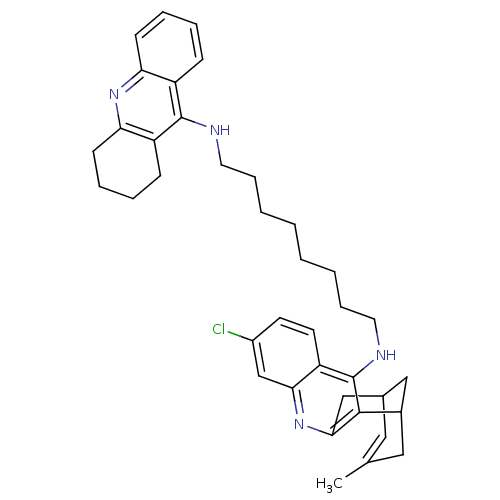 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{8-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10588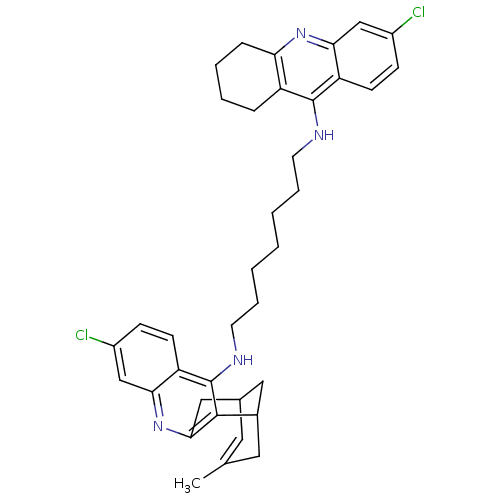 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{7-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10584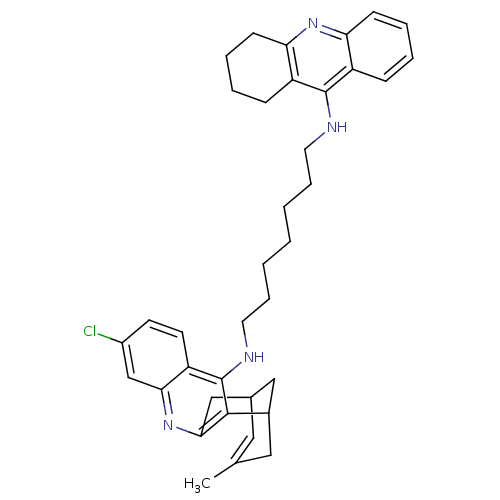 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{7-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10589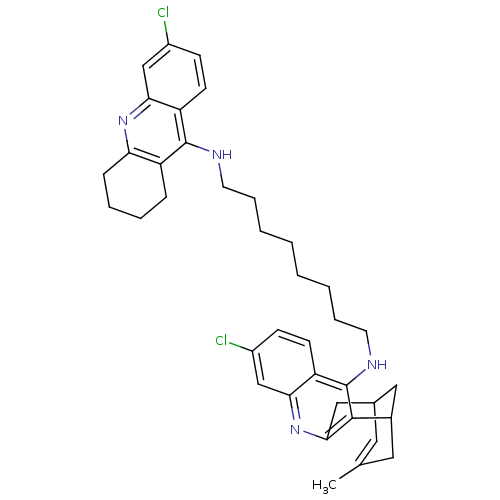 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{8-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10586 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379273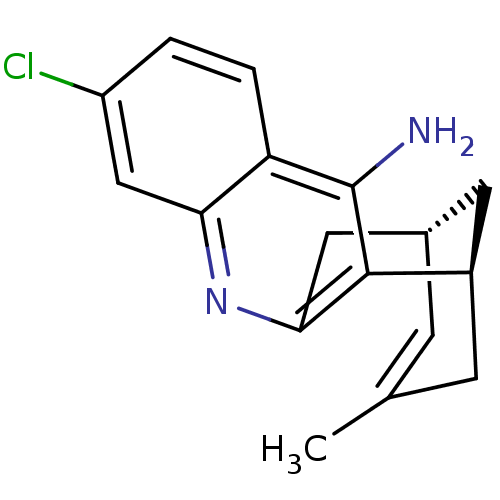 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10587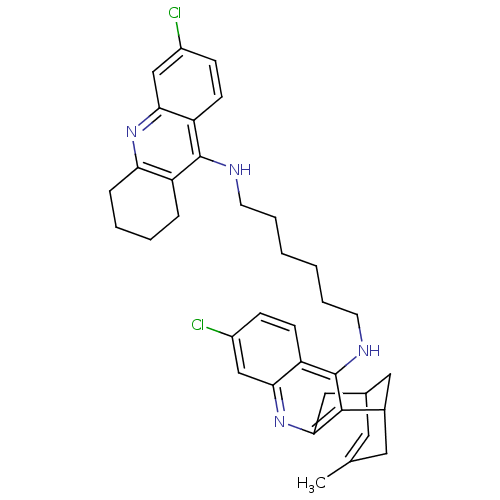 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094626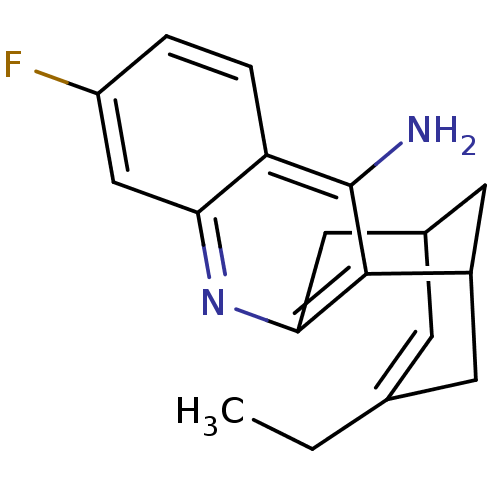 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10583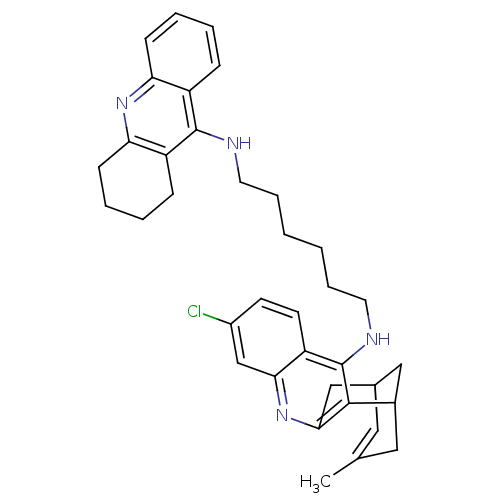 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271141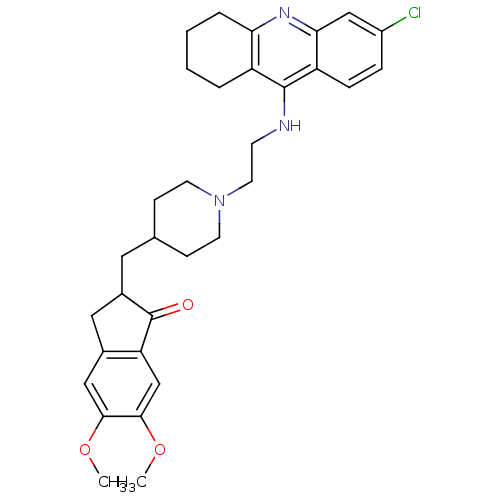 (6-Chloro-9-[(2-{4-[(5,6-dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9063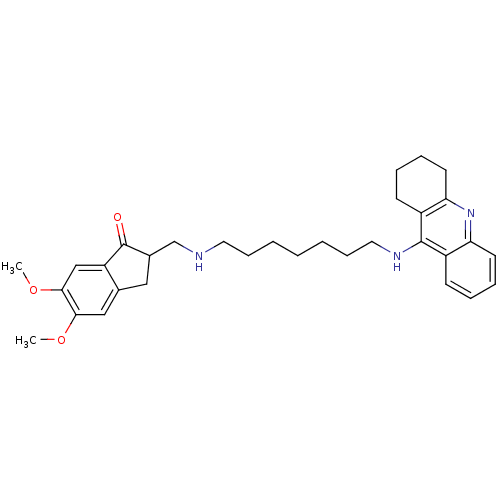 (5,6-Dimethoxy-2-{[7-(1,2,3,4-tetrahydro-acridin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271141 (6-Chloro-9-[(2-{4-[(5,6-dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50200340 ((+/-)-huprine Y-HCl | (+/-)-huprineY hydrochloride...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10587 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369748 (CHEMBL208599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369748 (CHEMBL208599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50200340 ((+/-)-huprine Y-HCl | (+/-)-huprineY hydrochloride...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE | J Med Chem 49: 6833-40 (2006) Article DOI: 10.1021/jm060945c BindingDB Entry DOI: 10.7270/Q29K4C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10582 (15-ethyl-N-[8-(1,2,3,4-tetrahydroacridin-9-ylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271190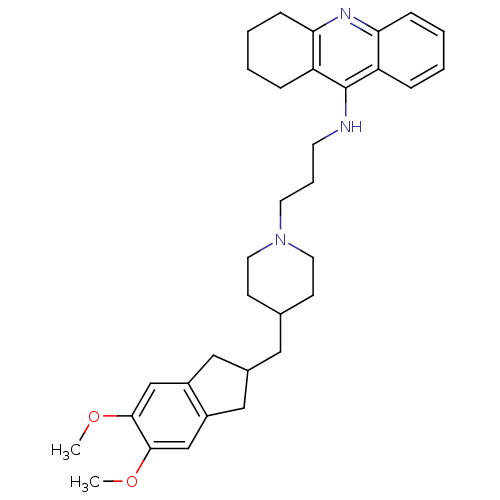 (9-[(3-{4-[(5,6-dimethoxyindan-2-yl)methyl]piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271192 (6-Chloro-9-[(3-{4-[(5,6-dimethoxyindan-2-yl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271140 (9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)methyl]pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10583 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271192 (6-Chloro-9-[(3-{4-[(5,6-dimethoxyindan-2-yl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase of bovine erythrocytes | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50200334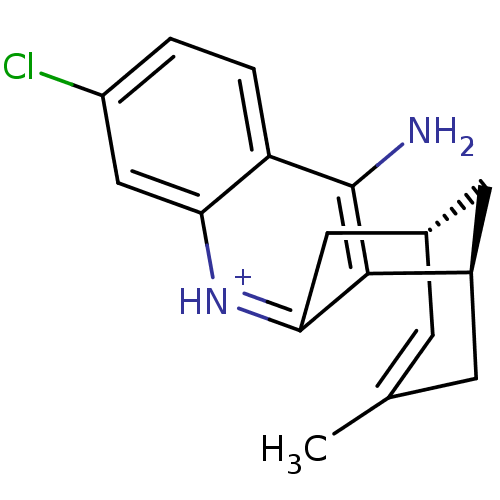 ((-)-(7S,11S)-huprine H3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE | J Med Chem 49: 6833-40 (2006) Article DOI: 10.1021/jm060945c BindingDB Entry DOI: 10.7270/Q29K4C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50200343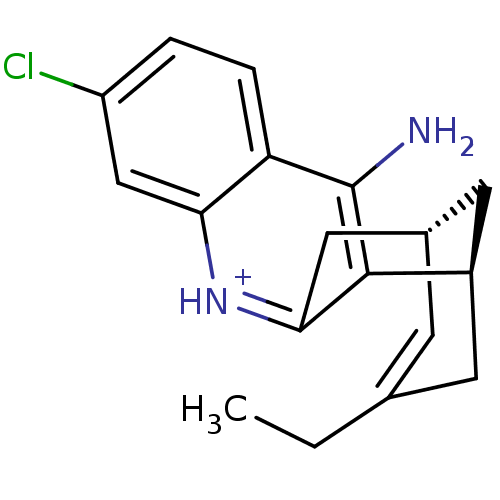 ((-)-(7S,11S)-huprine H7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE | J Med Chem 49: 6833-40 (2006) Article DOI: 10.1021/jm060945c BindingDB Entry DOI: 10.7270/Q29K4C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10589 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{8-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50094630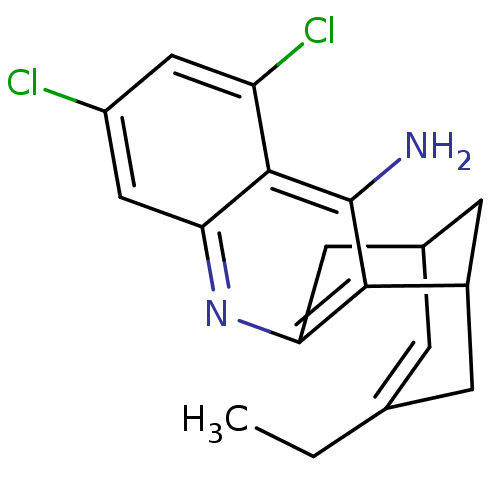 (5,7-dichloro-15-ethyl-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10585 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{8-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50377921 (SODIUM NITROPRUSSIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50094631 (15-ethyl-5,7-difluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase isolated from Human erythrocytes. | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271191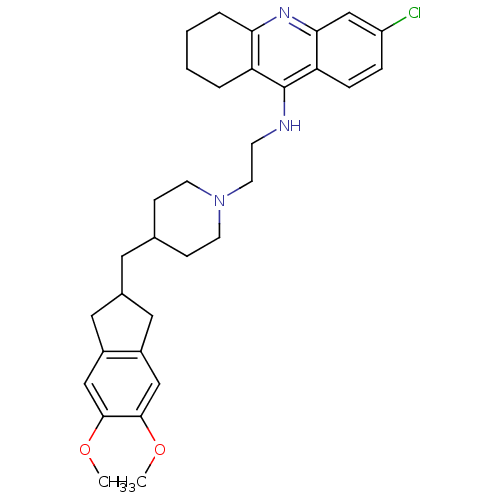 (6-Chloro-9-[(2-{4-[(5,6-dimethoxyindan-2-yl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10584 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{7-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50094626 ((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 0 minutes of incubation | J Med Chem 43: 4657-66 (2001) BindingDB Entry DOI: 10.7270/Q2Q52Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 359 total ) | Next | Last >> |