

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50039655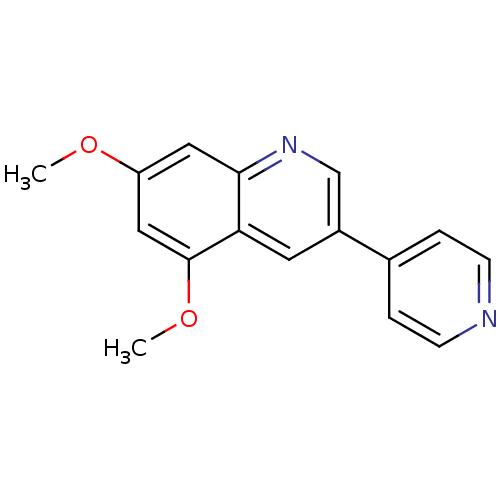 (5,7-Dimethoxy-3-pyridin-4-yl-quinoline | CHEMBL684...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Inhibition of PDGF-stimulated autophosphorylation of PDGF-receptor | J Med Chem 37: 2627-9 (1994) BindingDB Entry DOI: 10.7270/Q2CR5SDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229939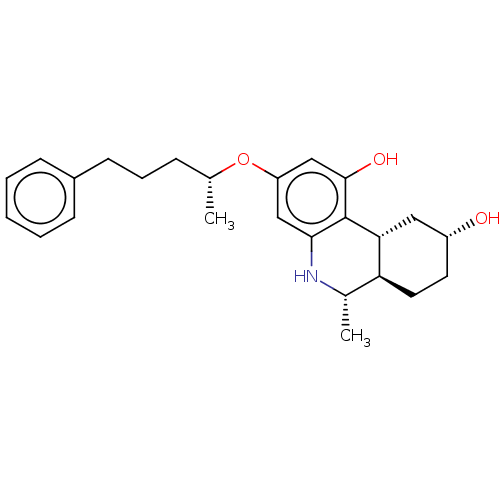 (CHEMBL423481) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042631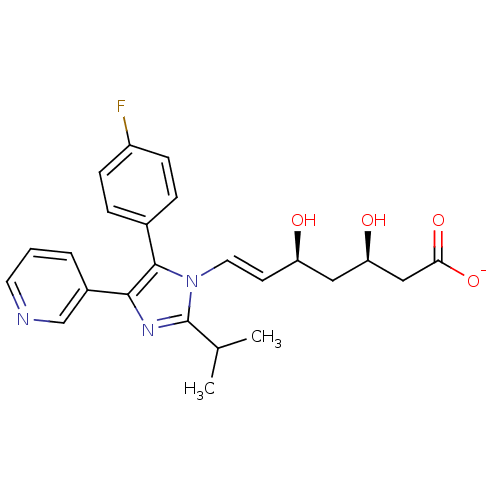 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042625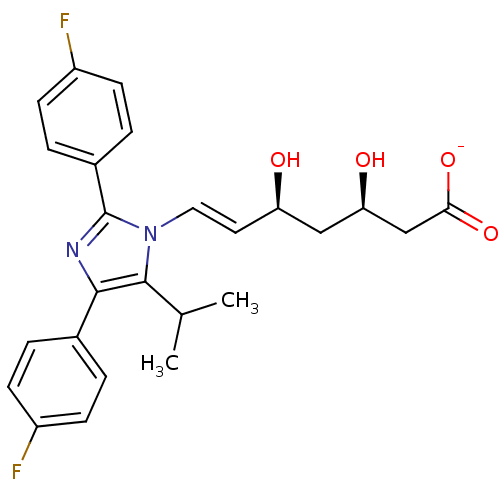 ((E)-(3R,5S)-7-[2,4-Bis-(4-fluoro-phenyl)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042607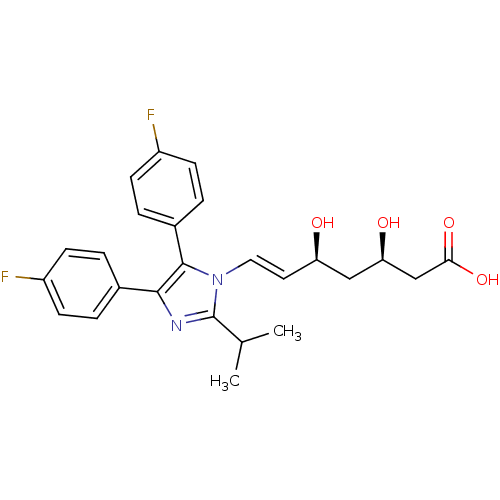 ((E)-(3R,5S)-7-[4,5-Bis-(4-fluoro-phenyl)-2-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042614 (CHEMBL120932 | Sodium; 7-[4,5-bis-(4-fluoro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281061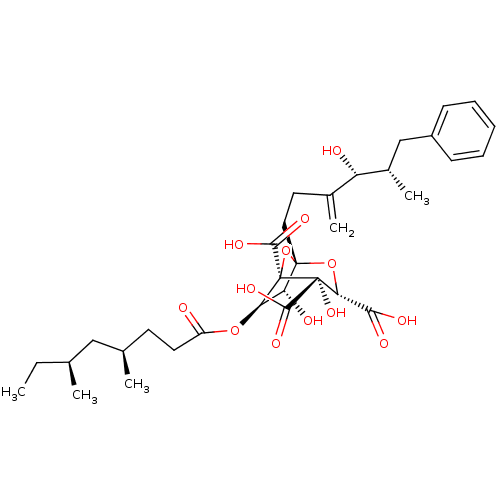 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of Squalene synthase activity, measured using juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462606 (CHEMBL4249022) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042620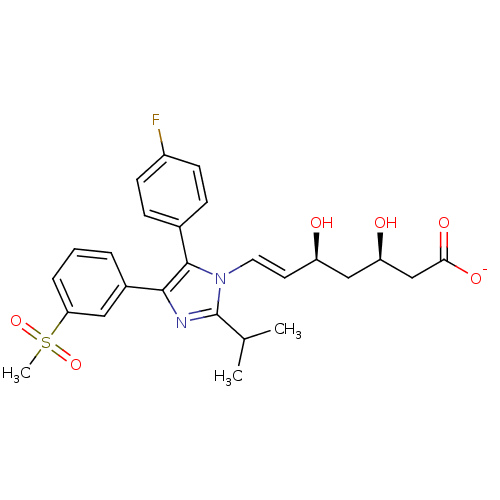 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042615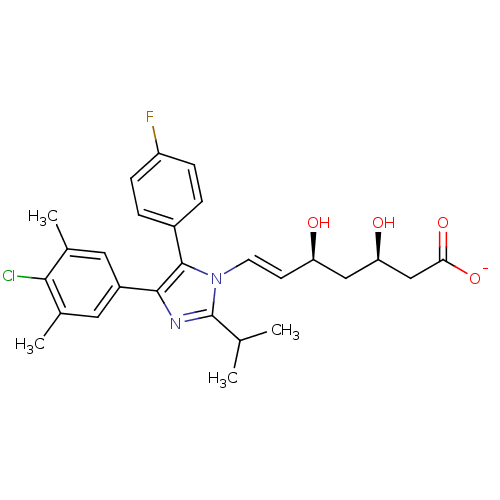 ((E)-(3R,5S)-7-[4-(4-Chloro-3,5-dimethyl-phenyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229940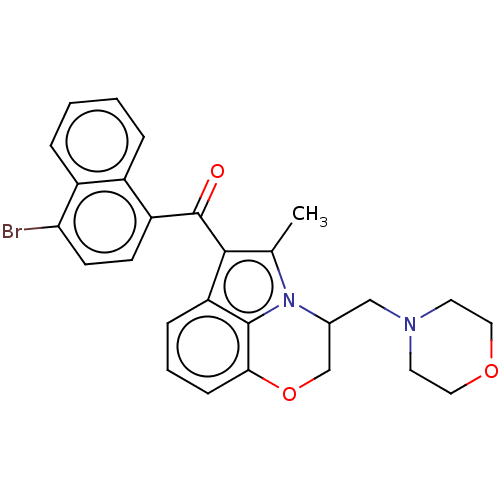 (CHEMBL286248) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462612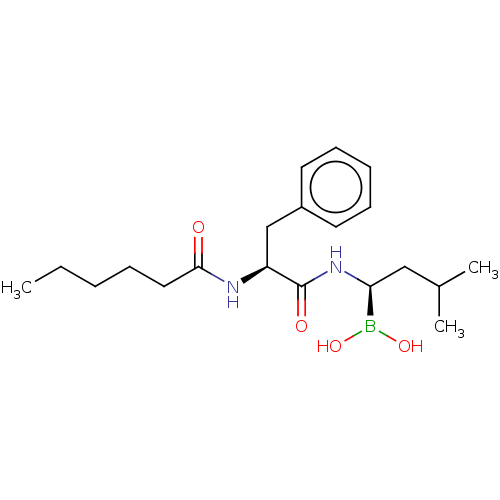 (CHEMBL4246480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50267307 (CHEMBL4085842) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant cytoplasmic GST-tagged EGFR (668 to 1210 residues) expressed in baculovirus using TK-substrate-biotin preincubated fo... | Eur J Med Chem 135: 12-23 (2017) Article DOI: 10.1016/j.ejmech.2017.04.036 BindingDB Entry DOI: 10.7270/Q28P630H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042629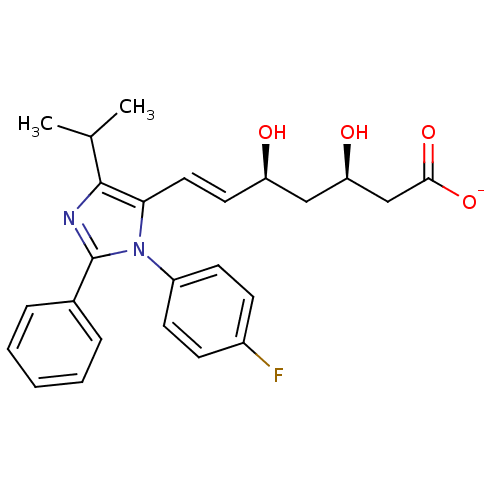 (CHEMBL121309 | Sodium; 7-[3-(4-fluoro-phenyl)-5-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037287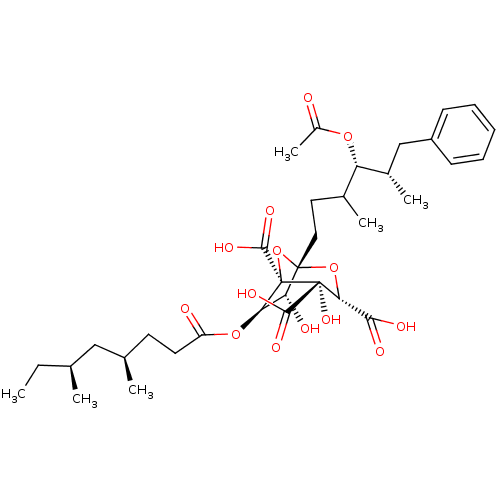 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042622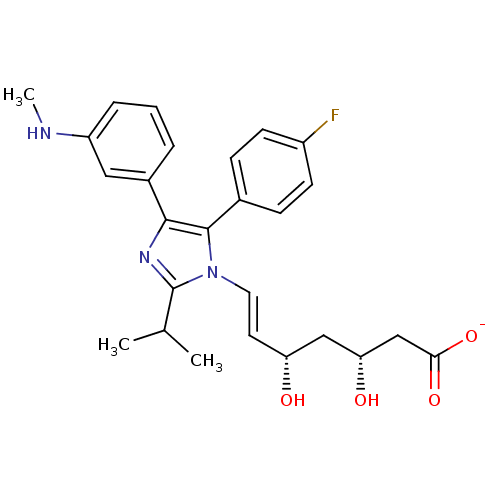 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042601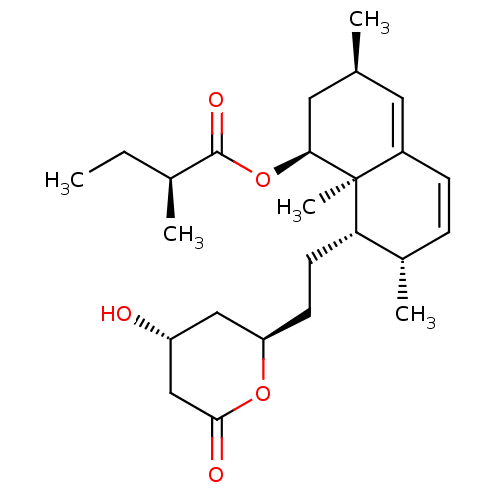 (2-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50267306 (CHEMBL4096021) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant cytoplasmic GST-tagged EGFR (668 to 1210 residues) expressed in baculovirus using TK-substrate-biotin preincubated fo... | Eur J Med Chem 135: 12-23 (2017) Article DOI: 10.1016/j.ejmech.2017.04.036 BindingDB Entry DOI: 10.7270/Q28P630H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462622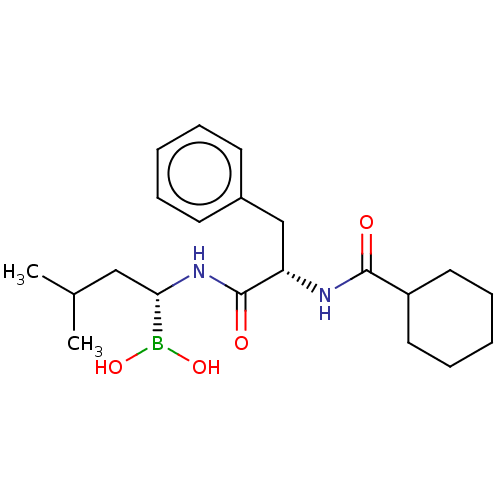 (CHEMBL4238198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462613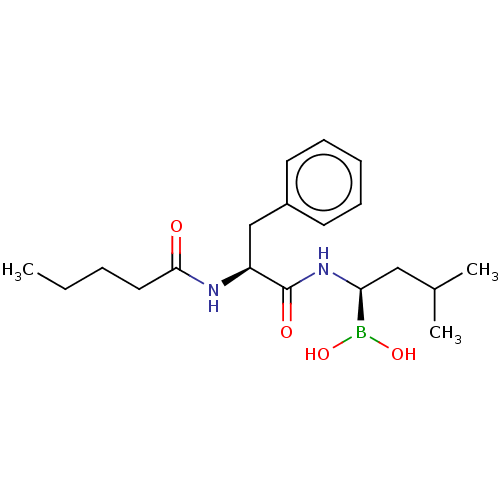 (CHEMBL4246864) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281068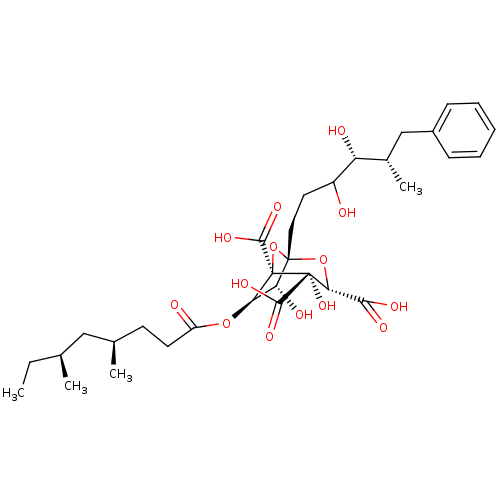 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-3,4-Dihydroxy-5-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of Squalene synthase activity, measured using juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042612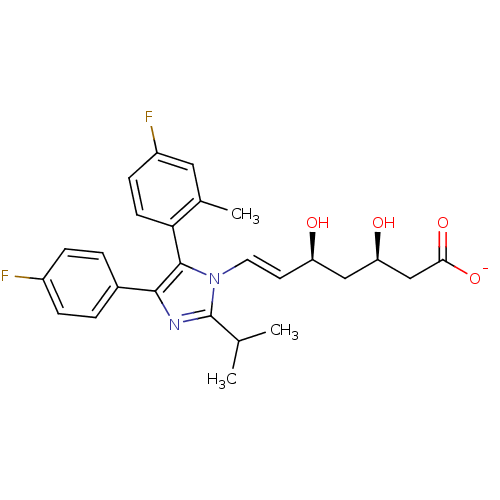 (CHEMBL121610 | Sodium; 7-[5-(4-fluoro-2-methyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462611 (CHEMBL4241473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462620 (CHEMBL4251272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037286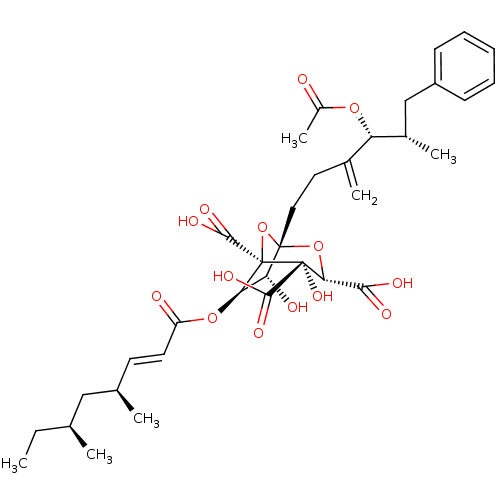 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037285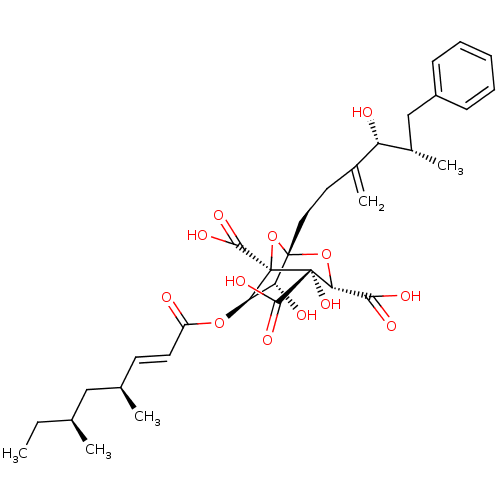 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281062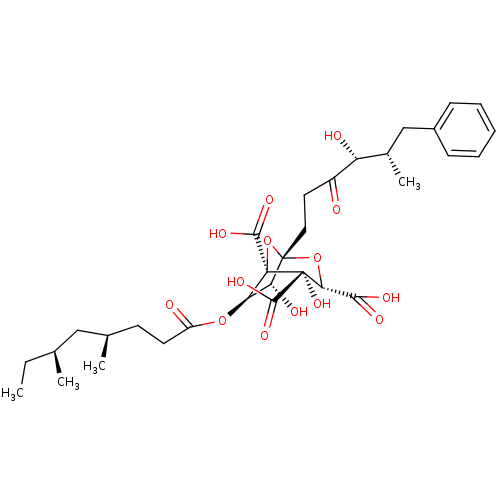 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of Squalene synthase activity, measured using juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037285 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Squalene synthase activity in juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042611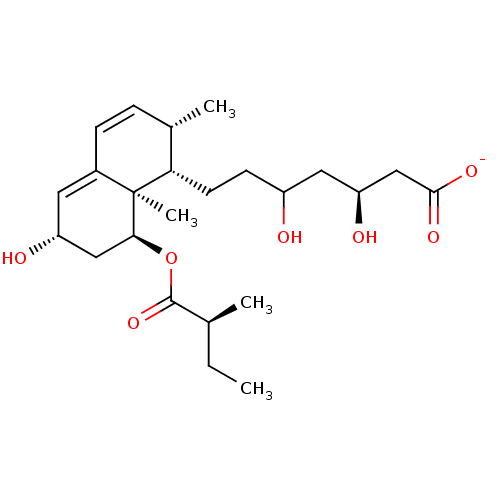 (CHEMBL333003 | Sodium; 3,5-dihydroxy-7-[6-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462605 (CHEMBL4245244) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462619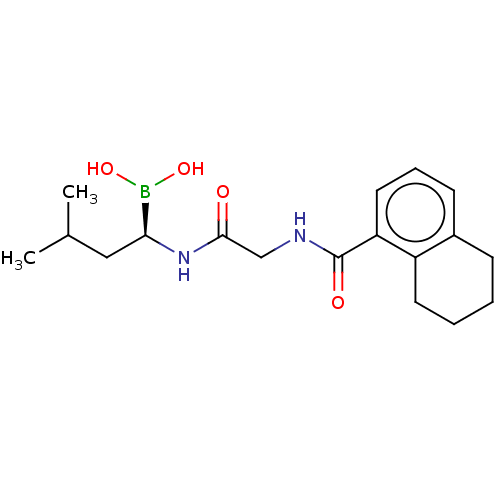 (CHEMBL4238378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Rattus norvegicus) | BDBM50604669 (CHEMBL5208485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01842 BindingDB Entry DOI: 10.7270/Q22V2M6R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462603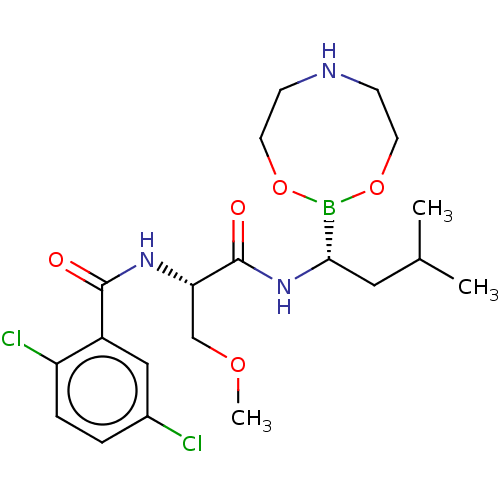 (CHEMBL4248209 | US11542283, Compound V-8A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042616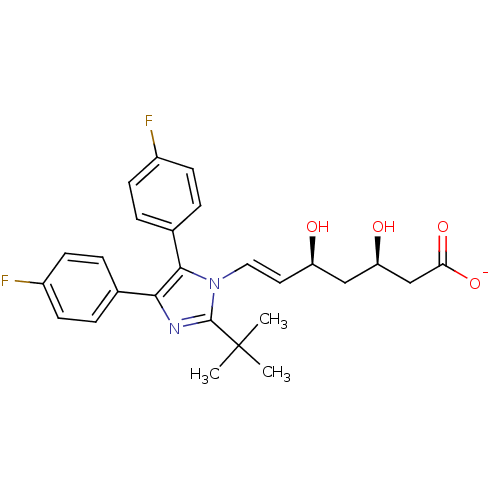 ((E)-(3R,5S)-7-[2-tert-Butyl-4,5-bis-(4-fluoro-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229943 (CHEMBL27388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50398609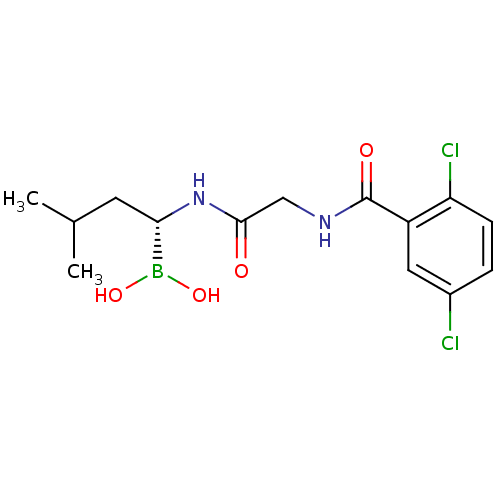 (CHEMBL2141296 | IXAZOMIB CITRATE | Ixazomib | MLN2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037279 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM16596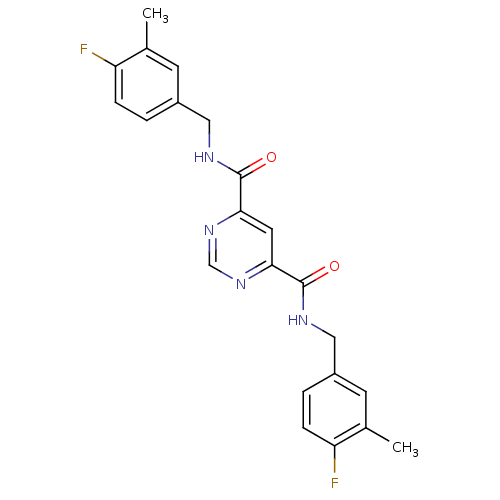 (4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Atlantic University Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP13 expressed in Escherichia coli | Bioorg Med Chem 17: 990-1005 (2009) Article DOI: 10.1016/j.bmc.2008.03.004 BindingDB Entry DOI: 10.7270/Q2GB24ZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042643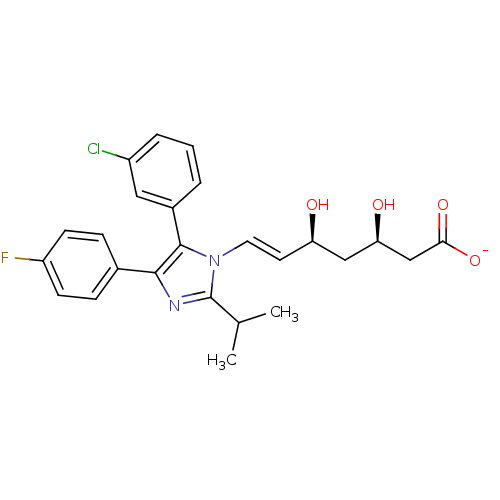 ((E)-(3R,5S)-7-[5-(3-Chloro-phenyl)-4-(4-fluoro-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462604 (CHEMBL4242666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462625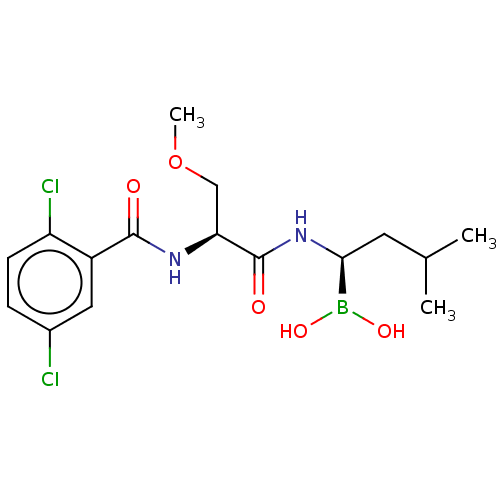 (CHEMBL4243375 | US11542283, Compound IV-8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462621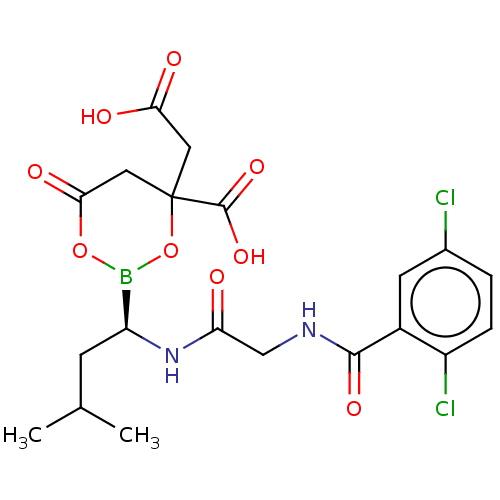 (CHEMBL1813256 | MLN-9708 | US11542283, Compound ML...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50267291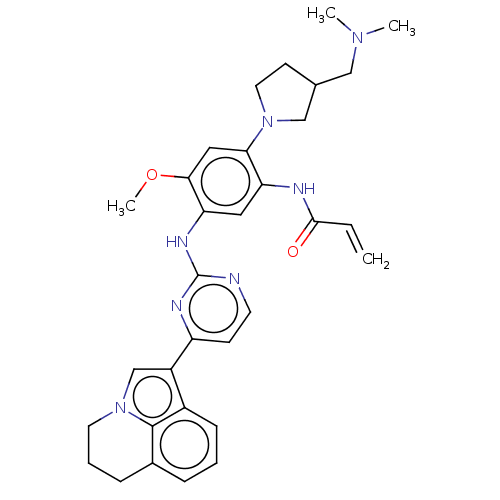 (CHEMBL4098967) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant cytoplasmic GST-tagged EGFR (668 to 1210 residues) expressed in baculovirus using TK-substrate-biotin preincubated fo... | Eur J Med Chem 135: 12-23 (2017) Article DOI: 10.1016/j.ejmech.2017.04.036 BindingDB Entry DOI: 10.7270/Q28P630H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50462610 (CHEMBL4249503) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Forestry University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate incubated for 10 mins followed by substra... | Bioorg Med Chem 26: 3975-3981 (2018) Article DOI: 10.1016/j.bmc.2018.06.020 BindingDB Entry DOI: 10.7270/Q2Q81GQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 403 total ) | Next | Last >> |