 Found 119 hits with Last Name = 'banner' and Initial = 'b'
Found 119 hits with Last Name = 'banner' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371874
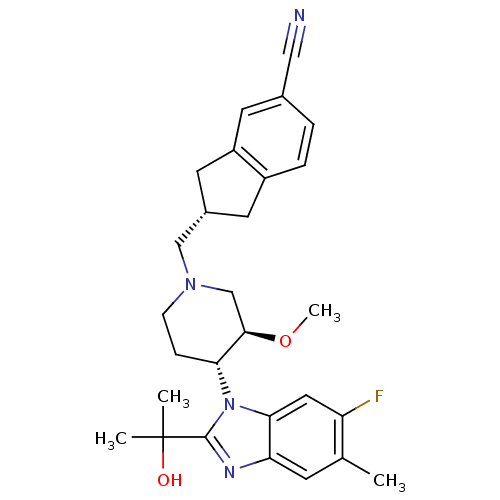
(CHEMBL257733)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(cc3C2)C#N)CC[C@H]1n1c(nc2cc(C)c(F)cc12)C(C)(C)O Show InChI InChI=1S/C28H33FN4O2/c1-17-9-23-25(13-22(17)29)33(27(31-23)28(2,3)34)24-7-8-32(16-26(24)35-4)15-19-11-20-6-5-18(14-30)10-21(20)12-19/h5-6,9-10,13,19,24,26,34H,7-8,11-12,15-16H2,1-4H3/t19-,24+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371872
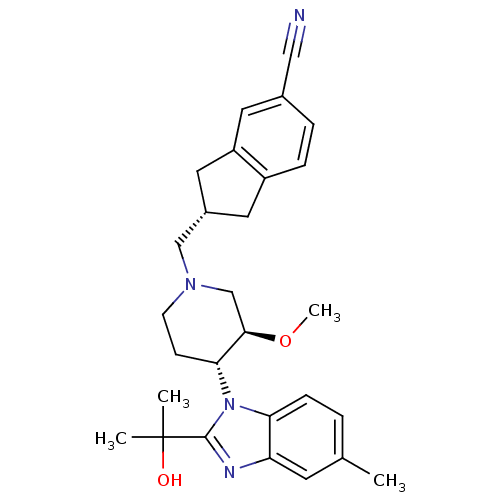
(CHEMBL257280)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(cc3C2)C#N)CC[C@H]1n1c(nc2cc(C)ccc12)C(C)(C)O Show InChI InChI=1S/C28H34N4O2/c1-18-5-8-24-23(11-18)30-27(28(2,3)33)32(24)25-9-10-31(17-26(25)34-4)16-20-13-21-7-6-19(15-29)12-22(21)14-20/h5-8,11-12,20,25-26,33H,9-10,13-14,16-17H2,1-4H3/t20-,25+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371868

(CHEMBL269939)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(Br)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C25H30BrN3O/c1-16-4-7-23-22(10-16)27-17(2)29(23)24-8-9-28(15-25(24)30-3)14-18-11-19-5-6-21(26)13-20(19)12-18/h4-7,10,13,18,24-25H,8-9,11-12,14-15H2,1-3H3/t18-,24+,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371871
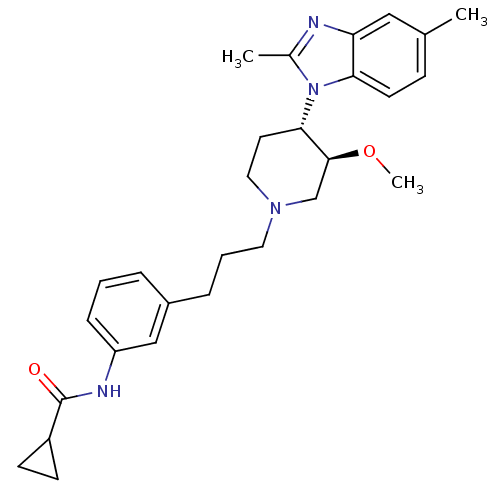
(CHEMBL255112)Show SMILES CO[C@H]1CN(CCCc2cccc(NC(=O)C3CC3)c2)CC[C@@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C28H36N4O2/c1-19-9-12-25-24(16-19)29-20(2)32(25)26-13-15-31(18-27(26)34-3)14-5-7-21-6-4-8-23(17-21)30-28(33)22-10-11-22/h4,6,8-9,12,16-17,22,26-27H,5,7,10-11,13-15,18H2,1-3H3,(H,30,33)/t26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371866
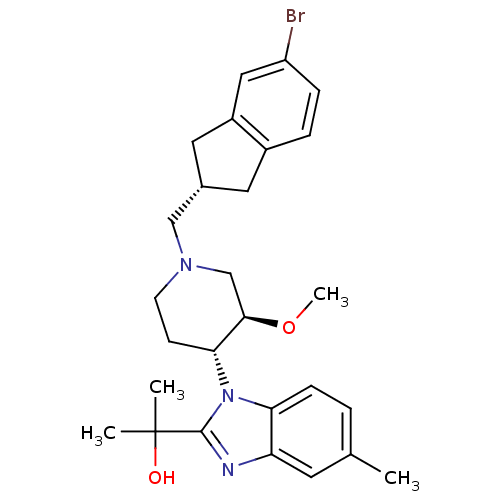
(CHEMBL257906)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(Br)cc3C2)CC[C@H]1n1c(nc2cc(C)ccc12)C(C)(C)O Show InChI InChI=1S/C27H34BrN3O2/c1-17-5-8-23-22(11-17)29-26(27(2,3)32)31(23)24-9-10-30(16-25(24)33-4)15-18-12-19-6-7-21(28)14-20(19)13-18/h5-8,11,14,18,24-25,32H,9-10,12-13,15-16H2,1-4H3/t18-,24+,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371869
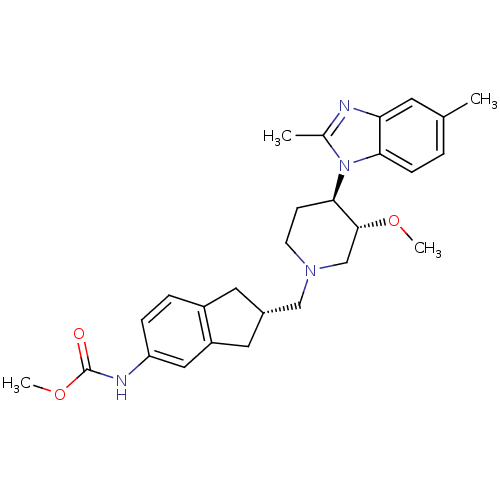
(CHEMBL270151)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(NC(=O)OC)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C27H34N4O3/c1-17-5-8-24-23(11-17)28-18(2)31(24)25-9-10-30(16-26(25)33-3)15-19-12-20-6-7-22(14-21(20)13-19)29-27(32)34-4/h5-8,11,14,19,25-26H,9-10,12-13,15-16H2,1-4H3,(H,29,32)/t19-,25+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371873
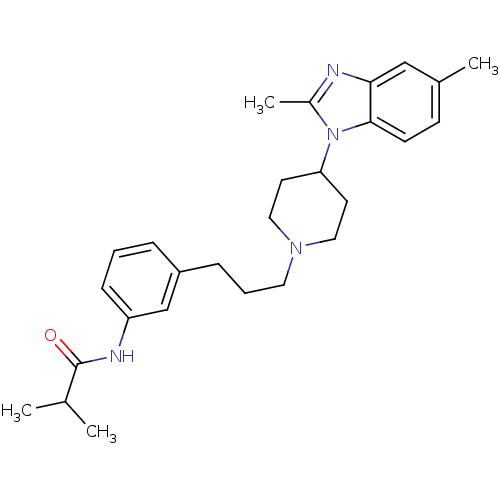
(CHEMBL403730)Show SMILES CC(C)C(=O)Nc1cccc(CCCN2CCC(CC2)n2c(C)nc3cc(C)ccc23)c1 Show InChI InChI=1S/C27H36N4O/c1-19(2)27(32)29-23-9-5-7-22(18-23)8-6-14-30-15-12-24(13-16-30)31-21(4)28-25-17-20(3)10-11-26(25)31/h5,7,9-11,17-19,24H,6,8,12-16H2,1-4H3,(H,29,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371870
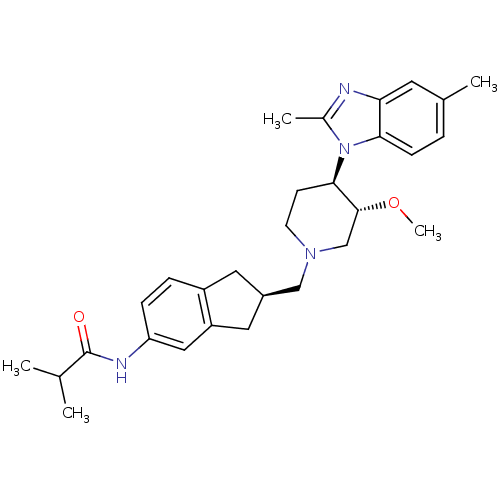
(CHEMBL258260)Show SMILES CO[C@@H]1CN(C[C@@H]2Cc3ccc(NC(=O)C(C)C)cc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C29H38N4O2/c1-18(2)29(34)31-24-8-7-22-13-21(14-23(22)15-24)16-32-11-10-27(28(17-32)35-5)33-20(4)30-25-12-19(3)6-9-26(25)33/h6-9,12,15,18,21,27-28H,10-11,13-14,16-17H2,1-5H3,(H,31,34)/t21-,27-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371867
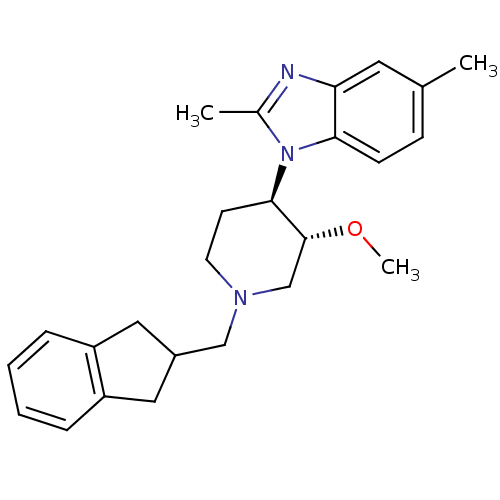
(CHEMBL257905)Show SMILES CO[C@@H]1CN(CC2Cc3ccccc3C2)CC[C@H]1n1c(C)nc2cc(C)ccc12 Show InChI InChI=1S/C25H31N3O/c1-17-8-9-23-22(12-17)26-18(2)28(23)24-10-11-27(16-25(24)29-3)15-19-13-20-6-4-5-7-21(20)14-19/h4-9,12,19,24-25H,10-11,13-16H2,1-3H3/t24-,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50009075
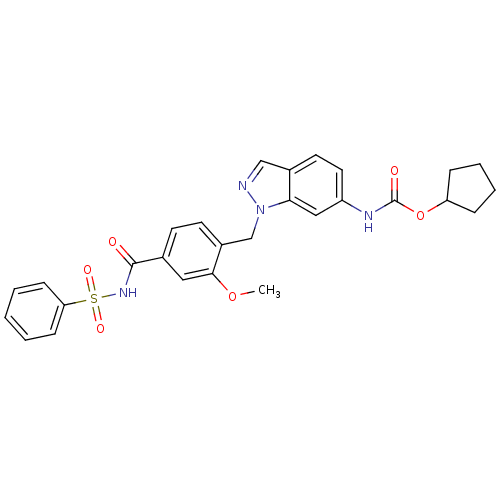
(CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...)Show SMILES COc1cc(ccc1Cn1ncc2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H28N4O6S/c1-37-26-15-19(27(33)31-39(35,36)24-9-3-2-4-10-24)11-12-21(26)18-32-25-16-22(14-13-20(25)17-29-32)30-28(34)38-23-7-5-6-8-23/h2-4,9-17,23H,5-8,18H2,1H3,(H,30,34)(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound to inhibit Inophore-induced arachidonic acid metabolism (inhibition of TXB2 formation) in rat |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402299

(CHEMBL2206064)Show SMILES CNc1nc(N)nc2nc(ccc12)-c1c(Oc2ccccc2)cccc1C(F)(F)F Show InChI InChI=1S/C21H16F3N5O/c1-26-18-13-10-11-15(27-19(13)29-20(25)28-18)17-14(21(22,23)24)8-5-9-16(17)30-12-6-3-2-4-7-12/h2-11H,1H3,(H3,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402291

(CHEMBL2206071)Show SMILES CNc1nc(N)nc2nc(c(cc12)C(O)=O)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C16H12F3N5O2/c1-21-12-9-6-8(14(25)26)11(22-13(9)24-15(20)23-12)7-4-2-3-5-10(7)16(17,18)19/h2-6H,1H3,(H,25,26)(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402302
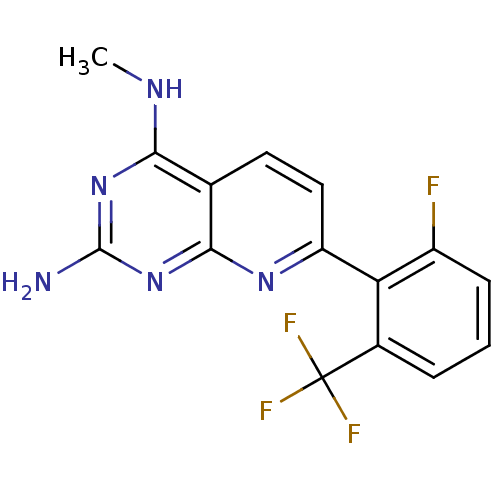
(CHEMBL2205521)Show InChI InChI=1S/C15H11F4N5/c1-21-12-7-5-6-10(22-13(7)24-14(20)23-12)11-8(15(17,18)19)3-2-4-9(11)16/h2-6H,1H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402292
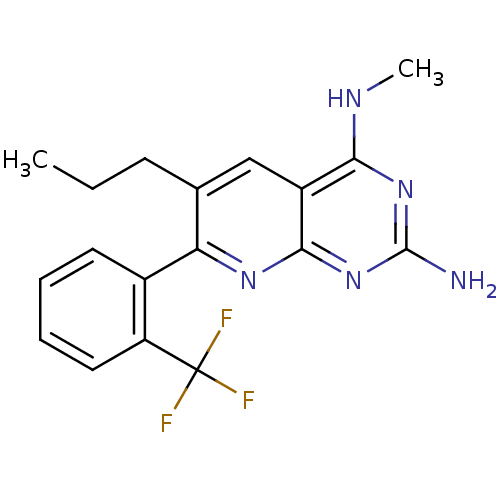
(CHEMBL2206070)Show InChI InChI=1S/C18H18F3N5/c1-3-6-10-9-12-15(23-2)25-17(22)26-16(12)24-14(10)11-7-4-5-8-13(11)18(19,20)21/h4-5,7-9H,3,6H2,1-2H3,(H3,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM7962
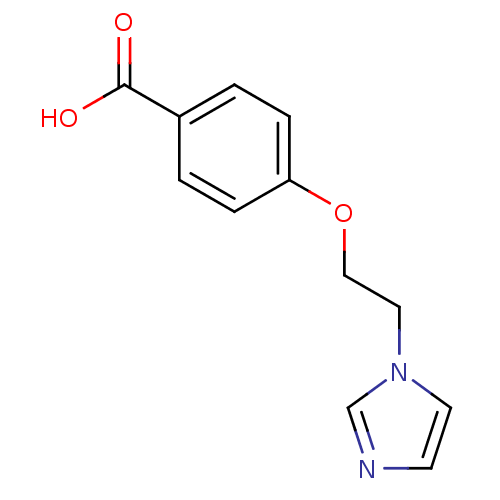
(4-(2-Imidazol-1-yl-ethoxy)-benzoic acid; hydrochlo...)Show InChI InChI=1S/C12H12N2O3/c15-12(16)10-1-3-11(4-2-10)17-8-7-14-6-5-13-9-14/h1-6,9H,7-8H2,(H,15,16) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound to inhibit Inophore-induced arachidonic acid metabolism (inhibition of TXB2 formation) in rat |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402300
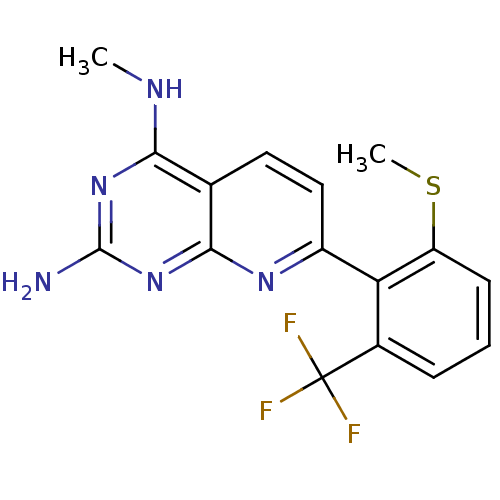
(CHEMBL2206063)Show InChI InChI=1S/C16H14F3N5S/c1-21-13-8-6-7-10(22-14(8)24-15(20)23-13)12-9(16(17,18)19)4-3-5-11(12)25-2/h3-7H,1-2H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402296
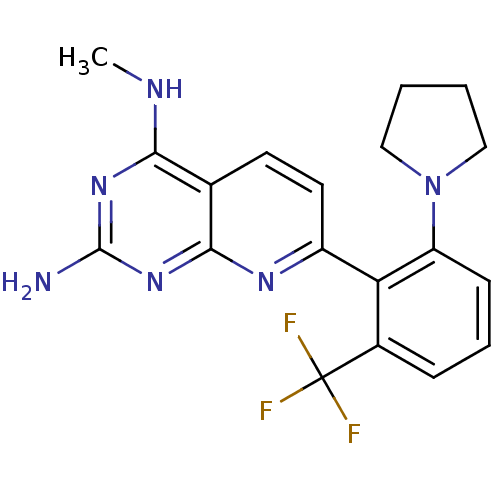
(CHEMBL2206067)Show SMILES CNc1nc(N)nc2nc(ccc12)-c1c(cccc1C(F)(F)F)N1CCCC1 Show InChI InChI=1S/C19H19F3N6/c1-24-16-11-7-8-13(25-17(11)27-18(23)26-16)15-12(19(20,21)22)5-4-6-14(15)28-9-2-3-10-28/h4-8H,2-3,9-10H2,1H3,(H3,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402316

(CHEMBL2206073)Show InChI InChI=1S/C15H12F3N5/c1-20-12-9-6-7-11(21-13(9)23-14(19)22-12)8-4-2-3-5-10(8)15(16,17)18/h2-7H,1H3,(H3,19,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402293

(CHEMBL2203315)Show InChI InChI=1S/C16H14F3N5/c1-8-7-10-13(21-2)23-15(20)24-14(10)22-12(8)9-5-3-4-6-11(9)16(17,18)19/h3-7H,1-2H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402303
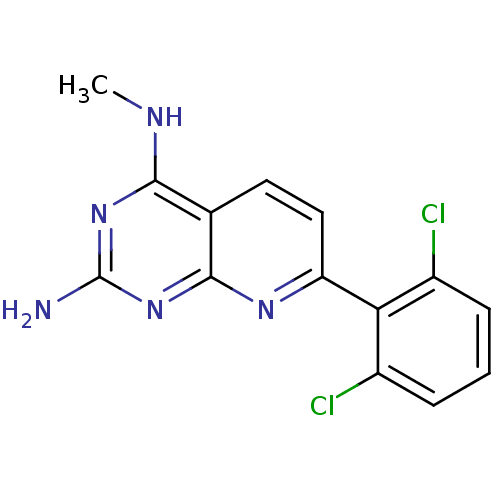
(CHEMBL2205520)Show InChI InChI=1S/C14H11Cl2N5/c1-18-12-7-5-6-10(19-13(7)21-14(17)20-12)11-8(15)3-2-4-9(11)16/h2-6H,1H3,(H3,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402308

(CHEMBL2205515)Show InChI InChI=1S/C14H12BrN5/c1-17-12-9-6-7-11(8-4-2-3-5-10(8)15)18-13(9)20-14(16)19-12/h2-7H,1H3,(H3,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402301
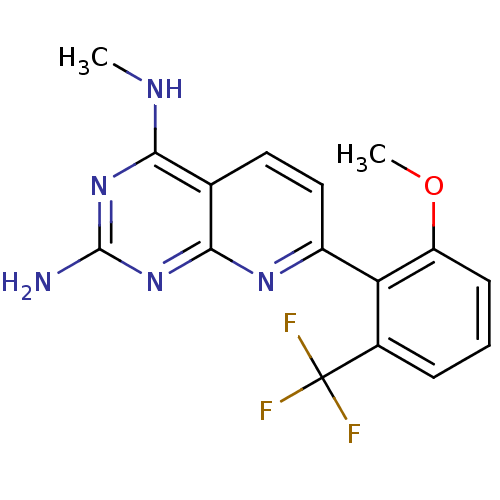
(CHEMBL2205522)Show InChI InChI=1S/C16H14F3N5O/c1-21-13-8-6-7-10(22-14(8)24-15(20)23-13)12-9(16(17,18)19)4-3-5-11(12)25-2/h3-7H,1-2H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402305
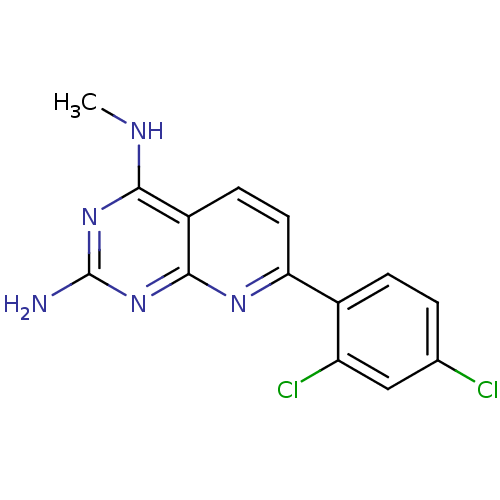
(CHEMBL2205518)Show InChI InChI=1S/C14H11Cl2N5/c1-18-12-9-4-5-11(19-13(9)21-14(17)20-12)8-3-2-7(15)6-10(8)16/h2-6H,1H3,(H3,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402294

(CHEMBL2206069)Show InChI InChI=1S/C18H19FN6/c1-21-16-11-7-8-13(22-17(11)24-18(20)23-16)15-12(19)5-4-6-14(15)25-9-2-3-10-25/h4-8H,2-3,9-10H2,1H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
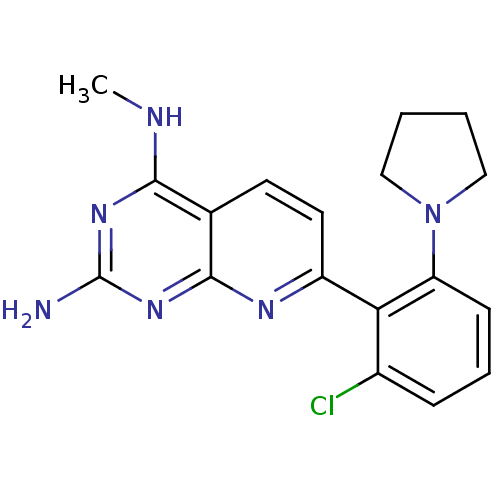
(Homo sapiens (Human)) | BDBM50402295
(CHEMBL2206068)Show InChI InChI=1S/C18H19ClN6/c1-21-16-11-7-8-13(22-17(11)24-18(20)23-16)15-12(19)5-4-6-14(15)25-9-2-3-10-25/h4-8H,2-3,9-10H2,1H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402304

(CHEMBL2205519)Show InChI InChI=1S/C14H11Cl2N5/c1-18-12-8-3-5-11(19-13(8)21-14(17)20-12)9-6-7(15)2-4-10(9)16/h2-6H,1H3,(H3,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402290

(CHEMBL2206072)Show SMILES CNc1nc(N)nc2nc(-c3ccccc3C(F)(F)F)c(cc12)S(C)(=O)=O Show InChI InChI=1S/C16H14F3N5O2S/c1-21-13-9-7-11(27(2,25)26)12(22-14(9)24-15(20)23-13)8-5-3-4-6-10(8)16(17,18)19/h3-7H,1-2H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50228307

(CGP-35949)Show SMILES CCCc1c(O)c(ccc1OCCCOc1cc(NC(=O)c2nn[nH]n2)c(C)cc1Cl)C(C)=O Show InChI InChI=1S/C23H26ClN5O5/c1-4-6-16-19(8-7-15(14(3)30)21(16)31)33-9-5-10-34-20-12-18(13(2)11-17(20)24)25-23(32)22-26-28-29-27-22/h7-8,11-12,31H,4-6,9-10H2,1-3H3,(H,25,32)(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402298

(CHEMBL2206065)Show SMILES CNc1nc(N)nc2nc(ccc12)-c1c(cccc1C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C19H19F3N6O/c1-24-16-11-5-6-13(25-17(11)27-18(23)26-16)15-12(19(20,21)22)3-2-4-14(15)28-7-9-29-10-8-28/h2-6H,7-10H2,1H3,(H3,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402307

(CHEMBL2205516)Show InChI InChI=1S/C14H12ClN5/c1-17-12-9-6-7-11(8-4-2-3-5-10(8)15)18-13(9)20-14(16)19-12/h2-7H,1H3,(H3,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
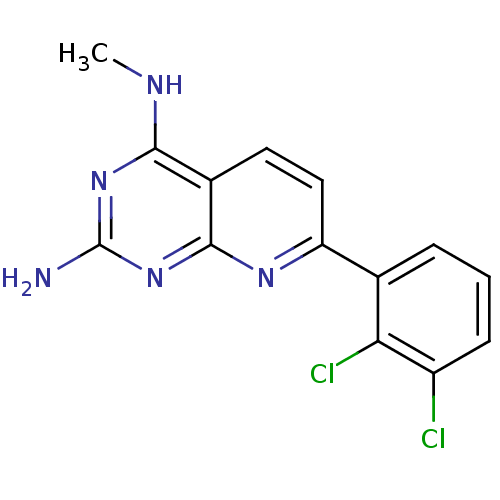
(Homo sapiens (Human)) | BDBM50402306
(CHEMBL2205517)Show InChI InChI=1S/C14H11Cl2N5/c1-18-12-8-5-6-10(19-13(8)21-14(17)20-12)7-3-2-4-9(15)11(7)16/h2-6H,1H3,(H3,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
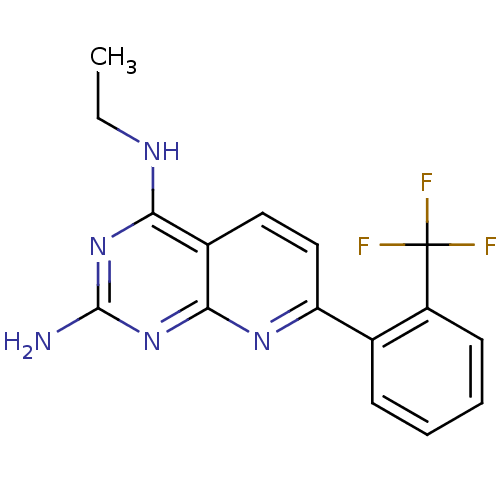
(Homo sapiens (Human)) | BDBM50402315
(CHEMBL2206074)Show InChI InChI=1S/C16H14F3N5/c1-2-21-13-10-7-8-12(22-14(10)24-15(20)23-13)9-5-3-4-6-11(9)16(17,18)19/h3-8H,2H2,1H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402297
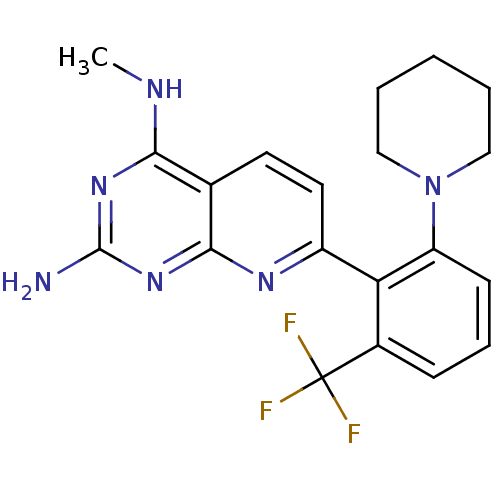
(CHEMBL2206066)Show SMILES CNc1nc(N)nc2nc(ccc12)-c1c(cccc1C(F)(F)F)N1CCCCC1 Show InChI InChI=1S/C20H21F3N6/c1-25-17-12-8-9-14(26-18(12)28-19(24)27-17)16-13(20(21,22)23)6-5-7-15(16)29-10-3-2-4-11-29/h5-9H,2-4,10-11H2,1H3,(H3,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
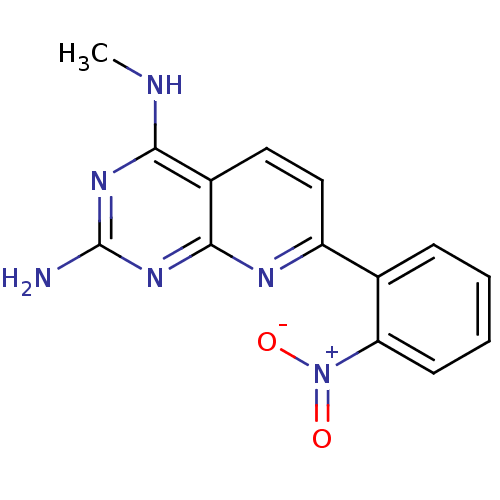
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402310
(CHEMBL2205513)Show InChI InChI=1S/C14H12N6O2/c1-16-12-9-6-7-10(17-13(9)19-14(15)18-12)8-4-2-3-5-11(8)20(21)22/h2-7H,1H3,(H3,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
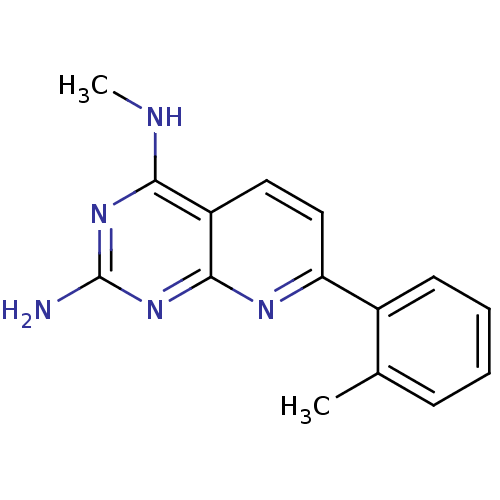
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402312
(CHEMBL2206077)Show InChI InChI=1S/C15H15N5/c1-9-5-3-4-6-10(9)12-8-7-11-13(17-2)19-15(16)20-14(11)18-12/h3-8H,1-2H3,(H3,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402313

(CHEMBL2206076)Show InChI InChI=1S/C16H14F3N5O/c17-16(18,19)11-4-2-1-3-9(11)12-6-5-10-13(21-7-8-25)23-15(20)24-14(10)22-12/h1-6,25H,7-8H2,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50228304
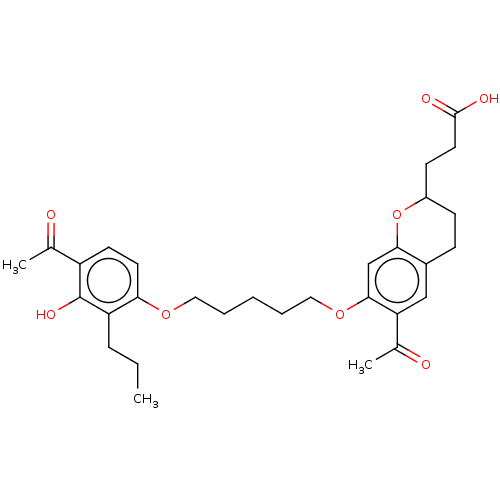
(CHEMBL417028)Show SMILES CCCc1c(O)c(ccc1OCCCCCOc1cc2OC(CCC(O)=O)CCc2cc1C(C)=O)C(C)=O Show InChI InChI=1S/C30H38O8/c1-4-8-24-26(13-12-23(19(2)31)30(24)35)36-15-6-5-7-16-37-28-18-27-21(17-25(28)20(3)32)9-10-22(38-27)11-14-29(33)34/h12-13,17-18,22,35H,4-11,14-16H2,1-3H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50017208
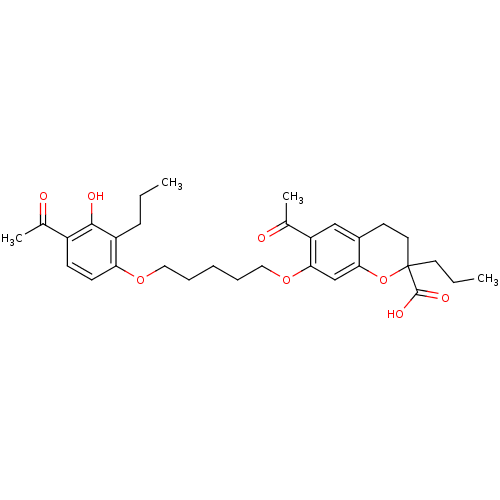
(6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...)Show SMILES CCCc1c(O)c(ccc1OCCCCCOc1cc2OC(CCC)(CCc2cc1C(C)=O)C(O)=O)C(C)=O Show InChI InChI=1S/C31H40O8/c1-5-10-24-26(12-11-23(20(3)32)29(24)34)37-16-8-7-9-17-38-28-19-27-22(18-25(28)21(4)33)13-15-31(39-27,14-6-2)30(35)36/h11-12,18-19,34H,5-10,13-17H2,1-4H3,(H,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound to block binding of [3H]-leukotriene D4 to Cysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50228313

(CHEMBL55714)Show SMILES CCCc1c(O)c(ccc1OCCCCCOc1cc2OC(CCc2cc1C(C)=O)C(=O)NS(=O)(=O)c1ccccc1)C(C)=O Show InChI InChI=1S/C34H39NO9S/c1-4-11-27-29(17-15-26(22(2)36)33(27)38)42-18-9-6-10-19-43-32-21-31-24(20-28(32)23(3)37)14-16-30(44-31)34(39)35-45(40,41)25-12-7-5-8-13-25/h5,7-8,12-13,15,17,20-21,30,38H,4,6,9-11,14,16,18-19H2,1-3H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50000838
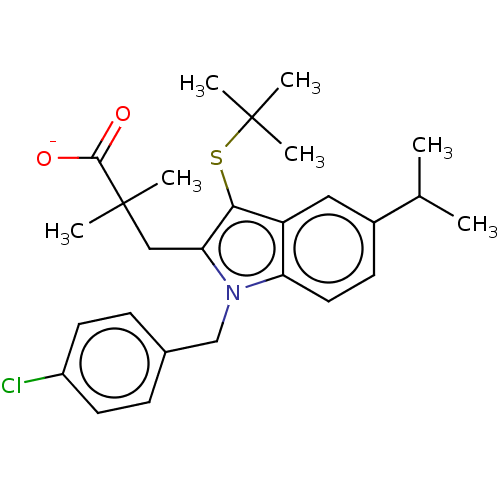
(CHEMBL416657 | Sodium; 3-[3-tert-butylsulfanyl-1-(...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C([O-])=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound to inhibit Inophore-induced arachidonic acid metabolism (inhibition of TXB2 formation) in rat |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402309
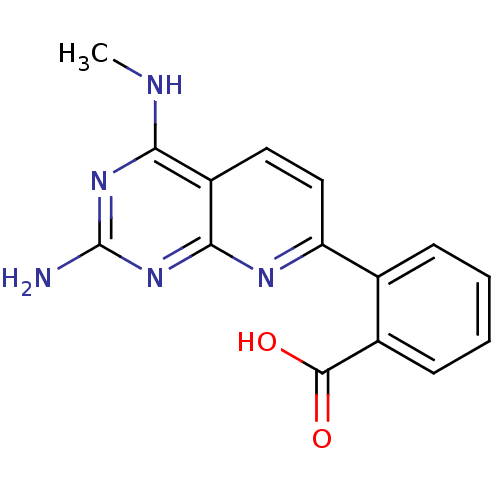
(CHEMBL2205514)Show InChI InChI=1S/C15H13N5O2/c1-17-12-10-6-7-11(18-13(10)20-15(16)19-12)8-4-2-3-5-9(8)14(21)22/h2-7H,1H3,(H,21,22)(H3,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402314
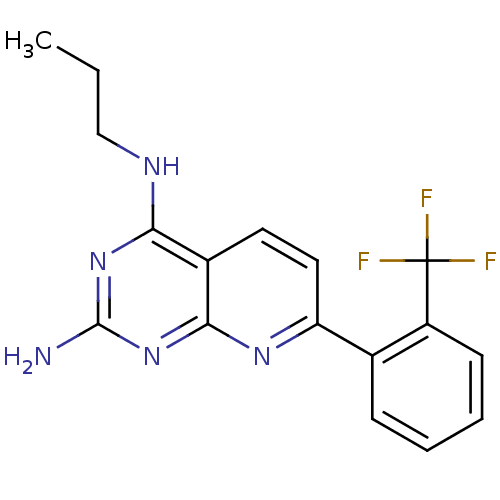
(CHEMBL2206075)Show InChI InChI=1S/C17H16F3N5/c1-2-9-22-14-11-7-8-13(23-15(11)25-16(21)24-14)10-5-3-4-6-12(10)17(18,19)20/h3-8H,2,9H2,1H3,(H3,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402311
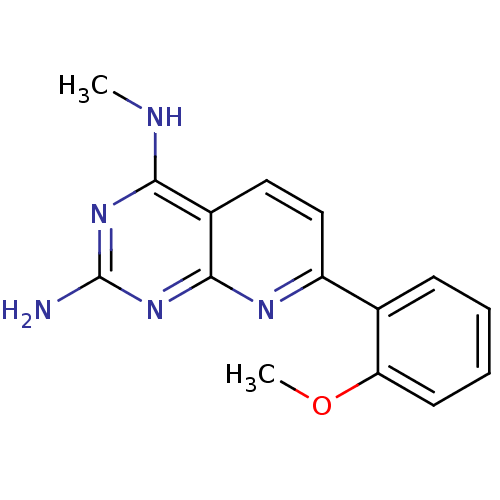
(CHEMBL2205512)Show InChI InChI=1S/C15H15N5O/c1-17-13-10-7-8-11(18-14(10)20-15(16)19-13)9-5-3-4-6-12(9)21-2/h3-8H,1-2H3,(H3,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009071

(6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...)Show SMILES CCCc1c(O)c(ccc1OCCCCCOc1cc2OC(CCc2cc1C(C)=O)C(O)=O)C(C)=O Show InChI InChI=1S/C28H34O8/c1-4-8-21-23(12-10-20(17(2)29)27(21)31)34-13-6-5-7-14-35-26-16-25-19(15-22(26)18(3)30)9-11-24(36-25)28(32)33/h10,12,15-16,24,31H,4-9,11,13-14H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound to block binding of [3H]-leukotriene D4 to Cysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50006812
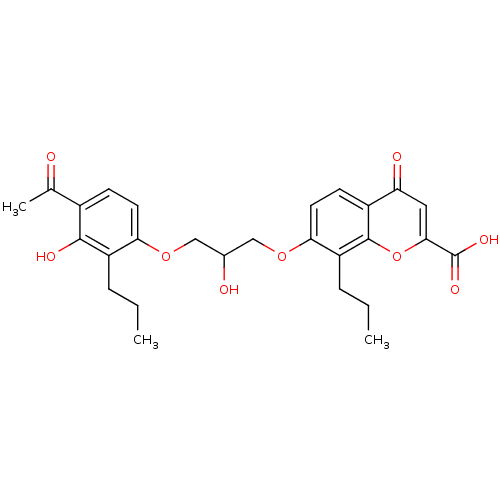
(7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydro...)Show SMILES CCCc1c(OCC(O)COc2ccc3c(oc(cc3=O)C(O)=O)c2CCC)ccc(C(C)=O)c1O Show InChI InChI=1S/C27H30O9/c1-4-6-19-22(10-8-17(15(3)28)25(19)31)34-13-16(29)14-35-23-11-9-18-21(30)12-24(27(32)33)36-26(18)20(23)7-5-2/h8-12,16,29,31H,4-7,13-14H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50228311
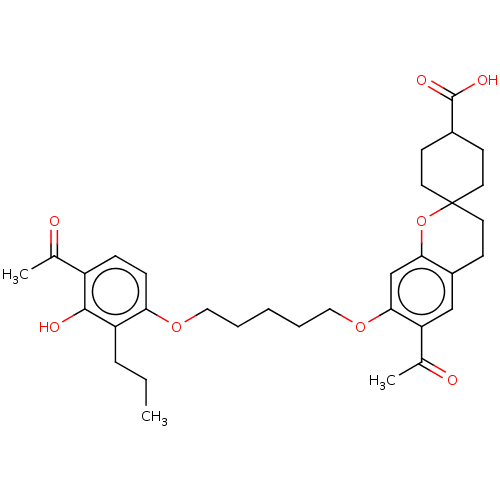
(CHEMBL53333)Show SMILES CCCc1c(O)c(ccc1OCCCCCOc1cc2OC3(CCC(CC3)C(O)=O)CCc2cc1C(C)=O)C(C)=O |(11.43,-34.65,;11.43,-33.09,;12.75,-32.34,;12.75,-30.8,;11.43,-30.03,;10.08,-30.8,;11.43,-28.49,;12.75,-27.7,;14.1,-28.47,;14.1,-30.03,;15.43,-30.8,;16.76,-30.01,;18.11,-30.78,;19.42,-30.01,;20.77,-30.78,;22.1,-30.01,;23.43,-30.78,;24.77,-30,;26.09,-30.78,;27.43,-30,;28.75,-30.75,;30.07,-29.98,;30.06,-31.52,;31.38,-32.29,;32.7,-31.54,;32.72,-30,;31.39,-29.21,;34.04,-32.32,;35.39,-31.55,;34.02,-33.86,;30.07,-28.44,;28.75,-27.67,;27.41,-28.46,;26.06,-27.7,;24.75,-28.47,;23.4,-27.7,;22.08,-28.49,;23.4,-26.16,;10.08,-27.72,;8.77,-28.49,;10.08,-26.18,)| Show InChI InChI=1S/C33H42O8/c1-4-8-26-28(10-9-25(21(2)34)31(26)36)39-17-6-5-7-18-40-30-20-29-24(19-27(30)22(3)35)13-16-33(41-29)14-11-23(12-15-33)32(37)38/h9-10,19-20,23,36H,4-8,11-18H2,1-3H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50017201
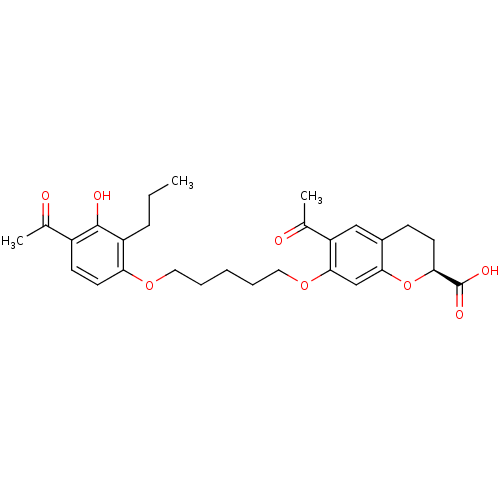
(6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...)Show SMILES CCCc1c(O)c(ccc1OCCCCCOc1cc2O[C@@H](CCc2cc1C(C)=O)C(O)=O)C(C)=O Show InChI InChI=1S/C28H34O8/c1-4-8-21-23(12-10-20(17(2)29)27(21)31)34-13-6-5-7-14-35-26-16-25-19(15-22(26)18(3)30)9-11-24(36-25)28(32)33/h10,12,15-16,24,31H,4-9,11,13-14H2,1-3H3,(H,32,33)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Invitro activity of the compound to inhibit binding of [3H]- leukotriene D4 to receptor sites in guinea pig lung membrane |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50228301
(CHEMBL54688)Show SMILES CCCc1c(O)c(ccc1OCCCCCOc1ccc2CCC(Oc2c1C(C)=O)C(O)=O)C(C)=O Show InChI InChI=1S/C28H34O8/c1-4-8-21-22(14-11-20(17(2)29)26(21)31)34-15-6-5-7-16-35-23-12-9-19-10-13-24(28(32)33)36-27(19)25(23)18(3)30/h9,11-12,14,24,31H,4-8,10,13,15-16H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity to block binding of [3H]leukotriene D4 to LTD4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50017202
(6-Acetyl-7-[6-(4-acetyl-3-hydroxy-2-propyl-phenoxy...)Show SMILES CCCc1c(O)c(ccc1OCCCCCCOc1cc2OC(CCc2cc1C(C)=O)C(O)=O)C(C)=O Show InChI InChI=1S/C29H36O8/c1-4-9-22-24(13-11-21(18(2)30)28(22)32)35-14-7-5-6-8-15-36-27-17-26-20(16-23(27)19(3)31)10-12-25(37-26)29(33)34/h11,13,16-17,25,32H,4-10,12,14-15H2,1-3H3,(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound to block binding of [3H]-leukotriene D4 to Cysteinyl leukotriene D4 receptor sites in homogenized guinea pig lung |
J Med Chem 32: 1842-60 (1989)
BindingDB Entry DOI: 10.7270/Q28W3C8B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data