 Found 94 hits with Last Name = 'bao' and Initial = 'b'
Found 94 hits with Last Name = 'bao' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373939
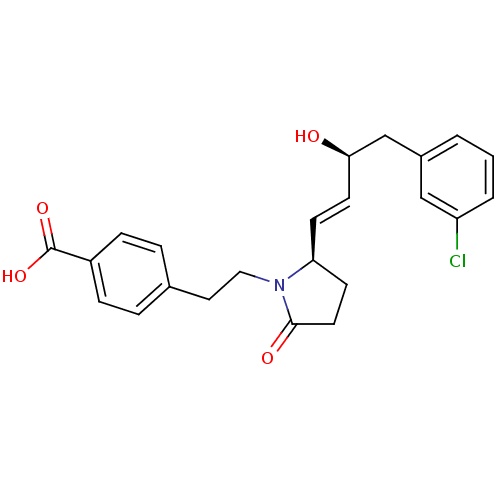
(CHEMBL258332)Show SMILES O[C@@H](Cc1cccc(Cl)c1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24ClNO4/c24-19-3-1-2-17(14-19)15-21(26)10-8-20-9-11-22(27)25(20)13-12-16-4-6-18(7-5-16)23(28)29/h1-8,10,14,20-21,26H,9,11-13,15H2,(H,28,29)/b10-8+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373942
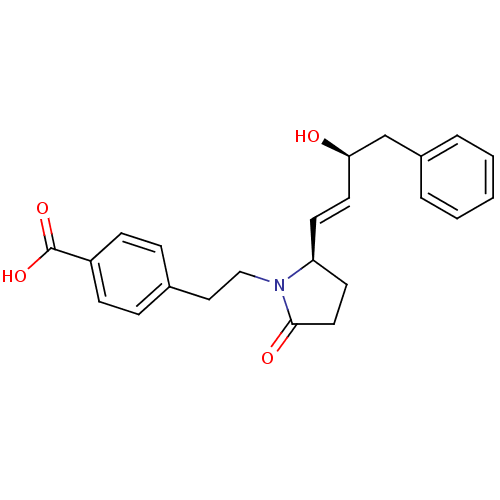
(CHEMBL272276)Show SMILES O[C@@H](Cc1ccccc1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO4/c25-21(16-18-4-2-1-3-5-18)12-10-20-11-13-22(26)24(20)15-14-17-6-8-19(9-7-17)23(27)28/h1-10,12,20-21,25H,11,13-16H2,(H,27,28)/b12-10+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50213980

(4-(2-((R)-2-((S)-3-hydroxyhept-1-enyl)-5-oxopyrrol...)Show SMILES CCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H27NO4/c1-2-3-4-18(22)11-9-17-10-12-19(23)21(17)14-13-15-5-7-16(8-6-15)20(24)25/h5-9,11,17-18,22H,2-4,10,12-14H2,1H3,(H,24,25)/b11-9+/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373941
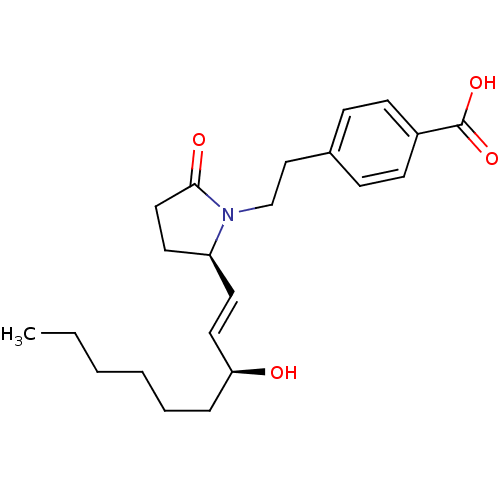
(CHEMBL257217)Show SMILES CCCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H31NO4/c1-2-3-4-5-6-20(24)13-11-19-12-14-21(25)23(19)16-15-17-7-9-18(10-8-17)22(26)27/h7-11,13,19-20,24H,2-6,12,14-16H2,1H3,(H,26,27)/b13-11+/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50156547
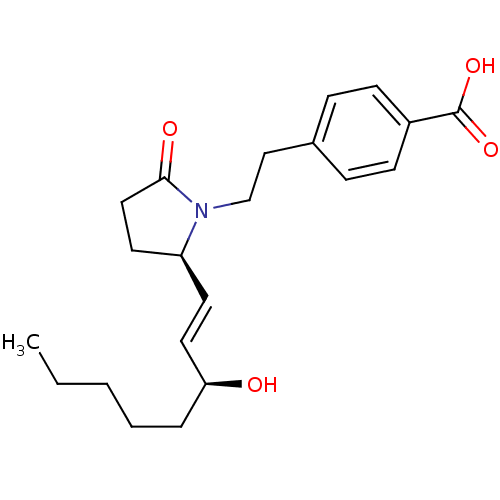
(4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C21H29NO4/c1-2-3-4-5-19(23)12-10-18-11-13-20(24)22(18)15-14-16-6-8-17(9-7-16)21(25)26/h6-10,12,18-19,23H,2-5,11,13-15H2,1H3,(H,25,26)/b12-10+/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373944
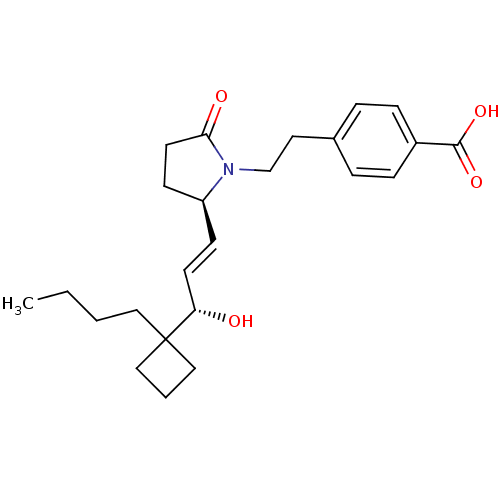
(CHEMBL272277)Show SMILES CCCCC1(CCC1)[C@@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H33NO4/c1-2-3-14-24(15-4-16-24)21(26)11-9-20-10-12-22(27)25(20)17-13-18-5-7-19(8-6-18)23(28)29/h5-9,11,20-21,26H,2-4,10,12-17H2,1H3,(H,28,29)/b11-9+/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373940
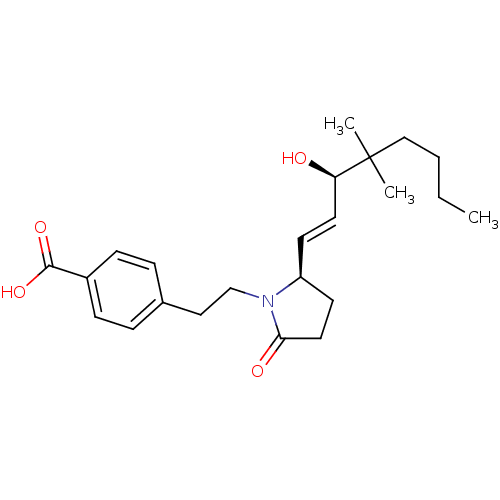
(CHEMBL257658)Show SMILES CCCCC(C)(C)[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H33NO4/c1-4-5-15-23(2,3)20(25)12-10-19-11-13-21(26)24(19)16-14-17-6-8-18(9-7-17)22(27)28/h6-10,12,19-20,25H,4-5,11,13-16H2,1-3H3,(H,27,28)/b12-10+/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373944

(CHEMBL272277)Show SMILES CCCCC1(CCC1)[C@@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H33NO4/c1-2-3-14-24(15-4-16-24)21(26)11-9-20-10-12-22(27)25(20)17-13-18-5-7-19(8-6-18)23(28)29/h5-9,11,20-21,26H,2-4,10,12-17H2,1H3,(H,28,29)/b11-9+/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373945

(CHEMBL271488)Show SMILES CCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H25NO4/c1-2-3-17(21)10-8-16-9-11-18(22)20(16)13-12-14-4-6-15(7-5-14)19(23)24/h4-8,10,16-17,21H,2-3,9,11-13H2,1H3,(H,23,24)/b10-8+/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373941

(CHEMBL257217)Show SMILES CCCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H31NO4/c1-2-3-4-5-6-20(24)13-11-19-12-14-21(25)23(19)16-15-17-7-9-18(10-8-17)22(26)27/h7-11,13,19-20,24H,2-6,12,14-16H2,1H3,(H,26,27)/b13-11+/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50156547

(4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C21H29NO4/c1-2-3-4-5-19(23)12-10-18-11-13-20(24)22(18)15-14-16-6-8-17(9-7-16)21(25)26/h6-10,12,18-19,23H,2-5,11,13-15H2,1H3,(H,25,26)/b12-10+/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373940

(CHEMBL257658)Show SMILES CCCCC(C)(C)[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H33NO4/c1-4-5-15-23(2,3)20(25)12-10-19-11-13-21(26)24(19)16-14-17-6-8-18(9-7-17)22(27)28/h6-10,12,19-20,25H,4-5,11,13-16H2,1-3H3,(H,27,28)/b12-10+/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50213980

(4-(2-((R)-2-((S)-3-hydroxyhept-1-enyl)-5-oxopyrrol...)Show SMILES CCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H27NO4/c1-2-3-4-18(22)11-9-17-10-12-19(23)21(17)14-13-15-5-7-16(8-6-15)20(24)25/h5-9,11,17-18,22H,2-4,10,12-14H2,1H3,(H,24,25)/b11-9+/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373943
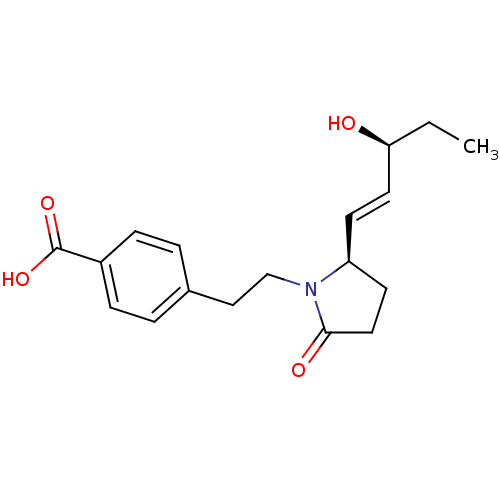
(CHEMBL272969)Show SMILES CC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H23NO4/c1-2-16(20)9-7-15-8-10-17(21)19(15)12-11-13-3-5-14(6-4-13)18(22)23/h3-7,9,15-16,20H,2,8,10-12H2,1H3,(H,22,23)/b9-7+/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373939

(CHEMBL258332)Show SMILES O[C@@H](Cc1cccc(Cl)c1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24ClNO4/c24-19-3-1-2-17(14-19)15-21(26)10-8-20-9-11-22(27)25(20)13-12-16-4-6-18(7-5-16)23(28)29/h1-8,10,14,20-21,26H,9,11-13,15H2,(H,28,29)/b10-8+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373942

(CHEMBL272276)Show SMILES O[C@@H](Cc1ccccc1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO4/c25-21(16-18-4-2-1-3-5-18)12-10-20-11-13-22(26)24(20)15-14-17-6-8-19(9-7-17)23(27)28/h1-10,12,20-21,25H,11,13-16H2,(H,27,28)/b12-10+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373943

(CHEMBL272969)Show SMILES CC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H23NO4/c1-2-16(20)9-7-15-8-10-17(21)19(15)12-11-13-3-5-14(6-4-13)18(22)23/h3-7,9,15-16,20H,2,8,10-12H2,1H3,(H,22,23)/b9-7+/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373945

(CHEMBL271488)Show SMILES CCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H25NO4/c1-2-3-17(21)10-8-16-9-11-18(22)20(16)13-12-14-4-6-15(7-5-14)19(23)24/h4-8,10,16-17,21H,2-3,9,11-13H2,1H3,(H,23,24)/b10-8+/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50386430

(CHEMBL2047536)Show SMILES Cc1c2CC[C@H](NC(=O)c3cc(nc4ccnn34)C(=O)NCc3ccc4OCC(=O)Nc4c3)c2ccc1C(O)=O |r| Show InChI InChI=1S/C28H24N6O6/c1-14-16-5-6-19(18(16)4-3-17(14)28(38)39)33-27(37)22-11-21(31-24-8-9-30-34(22)24)26(36)29-12-15-2-7-23-20(10-15)32-25(35)13-40-23/h2-4,7-11,19H,5-6,12-13H2,1H3,(H,29,36)(H,32,35)(H,33,37)(H,38,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Alantos Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 using MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 as substrate preincubated for 10 mins measured by fluorescence analys... |
J Med Chem 55: 709-16 (2012)
Article DOI: 10.1021/jm201152u
BindingDB Entry DOI: 10.7270/Q2668F73 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50386429

(CHEMBL2047537)Show SMILES Cc1c2CC[C@H](NC(=O)c3cc(nc4c(F)cnn34)C(=O)NCc3ccc(F)c(c3)C(F)(F)F)c2ccc1C(O)=O |r| Show InChI InChI=1S/C27H20F5N5O4/c1-12-14-5-7-20(16(14)4-3-15(12)26(40)41)36-25(39)22-9-21(35-23-19(29)11-34-37(22)23)24(38)33-10-13-2-6-18(28)17(8-13)27(30,31)32/h2-4,6,8-9,11,20H,5,7,10H2,1H3,(H,33,38)(H,36,39)(H,40,41)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Alantos Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 using MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 as substrate preincubated for 10 mins measured by fluorescence analys... |
J Med Chem 55: 709-16 (2012)
Article DOI: 10.1021/jm201152u
BindingDB Entry DOI: 10.7270/Q2668F73 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442739

(CHEMBL2442801)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2cccc(OCCCCCCC(=O)NO)c2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C34H38N2O10/c1-41-27-12-19(13-28(42-2)33(27)38)30-22-15-25-26(46-18-45-25)16-23(22)32(24-17-44-34(39)31(24)30)35-20-8-7-9-21(14-20)43-11-6-4-3-5-10-29(37)36-40/h7-9,12-16,24,30-32,35,38,40H,3-6,10-11,17-18H2,1-2H3,(H,36,37)/t24-,30+,31-,32+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442740
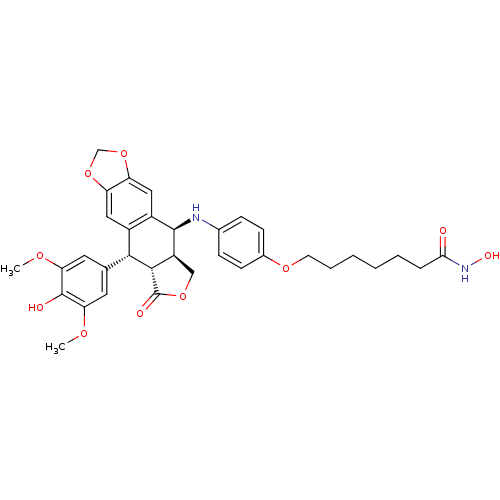
(CHEMBL2442800)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccc(OCCCCCCC(=O)NO)cc2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C34H38N2O10/c1-41-27-13-19(14-28(42-2)33(27)38)30-22-15-25-26(46-18-45-25)16-23(22)32(24-17-44-34(39)31(24)30)35-20-8-10-21(11-9-20)43-12-6-4-3-5-7-29(37)36-40/h8-11,13-16,24,30-32,35,38,40H,3-7,12,17-18H2,1-2H3,(H,36,37)/t24-,30+,31-,32+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442737
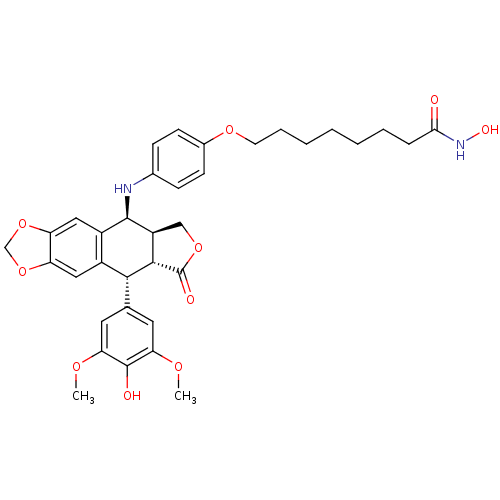
(CHEMBL2442691)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccc(OCCCCCCCC(=O)NO)cc2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C35H40N2O10/c1-42-28-14-20(15-29(43-2)34(28)39)31-23-16-26-27(47-19-46-26)17-24(23)33(25-18-45-35(40)32(25)31)36-21-9-11-22(12-10-21)44-13-7-5-3-4-6-8-30(38)37-41/h9-12,14-17,25,31-33,36,39,41H,3-8,13,18-19H2,1-2H3,(H,37,38)/t25-,31+,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442736

(CHEMBL2442692)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2cccc(OCCCCCCCC(=O)NO)c2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C35H40N2O10/c1-42-28-13-20(14-29(43-2)34(28)39)31-23-16-26-27(47-19-46-26)17-24(23)33(25-18-45-35(40)32(25)31)36-21-9-8-10-22(15-21)44-12-7-5-3-4-6-11-30(38)37-41/h8-10,13-17,25,31-33,36,39,41H,3-7,11-12,18-19H2,1-2H3,(H,37,38)/t25-,31+,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442721
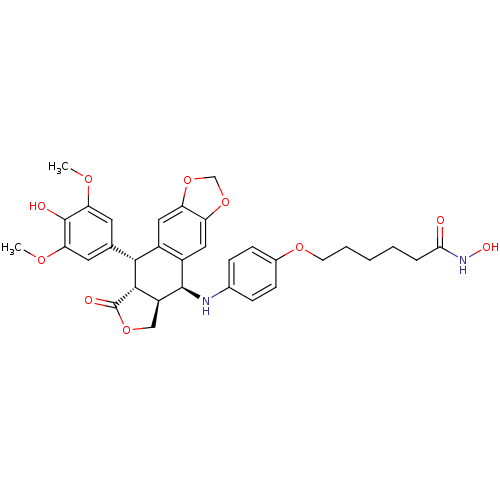
(CHEMBL2442797)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccc(OCCCCCC(=O)NO)cc2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C33H36N2O10/c1-40-26-12-18(13-27(41-2)32(26)37)29-21-14-24-25(45-17-44-24)15-22(21)31(23-16-43-33(38)30(23)29)34-19-7-9-20(10-8-19)42-11-5-3-4-6-28(36)35-39/h7-10,12-15,23,29-31,34,37,39H,3-6,11,16-17H2,1-2H3,(H,35,36)/t23-,29+,30-,31+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442720

(CHEMBL2442798)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2cccc(OCCCCCC(=O)NO)c2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C33H36N2O10/c1-40-26-11-18(12-27(41-2)32(26)37)29-21-14-24-25(45-17-44-24)15-22(21)31(23-16-43-33(38)30(23)29)34-19-7-6-8-20(13-19)42-10-5-3-4-9-28(36)35-39/h6-8,11-15,23,29-31,34,37,39H,3-5,9-10,16-17H2,1-2H3,(H,35,36)/t23-,29+,30-,31+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442723
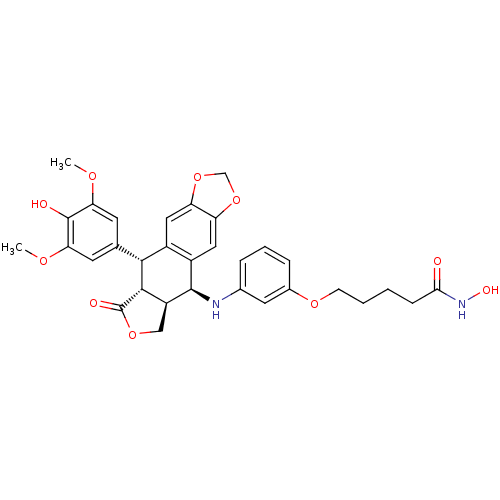
(CHEMBL2442795)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2cccc(OCCCCC(=O)NO)c2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C32H34N2O10/c1-39-25-10-17(11-26(40-2)31(25)36)28-20-13-23-24(44-16-43-23)14-21(20)30(22-15-42-32(37)29(22)28)33-18-6-5-7-19(12-18)41-9-4-3-8-27(35)34-38/h5-7,10-14,22,28-30,33,36,38H,3-4,8-9,15-16H2,1-2H3,(H,34,35)/t22-,28+,29-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442728
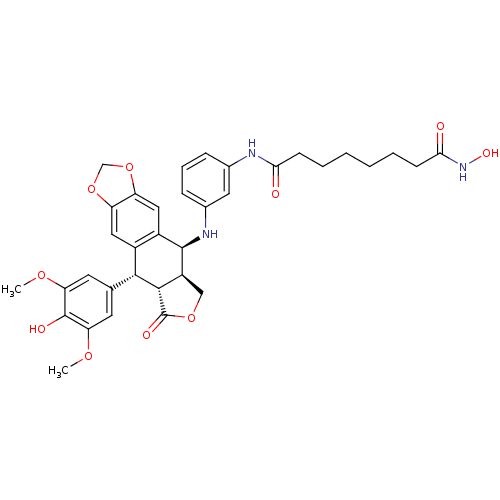
(CHEMBL2442790)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2cccc(NC(=O)CCCCCCC(=O)NO)c2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C35H39N3O10/c1-44-27-12-19(13-28(45-2)34(27)41)31-22-15-25-26(48-18-47-25)16-23(22)33(24-17-46-35(42)32(24)31)37-21-9-7-8-20(14-21)36-29(39)10-5-3-4-6-11-30(40)38-43/h7-9,12-16,24,31-33,37,41,43H,3-6,10-11,17-18H2,1-2H3,(H,36,39)(H,38,40)/t24-,31+,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC6 expressed in baculovirus infected High5 insect cells using Boc-Lys(eacety1)-AMC as substrate incubated for 3 hr... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115639
BindingDB Entry DOI: 10.7270/Q2CZ3BWM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442738
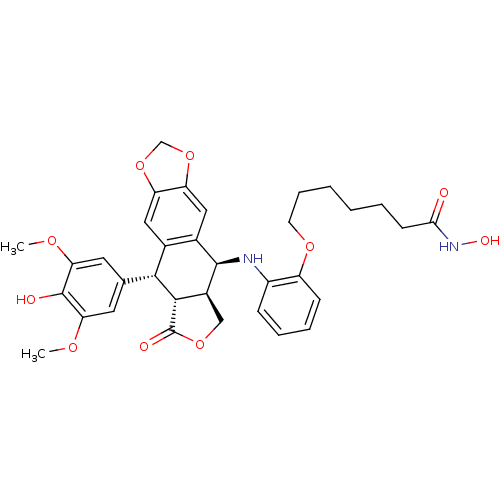
(CHEMBL2442802)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccccc2OCCCCCCC(=O)NO)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C34H38N2O10/c1-41-27-13-19(14-28(42-2)33(27)38)30-20-15-25-26(46-18-45-25)16-21(20)32(22-17-44-34(39)31(22)30)35-23-9-6-7-10-24(23)43-12-8-4-3-5-11-29(37)36-40/h6-7,9-10,13-16,22,30-32,35,38,40H,3-5,8,11-12,17-18H2,1-2H3,(H,36,37)/t22-,30+,31-,32+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442719
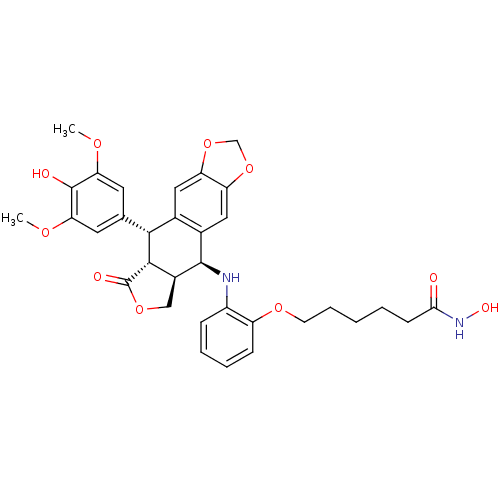
(CHEMBL2442799)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccccc2OCCCCCC(=O)NO)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C33H36N2O10/c1-40-26-12-18(13-27(41-2)32(26)37)29-19-14-24-25(45-17-44-24)15-20(19)31(21-16-43-33(38)30(21)29)34-22-8-5-6-9-23(22)42-11-7-3-4-10-28(36)35-39/h5-6,8-9,12-15,21,29-31,34,37,39H,3-4,7,10-11,16-17H2,1-2H3,(H,35,36)/t21-,29+,30-,31+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(e-acetyl)-AMC as substrate incubated ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115639
BindingDB Entry DOI: 10.7270/Q2CZ3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(e-acetyl)-AMC as substrate incubated ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115639
BindingDB Entry DOI: 10.7270/Q2CZ3BWM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50560545

(CHEMBL4761829)Show SMILES [I-].CC[N+]1=C(\C=C\C2=C3Oc4cc(O)cc(OCCCCCCCC(=O)NO)c4C=C3CCC2)C(C)(C)c2ccccc12 |c:2,6,29| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC6 expressed in baculovirus infected High5 insect cells using Boc-Lys(eacety1)-AMC as substrate incubated for 3 hr... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115639
BindingDB Entry DOI: 10.7270/Q2CZ3BWM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442727
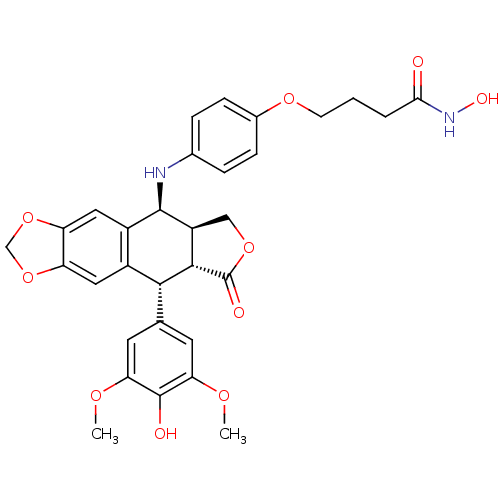
(CHEMBL2442791)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccc(OCCCC(=O)NO)cc2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C31H32N2O10/c1-38-24-10-16(11-25(39-2)30(24)35)27-19-12-22-23(43-15-42-22)13-20(19)29(21-14-41-31(36)28(21)27)32-17-5-7-18(8-6-17)40-9-3-4-26(34)33-37/h5-8,10-13,21,27-29,32,35,37H,3-4,9,14-15H2,1-2H3,(H,33,34)/t21-,27+,28-,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442735
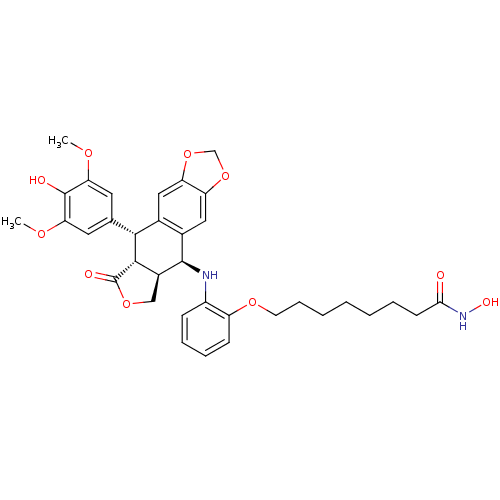
(CHEMBL2442693)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccccc2OCCCCCCCC(=O)NO)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C35H40N2O10/c1-42-28-14-20(15-29(43-2)34(28)39)31-21-16-26-27(47-19-46-26)17-22(21)33(23-18-45-35(40)32(23)31)36-24-10-7-8-11-25(24)44-13-9-5-3-4-6-12-30(38)37-41/h7-8,10-11,14-17,23,31-33,36,39,41H,3-6,9,12-13,18-19H2,1-2H3,(H,37,38)/t23-,31+,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442731
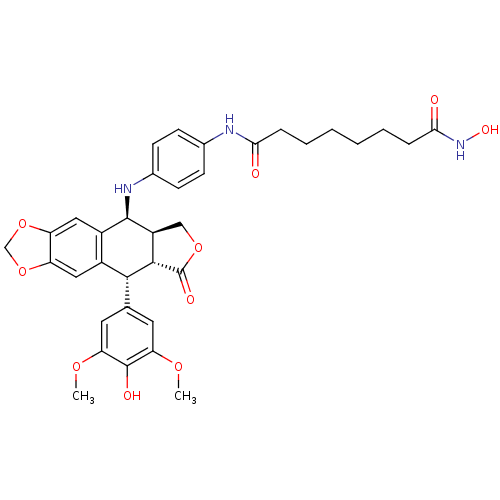
(CHEMBL2442698)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccc(NC(=O)CCCCCCC(=O)NO)cc2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C35H39N3O10/c1-44-27-13-19(14-28(45-2)34(27)41)31-22-15-25-26(48-18-47-25)16-23(22)33(24-17-46-35(42)32(24)31)37-21-11-9-20(10-12-21)36-29(39)7-5-3-4-6-8-30(40)38-43/h9-16,24,31-33,37,41,43H,3-8,17-18H2,1-2H3,(H,36,39)(H,38,40)/t24-,31+,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442724

(CHEMBL2442794)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccc(OCCCCC(=O)NO)cc2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C32H34N2O10/c1-39-25-11-17(12-26(40-2)31(25)36)28-20-13-23-24(44-16-43-23)14-21(20)30(22-15-42-32(37)29(22)28)33-18-6-8-19(9-7-18)41-10-4-3-5-27(35)34-38/h6-9,11-14,22,28-30,33,36,38H,3-5,10,15-16H2,1-2H3,(H,34,35)/t22-,28+,29-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50560545

(CHEMBL4761829)Show SMILES [I-].CC[N+]1=C(\C=C\C2=C3Oc4cc(O)cc(OCCCCCCCC(=O)NO)c4C=C3CCC2)C(C)(C)c2ccccc12 |c:2,6,29| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC1 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(e-acetyl)-AMC as substrate incubated ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115639
BindingDB Entry DOI: 10.7270/Q2CZ3BWM |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 1
(Rattus norvegicus) | BDBM50019143
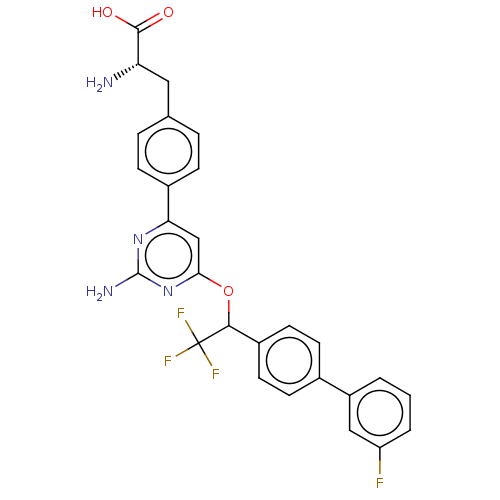
(CHEMBL511275 | US10683309, Example LP533401)Show SMILES N[C@@H](Cc1ccc(cc1)-c1cc(OC(c2ccc(cc2)-c2cccc(F)c2)C(F)(F)F)nc(N)n1)C(O)=O |r| Show InChI InChI=1S/C27H22F4N4O3/c28-20-3-1-2-19(13-20)16-8-10-18(11-9-16)24(27(29,30)31)38-23-14-22(34-26(33)35-23)17-6-4-15(5-7-17)12-21(32)25(36)37/h1-11,13-14,21,24H,12,32H2,(H,36,37)(H2,33,34,35)/t21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of TPH-1-mediated serotonin biosynthesis in rat RBL2H3 cells after 48 hrs by RP-HPLC analysis |
J Med Chem 57: 4692-709 (2014)
Article DOI: 10.1021/jm5002293
BindingDB Entry DOI: 10.7270/Q2CF9RP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442732
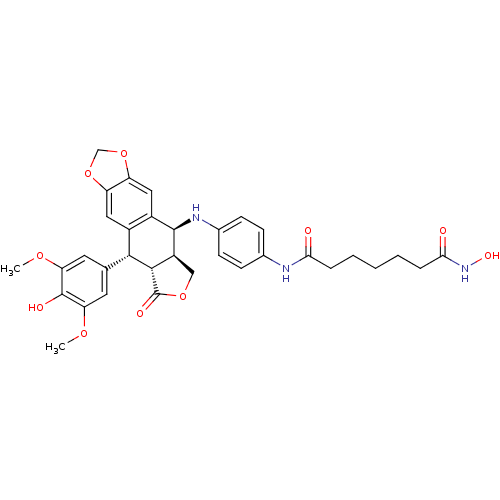
(CHEMBL2442697)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2ccc(NC(=O)CCCCCC(=O)NO)cc2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C34H37N3O10/c1-43-26-12-18(13-27(44-2)33(26)40)30-21-14-24-25(47-17-46-24)15-22(21)32(23-16-45-34(41)31(23)30)36-20-10-8-19(9-11-20)35-28(38)6-4-3-5-7-29(39)37-42/h8-15,23,30-32,36,40,42H,3-7,16-17H2,1-2H3,(H,35,38)(H,37,39)/t23-,30+,31-,32+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50560545

(CHEMBL4761829)Show SMILES [I-].CC[N+]1=C(\C=C\C2=C3Oc4cc(O)cc(OCCCCCCCC(=O)NO)c4C=C3CCC2)C(C)(C)c2ccccc12 |c:2,6,29| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(e-acetyl)-AMC as substrate incubated ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115639
BindingDB Entry DOI: 10.7270/Q2CZ3BWM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442729
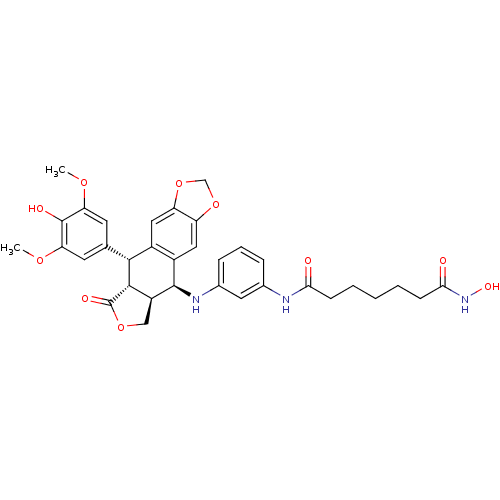
(CHEMBL2442789)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2cccc(NC(=O)CCCCCC(=O)NO)c2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C34H37N3O10/c1-43-26-11-18(12-27(44-2)33(26)40)30-21-14-24-25(47-17-46-24)15-22(21)32(23-16-45-34(41)31(23)30)36-20-8-6-7-19(13-20)35-28(38)9-4-3-5-10-29(39)37-42/h6-8,11-15,23,30-32,36,40,42H,3-5,9-10,16-17H2,1-2H3,(H,35,38)(H,37,39)/t23-,30+,31-,32+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50386430

(CHEMBL2047536)Show SMILES Cc1c2CC[C@H](NC(=O)c3cc(nc4ccnn34)C(=O)NCc3ccc4OCC(=O)Nc4c3)c2ccc1C(O)=O |r| Show InChI InChI=1S/C28H24N6O6/c1-14-16-5-6-19(18(16)4-3-17(14)28(38)39)33-27(37)22-11-21(31-24-8-9-30-34(22)24)26(36)29-12-15-2-7-23-20(10-15)32-25(35)13-40-23/h2-4,7-11,19H,5-6,12-13H2,1H3,(H,29,36)(H,32,35)(H,33,37)(H,38,39)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 655 | n/a | n/a | n/a | n/a | n/a | n/a |
Alantos Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP12 using MCA-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2.AcOH as substrate preincubated for 10 mins measured by fluorescence a... |
J Med Chem 55: 709-16 (2012)
Article DOI: 10.1021/jm201152u
BindingDB Entry DOI: 10.7270/Q2668F73 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50442726
(CHEMBL2442792)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](Nc2cccc(OCCCC(=O)NO)c2)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C31H32N2O10/c1-38-24-9-16(10-25(39-2)30(24)35)27-19-12-22-23(43-15-42-22)13-20(19)29(21-14-41-31(36)28(21)27)32-17-5-3-6-18(11-17)40-8-4-7-26(34)33-37/h3,5-6,9-13,21,27-29,32,35,37H,4,7-8,14-15H2,1-2H3,(H,33,34)/t21-,27+,28-,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 767 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 expressed in baculovirus infected insect high5 cells using Ac-Lys-Tyr-Lys (epsilon-acetyl)-AMC as substrate aft... |
Bioorg Med Chem 21: 6981-95 (2013)
Article DOI: 10.1016/j.bmc.2013.09.023
BindingDB Entry DOI: 10.7270/Q2CC124T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data