

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM50150119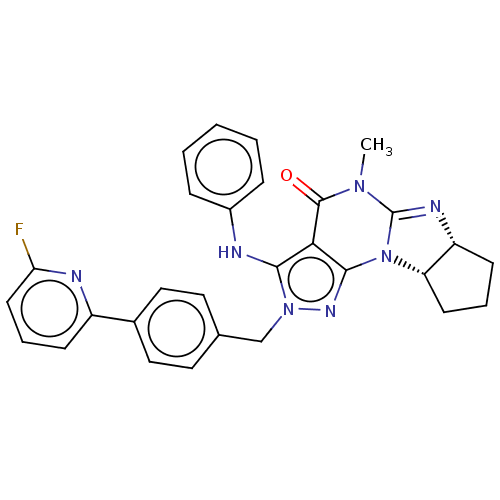 (CHEMBL3769414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of PDE1B (unknown origin) | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561654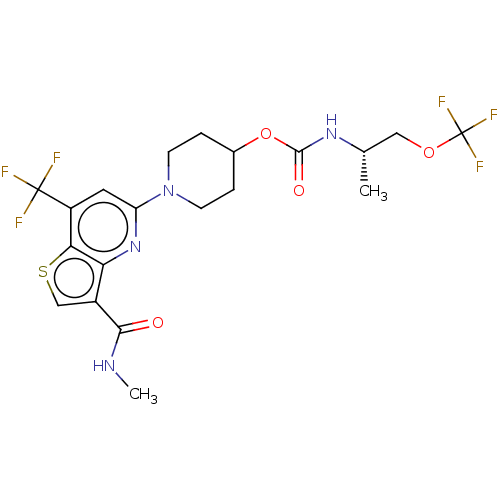 (CHEMBL4785914) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561654 (CHEMBL4785914) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Mus musculus) | BDBM50561654 (CHEMBL4785914) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse UGT8 assessed as redcution in GalCer accumulation | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Mus musculus) | BDBM50561654 (CHEMBL4785914) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse UGT8 assessed as redcution in SFT accumulation | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561671 (CHEMBL4796521) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561669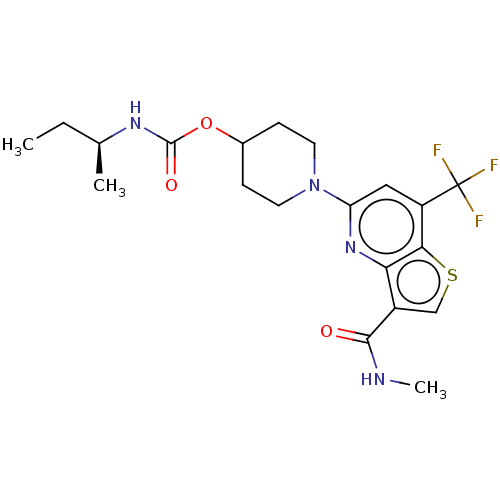 (CHEMBL4787939) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561671 (CHEMBL4796521) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561670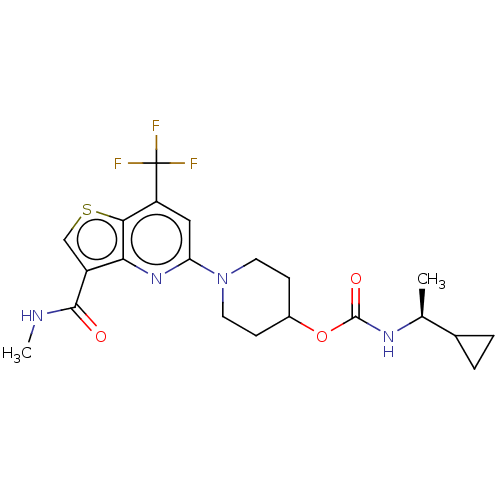 (CHEMBL4750073) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561669 (CHEMBL4787939) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM25142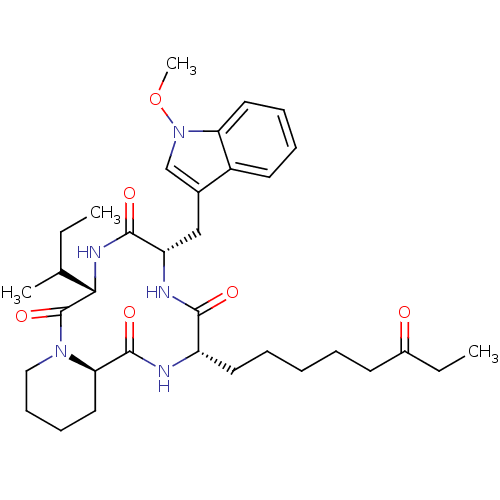 ((3S,6S,9S,15aR)-9-[(2R)-butan-2-yl]-6-[(1-methoxy-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561664 (CHEMBL4776963) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561670 (CHEMBL4750073) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561672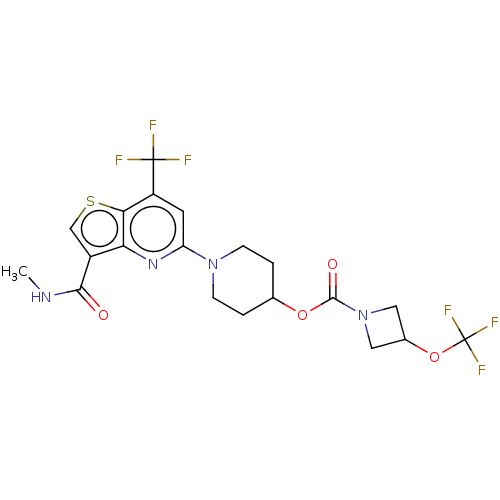 (CHEMBL4750432) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561663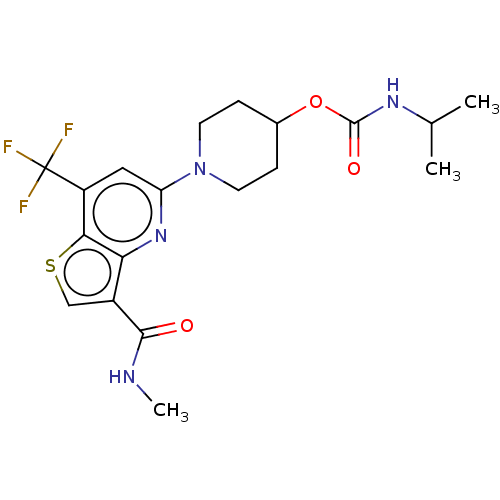 (CHEMBL4765010) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561663 (CHEMBL4765010) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561672 (CHEMBL4750432) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM289647 (11,11-Difluoro-6-(4-methoxybenzyl)-9-((tetrahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibitory activity against chymotrypsinogen; Range 1-10 uM | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561664 (CHEMBL4776963) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50239931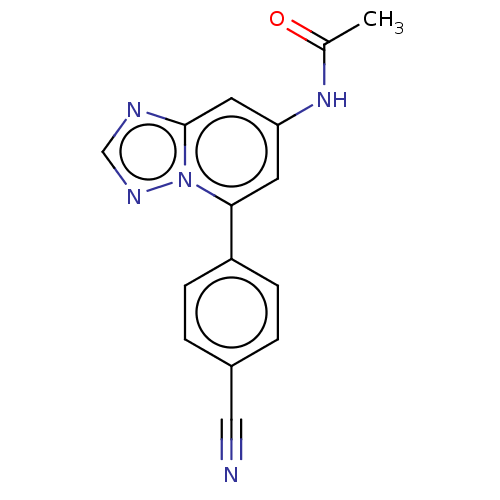 (CHEMBL4067995 | US10287286, Example 142) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... | J Med Chem 60: 5663-5672 (2017) Article DOI: 10.1021/acs.jmedchem.7b00352 BindingDB Entry DOI: 10.7270/Q2GQ70XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50239883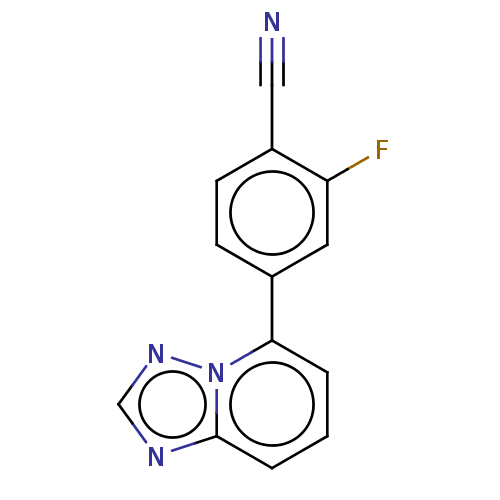 (CHEMBL4090769 | US10287286, Example 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... | J Med Chem 60: 5663-5672 (2017) Article DOI: 10.1021/acs.jmedchem.7b00352 BindingDB Entry DOI: 10.7270/Q2GQ70XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM289671 (9-(2,2-Difluorocyclopropanecarbonyl)-6-(4-methoxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged PDE1B (unknown origin) assessed as decrease in FAM-cAMP hydrolysis preincubated for 5 mins followed by FAM-cAMP ... | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50239889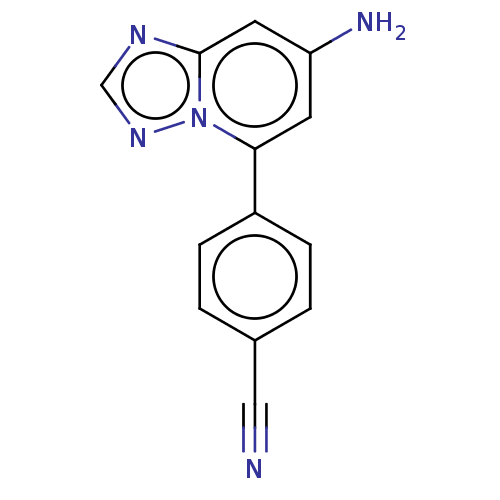 (CHEMBL4072842 | US10287286, Example 100) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... | J Med Chem 60: 5663-5672 (2017) Article DOI: 10.1021/acs.jmedchem.7b00352 BindingDB Entry DOI: 10.7270/Q2GQ70XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29602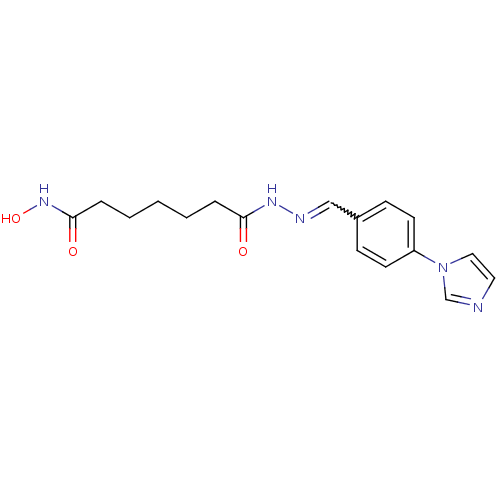 (benzylidenehydrazine derivative, 19) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561665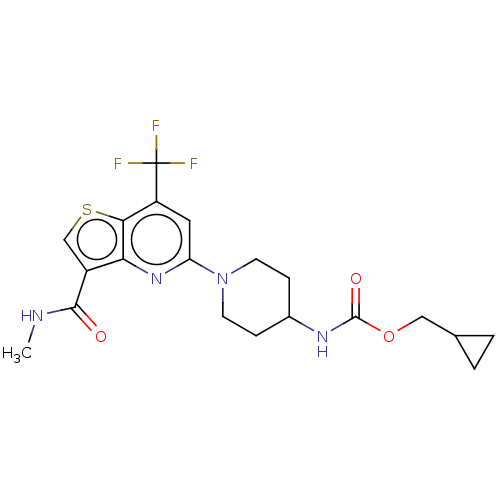 (CHEMBL4755797) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29593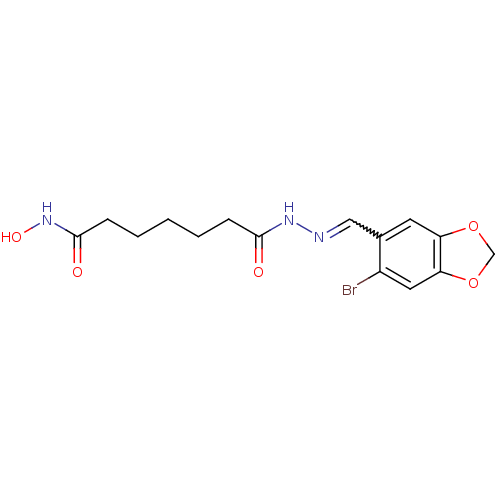 (benzylidenehydrazine derivative, 10) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29595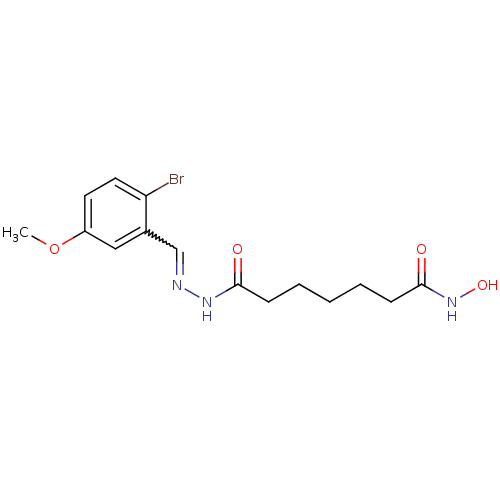 (benzylidenehydrazine derivative, 12) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50239888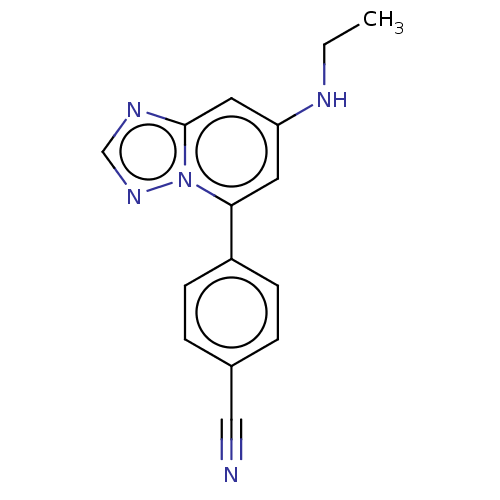 (CHEMBL4094292 | US10287286, Example 103) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... | J Med Chem 60: 5663-5672 (2017) Article DOI: 10.1021/acs.jmedchem.7b00352 BindingDB Entry DOI: 10.7270/Q2GQ70XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM289653 (6-(2-Fluoro-4-methoxybenzyl)-9-((tetrahydro-2H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged PDE1B (unknown origin) assessed as decrease in FAM-cAMP hydrolysis preincubated for 5 mins followed by FAM-cAMP ... | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM289621 (6-(4-Methoxybenzyl)-9-((tetrahydro-2H-pyran-4-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged PDE1B (unknown origin) assessed as decrease in FAM-cAMP hydrolysis preincubated for 5 mins followed by FAM-cAMP ... | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM289641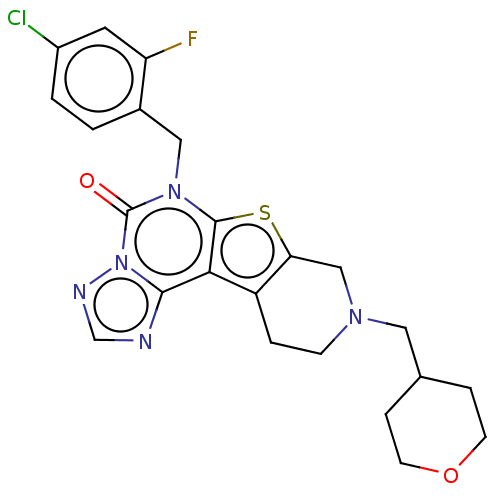 (6-(4-Chloro-2-fluorobenzyl)-9-((tetrahydro-2H-pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged PDE1B (unknown origin) assessed as decrease in FAM-cAMP hydrolysis preincubated for 5 mins followed by FAM-cAMP ... | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50239915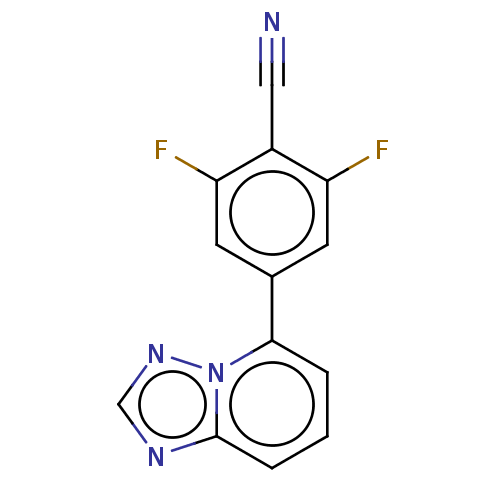 (CHEMBL4067559 | US10287286, Example 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... | J Med Chem 60: 5663-5672 (2017) Article DOI: 10.1021/acs.jmedchem.7b00352 BindingDB Entry DOI: 10.7270/Q2GQ70XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50239937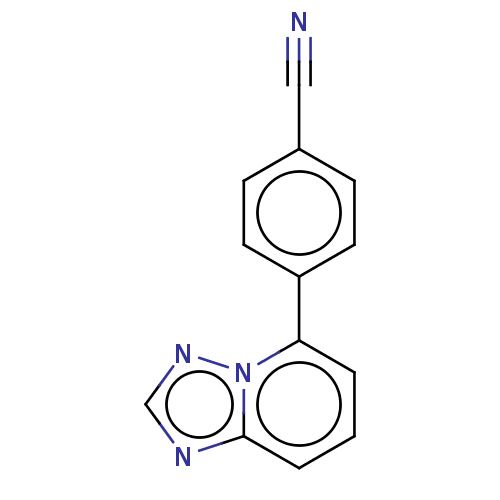 (CHEMBL4083851 | US10287286, Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... | J Med Chem 60: 5663-5672 (2017) Article DOI: 10.1021/acs.jmedchem.7b00352 BindingDB Entry DOI: 10.7270/Q2GQ70XZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29603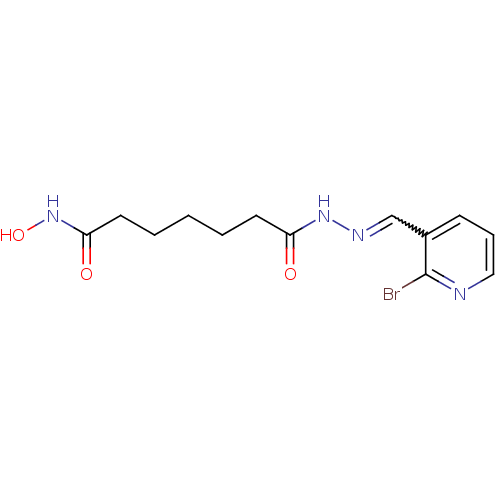 (benzylidenehydrazine derivative, 20) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29599 (benzylidenehydrazine derivative, 16) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29596 (benzylidenehydrazine derivative, 13) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561665 (CHEMBL4755797) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50239890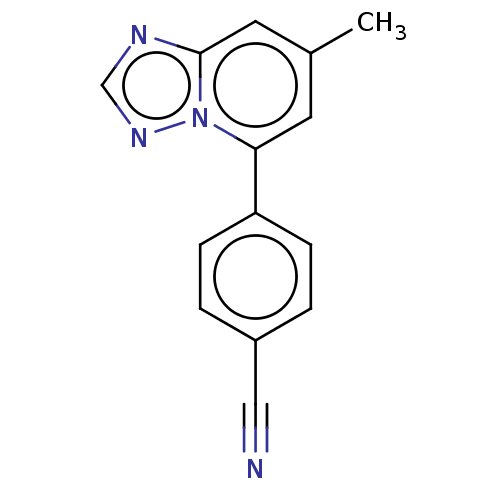 (CHEMBL4095005 | US10287286, Example 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... | J Med Chem 60: 5663-5672 (2017) Article DOI: 10.1021/acs.jmedchem.7b00352 BindingDB Entry DOI: 10.7270/Q2GQ70XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50059033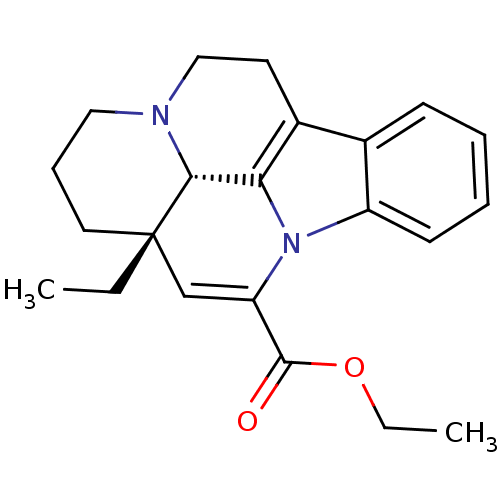 ((11aS,11bS)-11a-Ethyl-2,3,4,5,11a,11b-hexahydro-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29597 (benzylidenehydrazine derivative, 14) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM289617 (6-(4-Chlorobenzyl)-9-((tetrahydro-2H-pyran-4-yl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibitory activity against chymotrypsinogen; Range 1-10 uM | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29594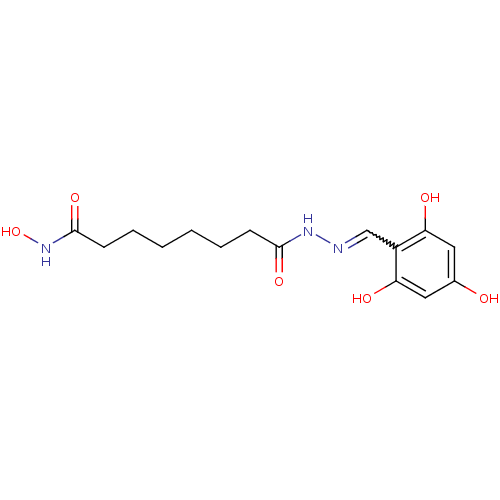 (benzylidenehydrazine derivative, 11) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561667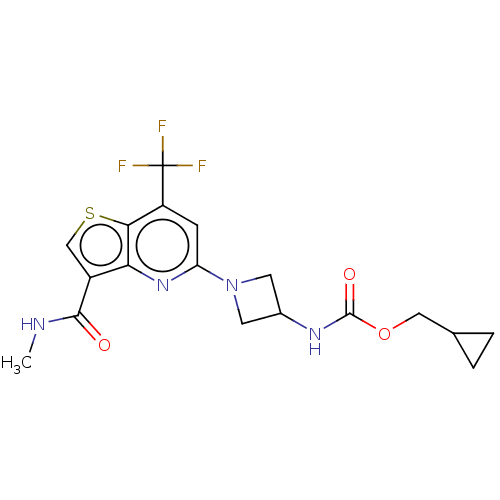 (CHEMBL4757982) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29607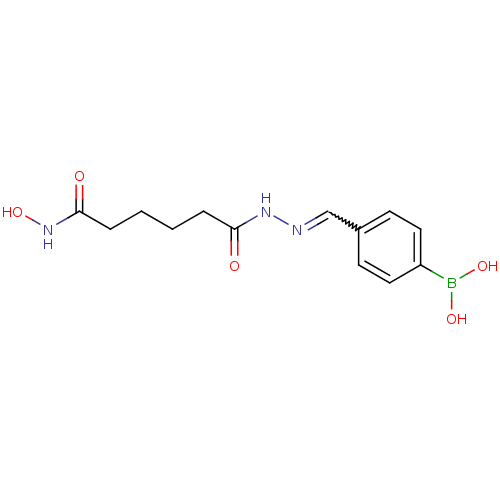 (benzylidenehydrazine derivative, 24) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM50059026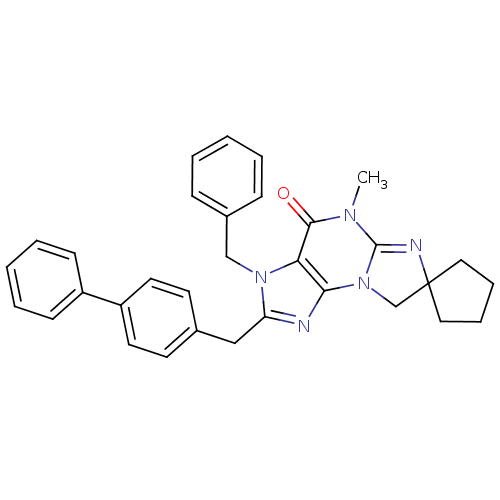 (5'-Methyl-2'-(biphenylmethyl)-3'-(phenylmethyl)spi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged PDE1B (unknown origin) assessed as decrease in FAM-cAMP hydrolysis preincubated for 5 mins followed by FAM-cAMP ... | J Med Chem 60: 3472-3483 (2017) Article DOI: 10.1021/acs.jmedchem.7b00302 BindingDB Entry DOI: 10.7270/Q2D79DP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (Homo sapiens) | BDBM50561667 (CHEMBL4757982) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Plasmodium falciparum (isolate 3D7)) | BDBM29598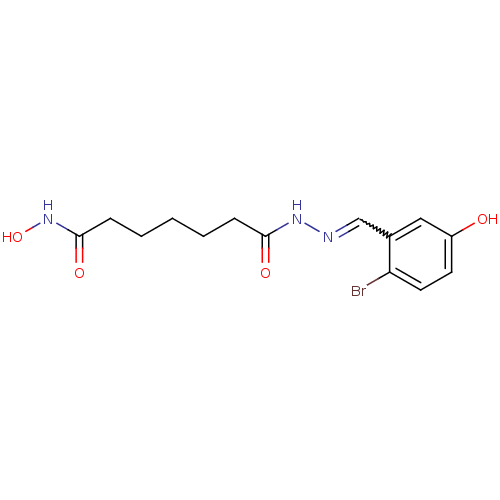 (benzylidenehydrazine derivative, 15) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Harvard Medical School | Assay Description Recombinant pfHDAC-1 was assayed with substrate in the presence of test compound. The substrate concentration was kept constant at 125 uM while the c... | J Med Chem 52: 2185-7 (2009) Article DOI: 10.1021/jm801654y BindingDB Entry DOI: 10.7270/Q26H4FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50239921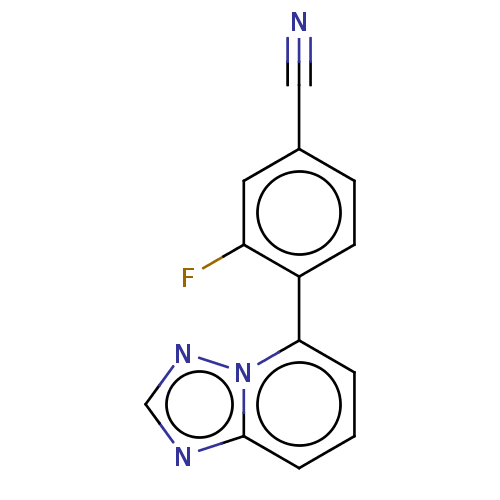 (CHEMBL4075128 | US10287286, Example 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... | J Med Chem 60: 5663-5672 (2017) Article DOI: 10.1021/acs.jmedchem.7b00352 BindingDB Entry DOI: 10.7270/Q2GQ70XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 256 total ) | Next | Last >> |