 Found 94 hits with Last Name = 'barlow' and Initial = 'dj'
Found 94 hits with Last Name = 'barlow' and Initial = 'dj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447108

(CHEMBL3112881)Show InChI InChI=1S/C11H7ClFNO2S/c12-11-7-2-1-6(13)5-9(7)17-8(11)3-4-10(15)14-16/h1-5,16H,(H,14,15)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC assessed as cleavage of SNAP-25 (141 to 206) after 30 mins by LC-MS analysis |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM23420
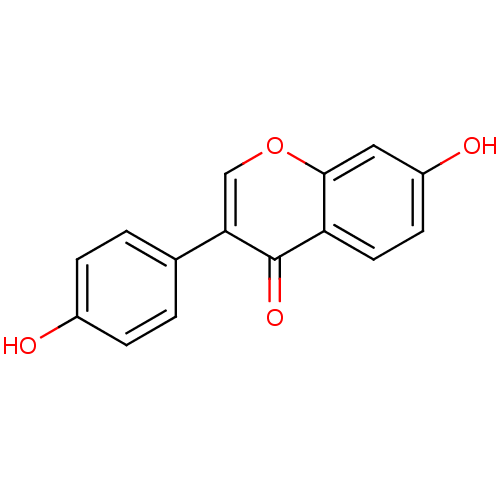
(7,4′-Dihydroxy-isoflavone (3a) | 7-hydroxy-3...)Show InChI InChI=1S/C15H10O4/c16-10-3-1-9(2-4-10)13-8-19-14-7-11(17)5-6-12(14)15(13)18/h1-8,16-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase expressed in CHO cells |
Bioorg Med Chem 16: 8466-70 (2008)
Article DOI: 10.1016/j.bmc.2008.08.034
BindingDB Entry DOI: 10.7270/Q2CV4JN9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50049391
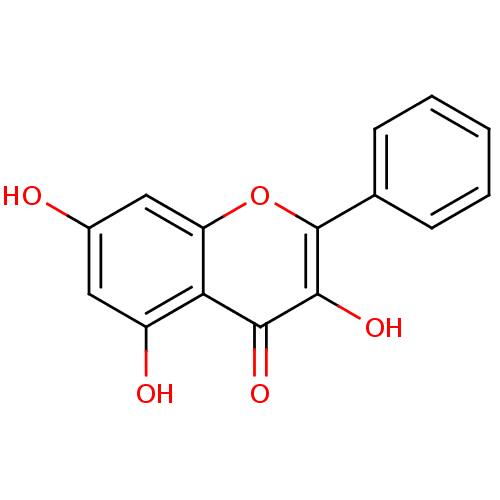
(3,5,7-Trihydroxyflavone | 3,5,7-triOH-flavone | 3,...)Show InChI InChI=1S/C15H10O5/c16-9-6-10(17)12-11(7-9)20-15(14(19)13(12)18)8-4-2-1-3-5-8/h1-7,16-17,19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase expressed in CHO cells |
Bioorg Med Chem 16: 8466-70 (2008)
Article DOI: 10.1016/j.bmc.2008.08.034
BindingDB Entry DOI: 10.7270/Q2CV4JN9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM19459

(5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase expressed in CHO cells |
Bioorg Med Chem 16: 8466-70 (2008)
Article DOI: 10.1016/j.bmc.2008.08.034
BindingDB Entry DOI: 10.7270/Q2CV4JN9 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50209771
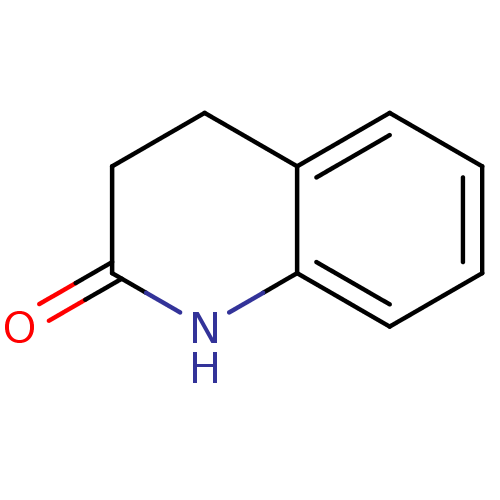
(3,4-dihydroquinolin-2(1H)-one | CHEMBL388582 | Dih...)Show InChI InChI=1S/C9H9NO/c11-9-6-5-7-3-1-2-4-8(7)10-9/h1-4H,5-6H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human MAPK p38alpha |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50308509

(4-(4-(4-bromophenyl)-3-(trifluoromethyl)-1H-pyrazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cc(c(n1)C(F)(F)F)-c1ccc(Br)cc1 Show InChI InChI=1S/C16H11BrF3N3O2S/c17-11-3-1-10(2-4-11)14-9-23(22-15(14)16(18,19)20)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of mouse COX2 |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50346088

((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@]1(CC[C@@H](CC1)C(O)=O)C#N |r,wU:17.22,14.25,(4.79,2.97,;3.46,3.74,;2.12,2.97,;.79,3.74,;-.54,2.97,;-.54,1.43,;.79,.66,;2.12,1.43,;3.46,.66,;3.46,-.88,;4.7,-1.79,;4.23,-3.25,;2.69,-3.25,;2.21,-1.79,;-1.88,.66,;-3.42,.71,;-4.23,-.6,;-3.51,-1.96,;-1.97,-2.01,;-1.16,-.7,;-4.33,-3.26,;-3.6,-4.62,;-5.87,-3.21,;-1.77,2.19,;-1.66,3.73,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23)/t14-,20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM15239
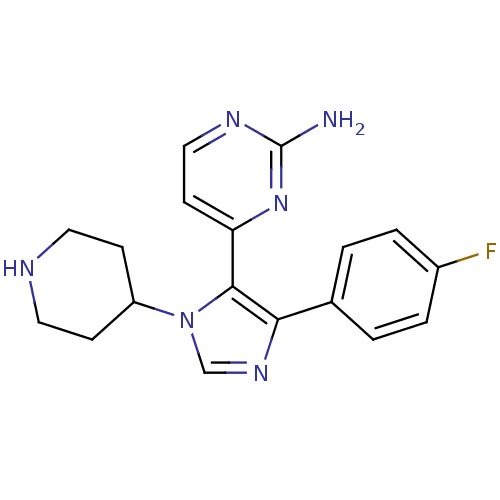
(4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...)Show InChI InChI=1S/C18H19FN6/c19-13-3-1-12(2-4-13)16-17(15-7-10-22-18(20)24-15)25(11-23-16)14-5-8-21-9-6-14/h1-4,7,10-11,14,21H,5-6,8-9H2,(H2,20,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human MAPK p38alpha |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346088

((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@]1(CC[C@@H](CC1)C(O)=O)C#N |r,wU:17.22,14.25,(4.79,2.97,;3.46,3.74,;2.12,2.97,;.79,3.74,;-.54,2.97,;-.54,1.43,;.79,.66,;2.12,1.43,;3.46,.66,;3.46,-.88,;4.7,-1.79,;4.23,-3.25,;2.69,-3.25,;2.21,-1.79,;-1.88,.66,;-3.42,.71,;-4.23,-.6,;-3.51,-1.96,;-1.97,-2.01,;-1.16,-.7,;-4.33,-3.26,;-3.6,-4.62,;-5.87,-3.21,;-1.77,2.19,;-1.66,3.73,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23)/t14-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM15238

(4-[1-(cyclopropylmethyl)-4-(4-fluorophenyl)-1H-imi...)Show InChI InChI=1S/C17H16FN5/c18-13-5-3-12(4-6-13)15-16(14-7-8-20-17(19)22-14)23(10-21-15)9-11-1-2-11/h3-8,10-11H,1-2,9H2,(H2,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human MAPK p38alpha |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13336
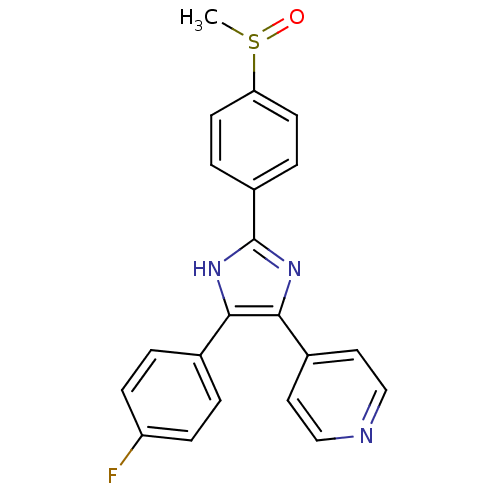
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human MAPK p38alpha |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of mouse COX2 |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50074933
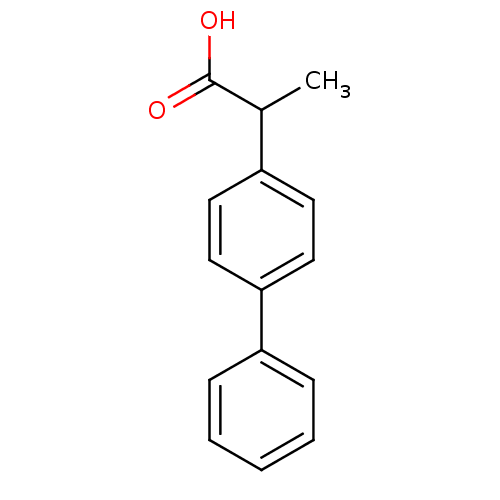
(2-biphenyl-4-yl-propionic acid | CHEMBL317434 | al...)Show InChI InChI=1S/C15H14O2/c1-11(15(16)17)12-7-9-14(10-8-12)13-5-3-2-4-6-13/h2-11H,1H3,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM15237

(4-[1-(cyclopropylmethyl)-4-(4-fluorophenyl)-1H-imi...)Show InChI InChI=1S/C18H16FN3/c19-16-5-3-14(4-6-16)17-18(15-7-9-20-10-8-15)22(12-21-17)11-13-1-2-13/h3-10,12-13H,1-2,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human MAPK p38alpha |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aromatase
(Homo sapiens (Human)) | BDBM50241408
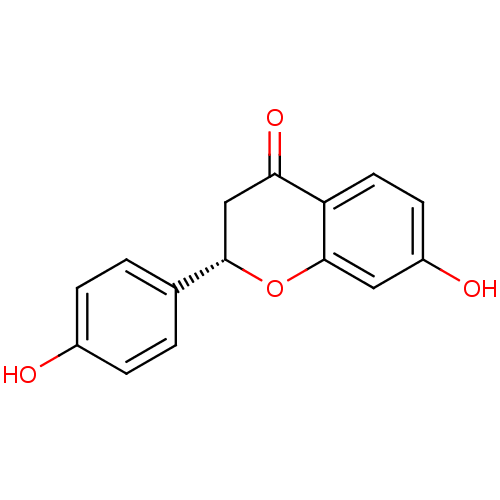
((2S)-liquiritigenin | 7-HYDROXY-2-(4-HYDROXY-PHENY...)Show InChI InChI=1S/C15H12O4/c16-10-3-1-9(2-4-10)14-8-13(18)12-6-5-11(17)7-15(12)19-14/h1-7,14,16-17H,8H2/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase by fluorometric assay |
Bioorg Med Chem 16: 8466-70 (2008)
Article DOI: 10.1016/j.bmc.2008.08.034
BindingDB Entry DOI: 10.7270/Q2CV4JN9 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14769

(6-(3,4-Dimethoxy-phenyl)-4,5-dimethyl-4,5-dihydro-...)Show InChI InChI=1S/C12H10F2N2O3/c1-18-10-6-7(2-4-9(10)19-12(13)14)8-3-5-11(17)16-15-8/h2-6,12H,1H3,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50308512

((5R)-5-(4-methoxy-3-propoxyphenyl)-5-methyl-1,3-ox...)Show InChI InChI=1S/C14H19NO4/c1-4-7-18-12-8-10(5-6-11(12)17-3)14(2)9-15-13(16)19-14/h5-6,8H,4,7,9H2,1-3H3,(H,15,16)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50074922
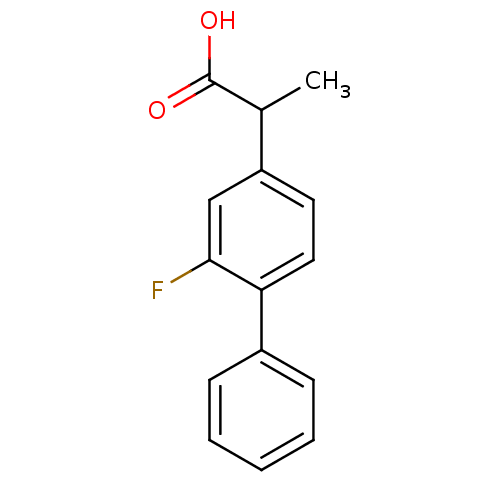
((+-)-2-fluoro-alpha-methyl-4-biphenylacetic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50074922

((+-)-2-fluoro-alpha-methyl-4-biphenylacetic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of mouse COX2 |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50308511
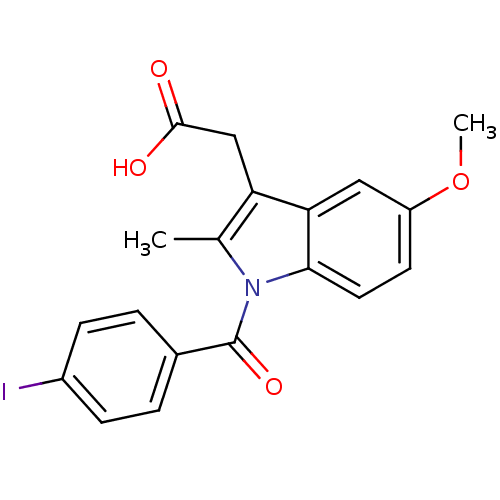
(1-(4-IODOBENZOYL)-5-METHOXY-2-METHYL INDOLE-3-ACET...)Show SMILES COc1ccc2n(C(=O)c3ccc(I)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16INO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447105

(CHEMBL3112883)Show SMILES Oc1c(ccc2cccnc12)C(NC(=O)c1ccccc1)c1ccccc1Cl Show InChI InChI=1S/C23H17ClN2O2/c24-19-11-5-4-10-17(19)21(26-23(28)16-7-2-1-3-8-16)18-13-12-15-9-6-14-25-20(15)22(18)27/h1-14,21,27H,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447153
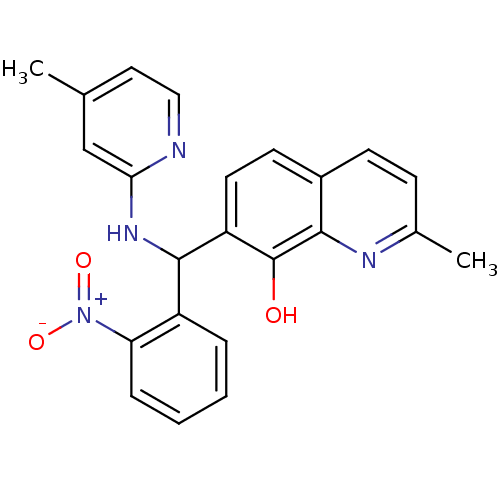
(CHEMBL3112887)Show SMILES Cc1ccnc(NC(c2ccc3ccc(C)nc3c2O)c2ccccc2[N+]([O-])=O)c1 Show InChI InChI=1S/C23H20N4O3/c1-14-11-12-24-20(13-14)26-22(17-5-3-4-6-19(17)27(29)30)18-10-9-16-8-7-15(2)25-21(16)23(18)28/h3-13,22,28H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM14771

((E)-{1-[3-(cyclopentyloxy)-4-methoxyphenyl]ethylid...)Show SMILES COc1ccc(cc1OC1CCCC1)C(C)=NOC(N)=O |w:16.18| Show InChI InChI=1S/C15H20N2O4/c1-10(17-21-15(16)18)11-7-8-13(19-2)14(9-11)20-12-5-3-4-6-12/h7-9,12H,3-6H2,1-2H3,(H2,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human MAPK p38alpha |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50308510
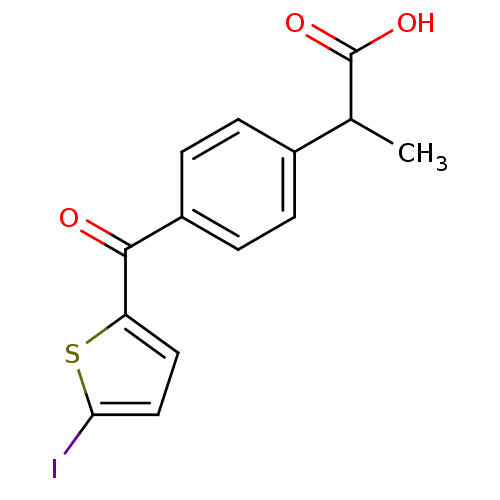
(CHEMBL589253 | Iodosuprofen)Show InChI InChI=1S/C14H11IO3S/c1-8(14(17)18)9-2-4-10(5-3-9)13(16)11-6-7-12(15)19-11/h2-8H,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of aromatase expressed in human H295R cells |
Bioorg Med Chem 16: 8466-70 (2008)
Article DOI: 10.1016/j.bmc.2008.08.034
BindingDB Entry DOI: 10.7270/Q2CV4JN9 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447128
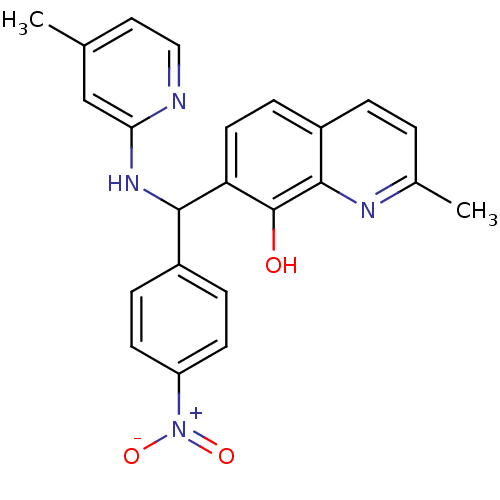
(CHEMBL3112908)Show SMILES Cc1ccnc(NC(c2ccc(cc2)[N+]([O-])=O)c2ccc3ccc(C)nc3c2O)c1 Show InChI InChI=1S/C23H20N4O3/c1-14-11-12-24-20(13-14)26-21(16-5-8-18(9-6-16)27(29)30)19-10-7-17-4-3-15(2)25-22(17)23(19)28/h3-13,21,28H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447149
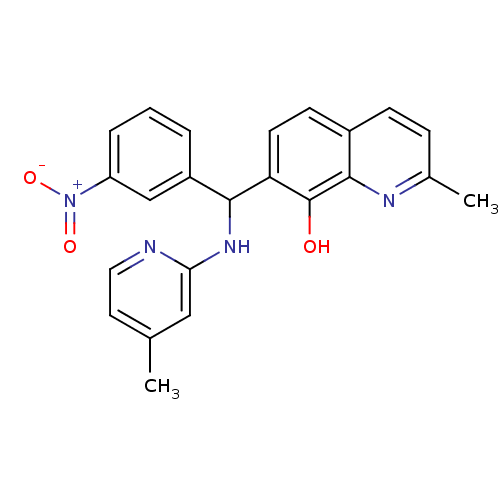
(CHEMBL3112891)Show SMILES Cc1ccnc(NC(c2cccc(c2)[N+]([O-])=O)c2ccc3ccc(C)nc3c2O)c1 Show InChI InChI=1S/C23H20N4O3/c1-14-10-11-24-20(12-14)26-21(17-4-3-5-18(13-17)27(29)30)19-9-8-16-7-6-15(2)25-22(16)23(19)28/h3-13,21,28H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447143
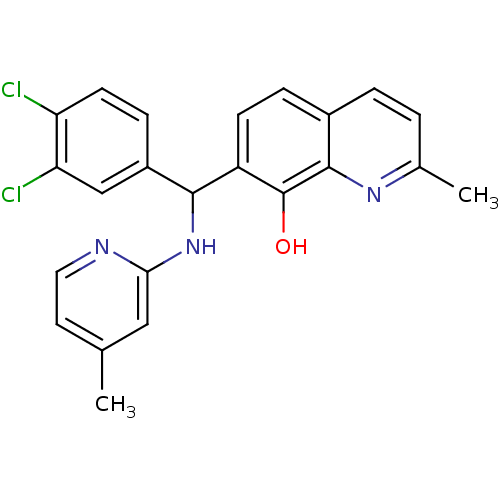
(CHEMBL3112896)Show SMILES Cc1ccnc(NC(c2ccc(Cl)c(Cl)c2)c2ccc3ccc(C)nc3c2O)c1 Show InChI InChI=1S/C23H19Cl2N3O/c1-13-9-10-26-20(11-13)28-21(16-6-8-18(24)19(25)12-16)17-7-5-15-4-3-14(2)27-22(15)23(17)29/h3-12,21,29H,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447148

(CHEMBL3112892)Show SMILES Cc1ccc2ccc(C(Nc3ccccn3)c3cccc(OCc4ccccc4)c3)c(O)c2n1 Show InChI InChI=1S/C29H25N3O2/c1-20-13-14-22-15-16-25(29(33)28(22)31-20)27(32-26-12-5-6-17-30-26)23-10-7-11-24(18-23)34-19-21-8-3-2-4-9-21/h2-18,27,33H,19H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447144

(CHEMBL3112895 | US9023354, AD4-12917)Show SMILES Cc1ccnc(NC(c2ccc(Cl)c(Cl)c2)c2ccc3cccnc3c2O)c1 Show InChI InChI=1S/C22H17Cl2N3O/c1-13-8-10-25-19(11-13)27-20(15-5-7-17(23)18(24)12-15)16-6-4-14-3-2-9-26-21(14)22(16)28/h2-12,20,28H,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447155
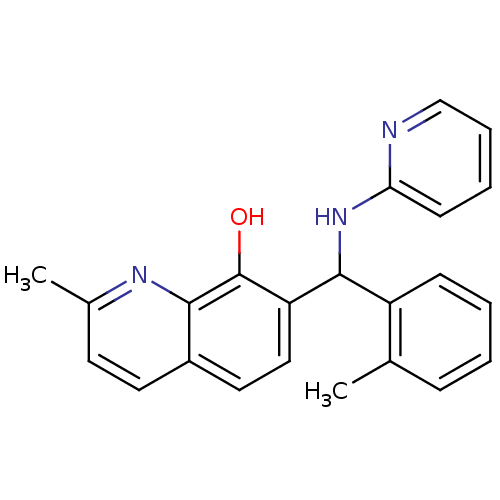
(CHEMBL3112885)Show InChI InChI=1S/C23H21N3O/c1-15-7-3-4-8-18(15)22(26-20-9-5-6-14-24-20)19-13-12-17-11-10-16(2)25-21(17)23(19)27/h3-14,22,27H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447123
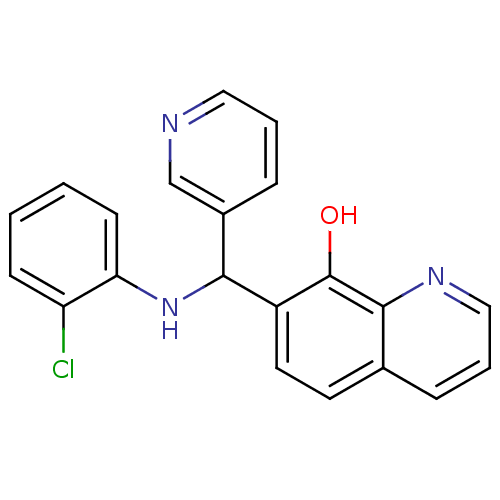
(CHEMBL3112913)Show InChI InChI=1S/C21H16ClN3O/c22-17-7-1-2-8-18(17)25-19(15-6-3-11-23-13-15)16-10-9-14-5-4-12-24-20(14)21(16)26/h1-13,19,25-26H | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447126
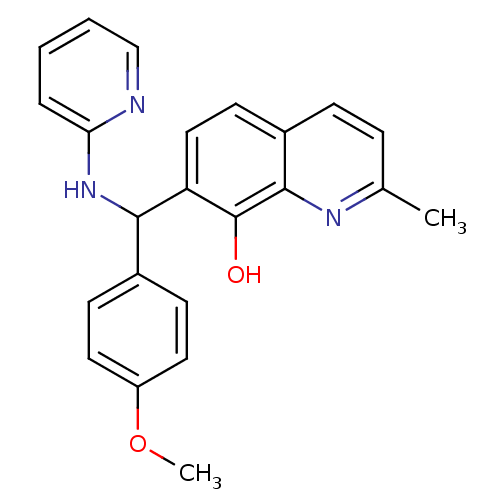
(CHEMBL3112910)Show InChI InChI=1S/C23H21N3O2/c1-15-6-7-17-10-13-19(23(27)22(17)25-15)21(26-20-5-3-4-14-24-20)16-8-11-18(28-2)12-9-16/h3-14,21,27H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447125
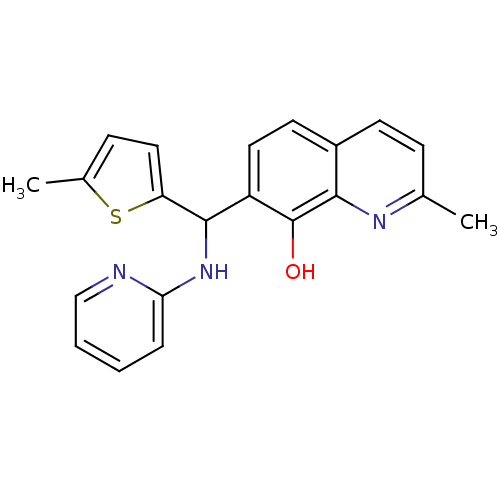
(CHEMBL3112911)Show InChI InChI=1S/C21H19N3OS/c1-13-6-8-15-9-10-16(21(25)19(15)23-13)20(17-11-7-14(2)26-17)24-18-5-3-4-12-22-18/h3-12,20,25H,1-2H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447145

(CHEMBL3112894)Show SMILES COc1cccc(c1)C(Nc1cc(C)ccn1)c1ccc2ccc(C)nc2c1O Show InChI InChI=1S/C24H23N3O2/c1-15-11-12-25-21(13-15)27-22(18-5-4-6-19(14-18)29-3)20-10-9-17-8-7-16(2)26-23(17)24(20)28/h4-14,22,28H,1-3H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447137
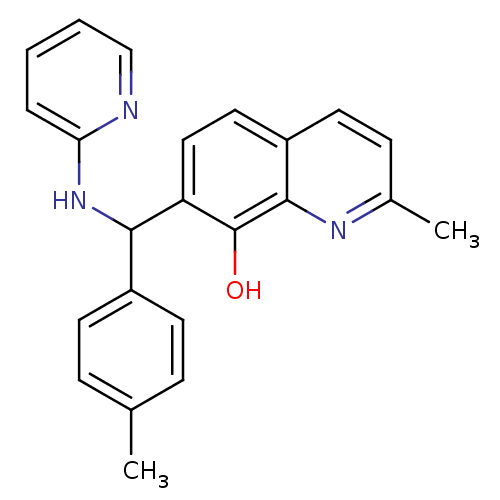
(CHEMBL3112901)Show InChI InChI=1S/C23H21N3O/c1-15-6-9-17(10-7-15)21(26-20-5-3-4-14-24-20)19-13-12-18-11-8-16(2)25-22(18)23(19)27/h3-14,21,27H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447151
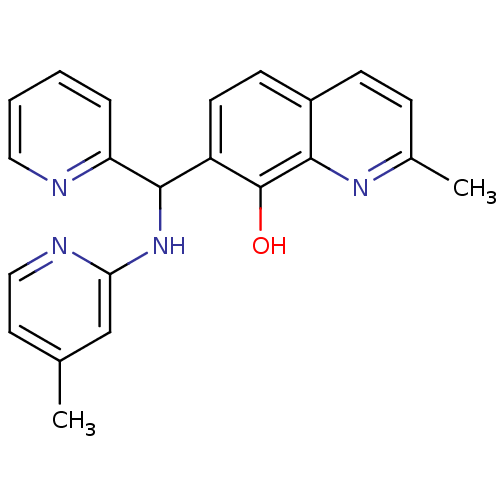
(CHEMBL3112889)Show InChI InChI=1S/C22H20N4O/c1-14-10-12-24-19(13-14)26-21(18-5-3-4-11-23-18)17-9-8-16-7-6-15(2)25-20(16)22(17)27/h3-13,21,27H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50339142

(CHEMBL1688558 | rac-7-(phenyl(pyridin-2-ylamino)me...)Show InChI InChI=1S/C21H17N3O/c25-21-17(12-11-16-9-6-14-23-20(16)21)19(15-7-2-1-3-8-15)24-18-10-4-5-13-22-18/h1-14,19,25H,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447113
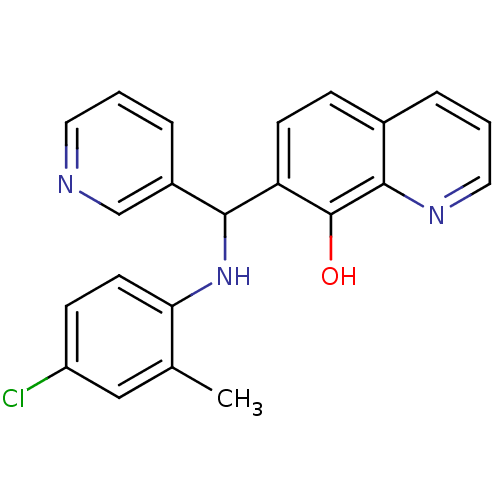
(CHEMBL3112923)Show InChI InChI=1S/C22H18ClN3O/c1-14-12-17(23)7-9-19(14)26-20(16-5-2-10-24-13-16)18-8-6-15-4-3-11-25-21(15)22(18)27/h2-13,20,26-27H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447131
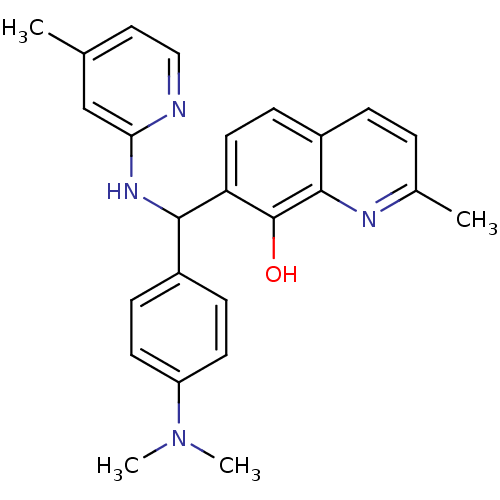
(CHEMBL3112906)Show SMILES CN(C)c1ccc(cc1)C(Nc1cc(C)ccn1)c1ccc2ccc(C)nc2c1O Show InChI InChI=1S/C25H26N4O/c1-16-13-14-26-22(15-16)28-23(18-7-10-20(11-8-18)29(3)4)21-12-9-19-6-5-17(2)27-24(19)25(21)30/h5-15,23,30H,1-4H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447138
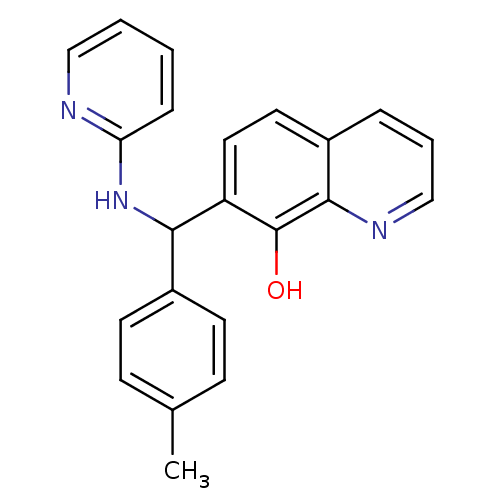
(CHEMBL1497004)Show InChI InChI=1S/C22H19N3O/c1-15-7-9-17(10-8-15)20(25-19-6-2-3-13-23-19)18-12-11-16-5-4-14-24-21(16)22(18)26/h2-14,20,26H,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447139

(CHEMBL3112900)Show SMILES Cc1ccc2ccc(C(Nc3ccccn3)c3ccc(F)cc3)c(O)c2n1 Show InChI InChI=1S/C22H18FN3O/c1-14-5-6-16-9-12-18(22(27)21(16)25-14)20(15-7-10-17(23)11-8-15)26-19-4-2-3-13-24-19/h2-13,20,27H,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447121

(CHEMBL3112915)Show SMILES CCOC(=O)c1ccc(NC(c2cccnc2)c2ccc3cccnc3c2O)cc1 Show InChI InChI=1S/C24H21N3O3/c1-2-30-24(29)17-7-10-19(11-8-17)27-21(18-6-3-13-25-15-18)20-12-9-16-5-4-14-26-22(16)23(20)28/h3-15,21,27-28H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase by fluorometric assay |
Bioorg Med Chem 16: 8466-70 (2008)
Article DOI: 10.1016/j.bmc.2008.08.034
BindingDB Entry DOI: 10.7270/Q2CV4JN9 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447117
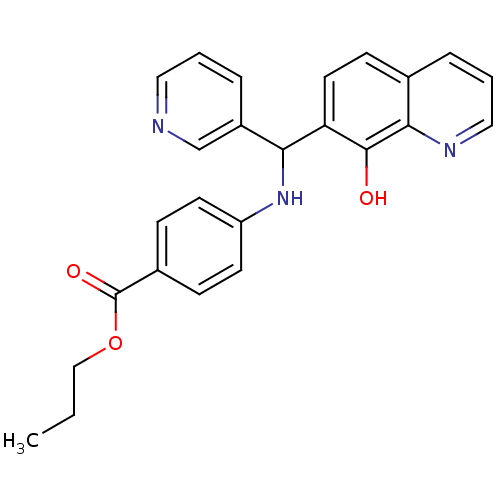
(CHEMBL3112919)Show SMILES CCCOC(=O)c1ccc(NC(c2cccnc2)c2ccc3cccnc3c2O)cc1 Show InChI InChI=1S/C25H23N3O3/c1-2-15-31-25(30)18-7-10-20(11-8-18)28-22(19-6-3-13-26-16-19)21-12-9-17-5-4-14-27-23(17)24(21)29/h3-14,16,22,28-29H,2,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM81384
(Oxyquinoline, D2, #3)Show SMILES COc1ccc(cc1OC)C(Nc1ccccn1)c1ccc2ccc(C)nc2c1O Show InChI InChI=1S/C24H23N3O3/c1-15-7-8-16-9-11-18(24(28)23(16)26-15)22(27-21-6-4-5-13-25-21)17-10-12-19(29-2)20(14-17)30-3/h4-14,22,28H,1-3H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC expressed in Escherichia coli assessed as cleavage of SNAPtide preincubated for 5 mins followed by SNAP... |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data