

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50046798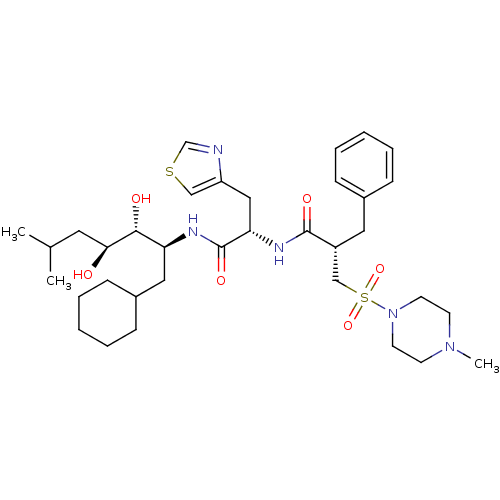 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against monkey plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046799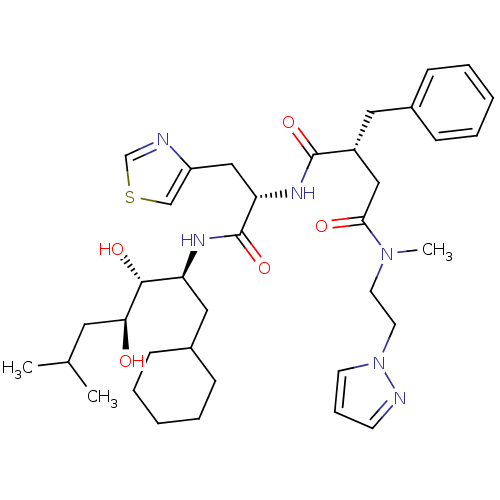 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046794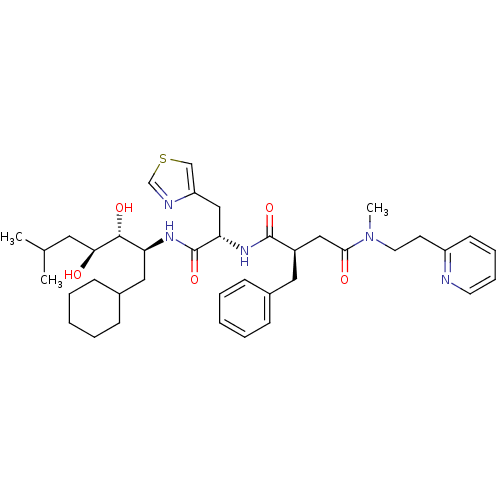 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against monkey plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046802 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046796 ((S)-N-[(S)-1-((1S,2R,3S)-1-Cyclohexylmethyl-2,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006157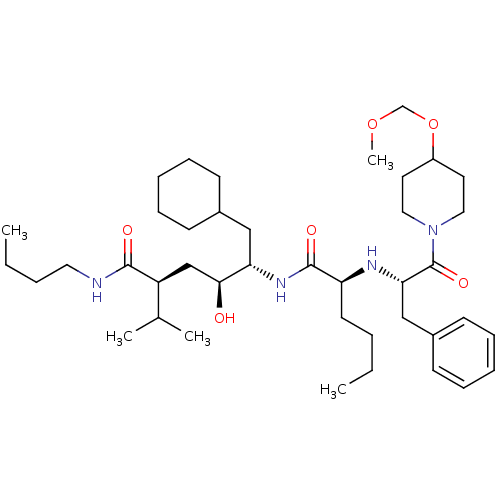 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006147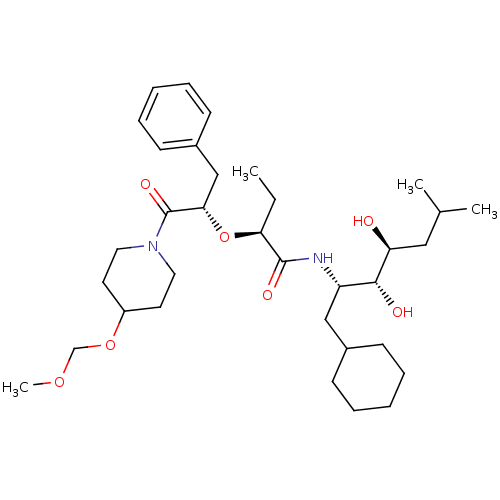 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 6.0. | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006189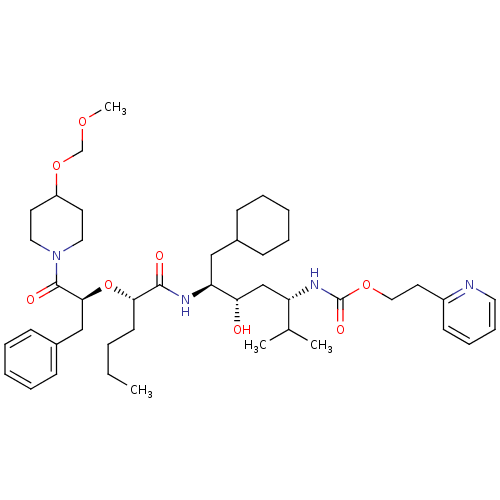 ((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006152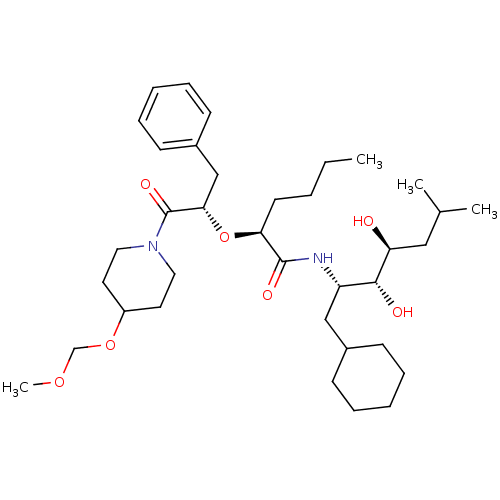 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006152 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006185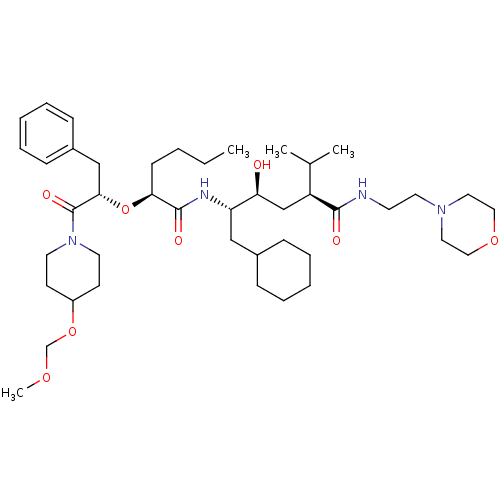 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006206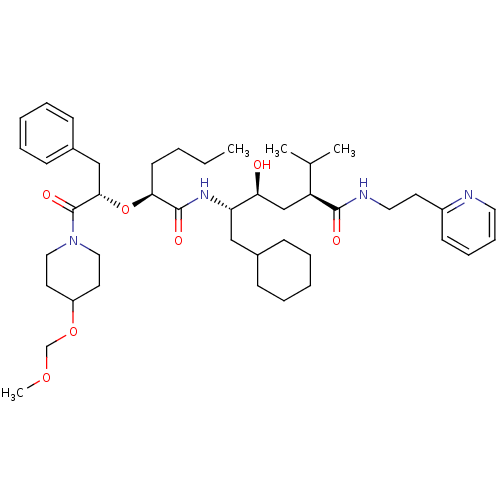 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046798 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006161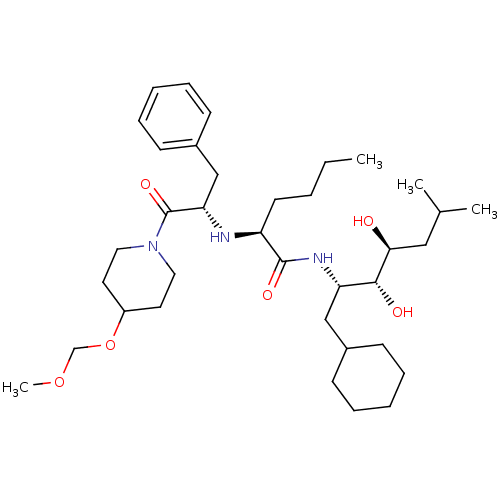 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006161 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046798 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036999 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006148 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006211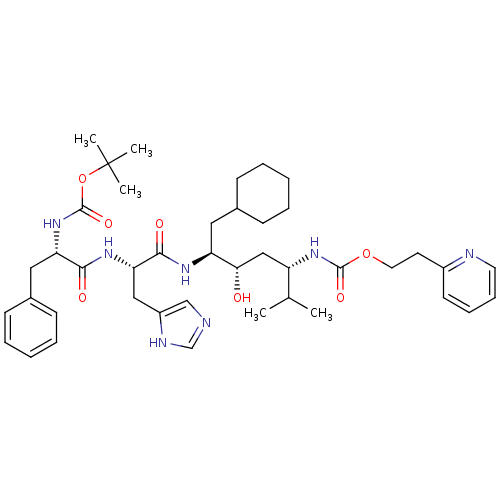 (CHEMBL49218 | {4-[2-(2-tert-Butoxycarbonylamino-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006204 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006176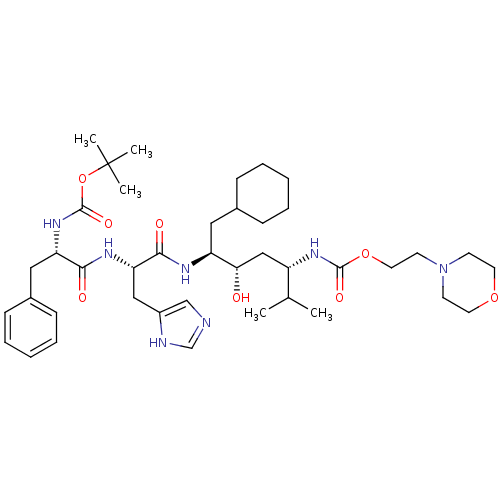 (CHEMBL264194 | {4-[2-(2-tert-Butoxycarbonylamino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006208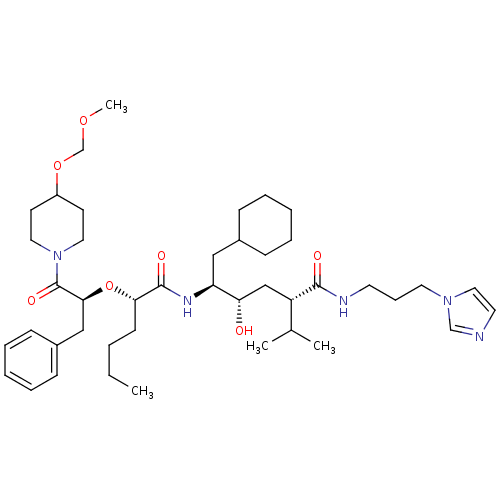 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006150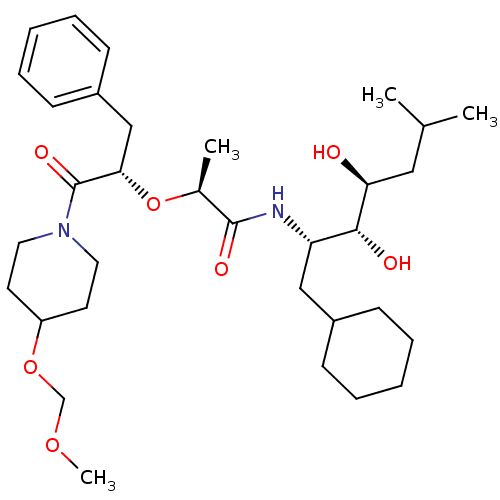 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011228 ((R)-2-Benzyl-N-[(S)-1-[(1S,2R)-1-cyclohexylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin at pH 7.4 | J Med Chem 36: 449-59 (1993) BindingDB Entry DOI: 10.7270/Q20K27M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046801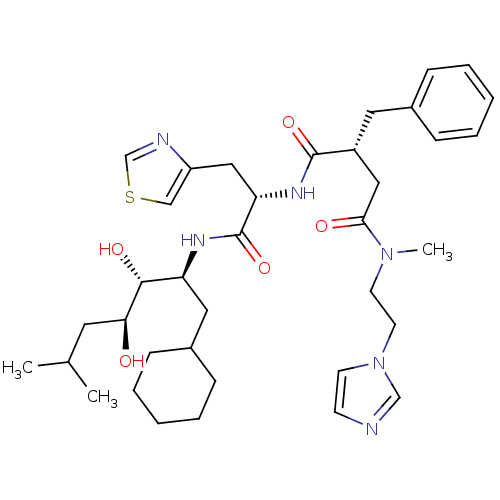 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036995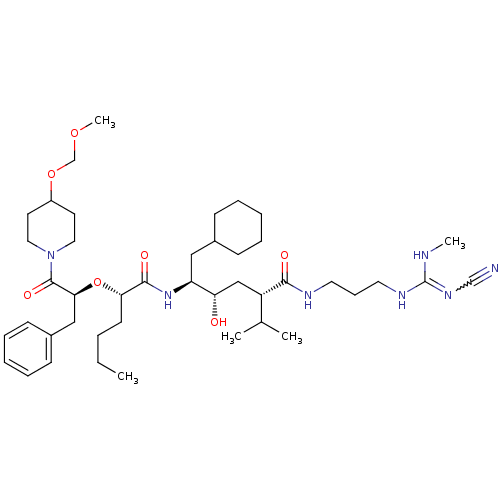 (1N-[3-methylamino(cyano imino)methylaminopropyl]-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036979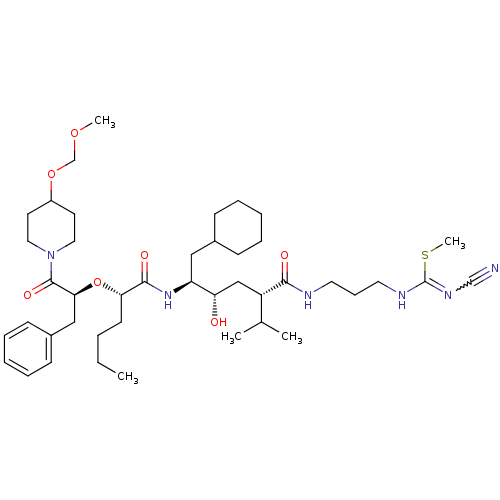 (1N-[3-cyanoimino(methylsulfanyl)methylaminopropyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006141 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 6.0 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006197 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006195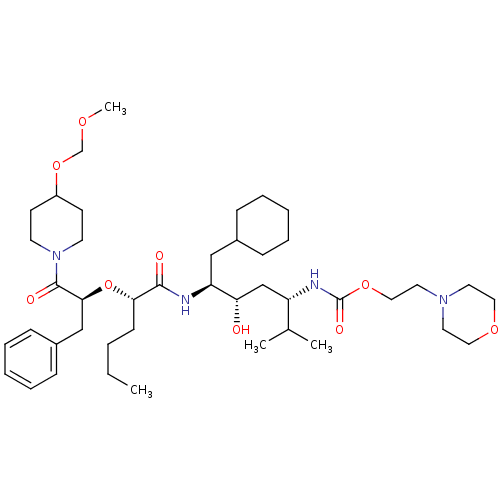 ((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006196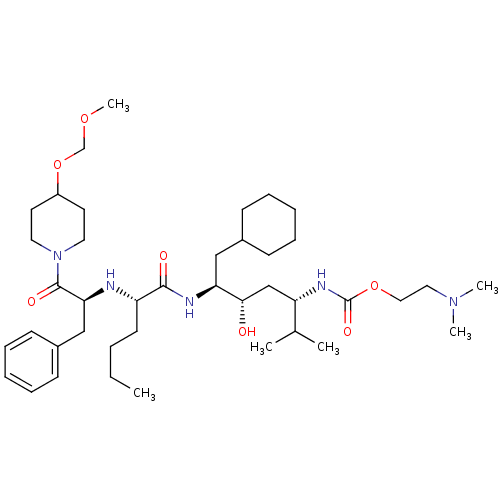 ((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006184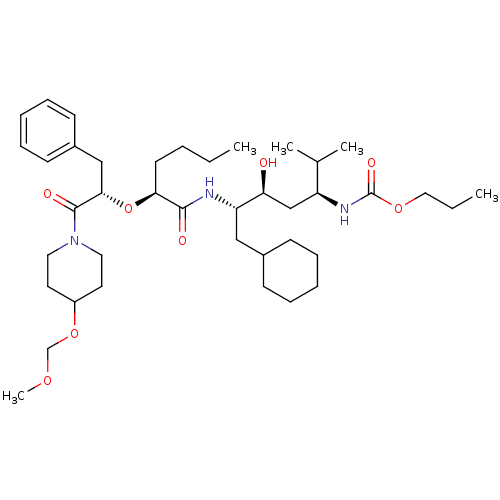 ((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006148 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036977 (1N-[3-amino(cyanoimino)methylaminopropyl]-5-[1-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046806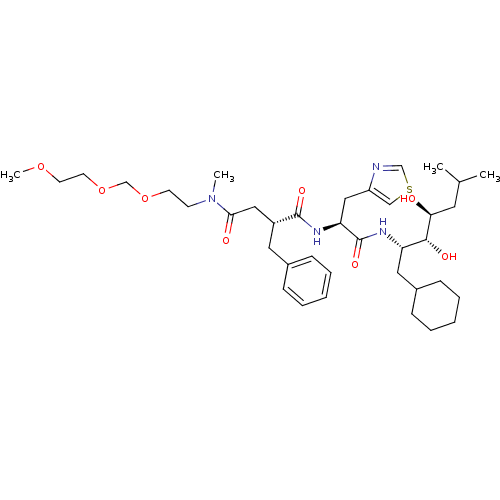 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046808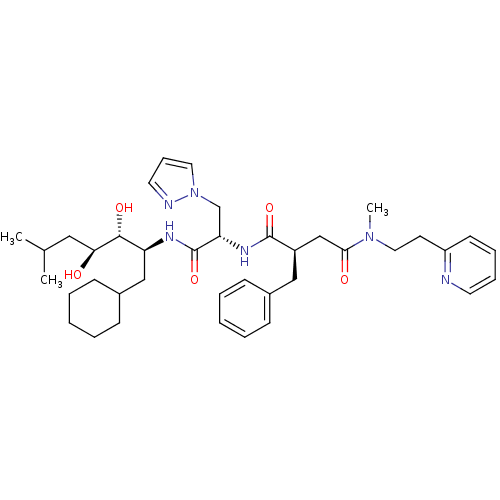 (2-Benzyl-N*1*-[1-(1-cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022647 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma renin at pH 7.4 | J Med Chem 36: 449-59 (1993) BindingDB Entry DOI: 10.7270/Q20K27M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036989 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006207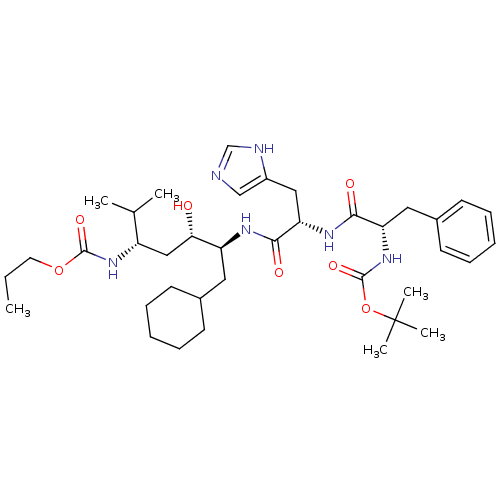 (CHEMBL301395 | {4-[2-(2-tert-Butoxycarbonylamino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036974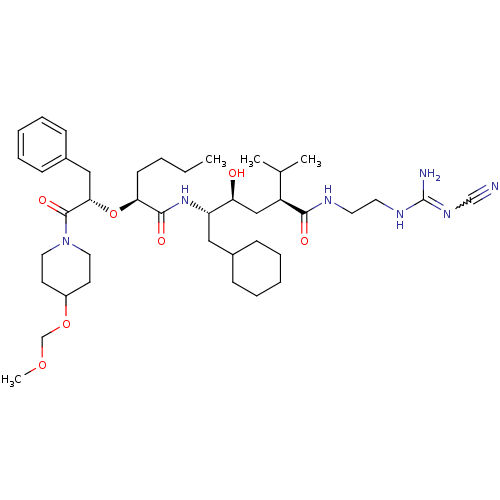 (1N-[2-amino(cyanoimino)methylaminoethyl]-5-[1-[1-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006157 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006174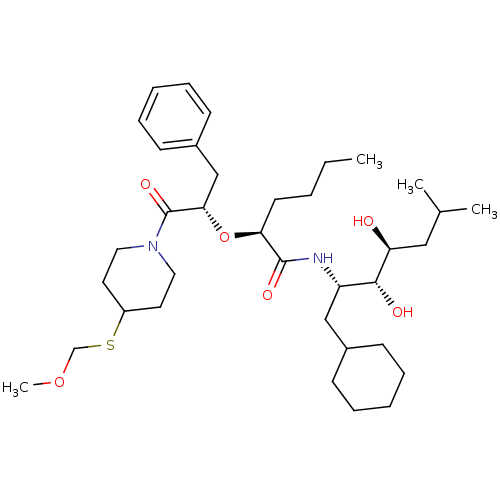 (2-[1-Benzyl-2-(4-methoxymethylsulfanyl-piperidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046805 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006200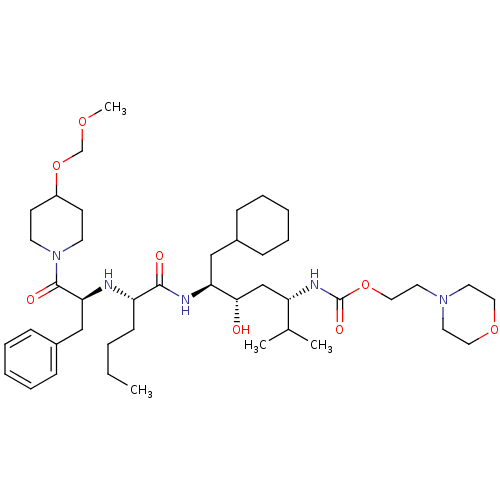 ((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at a pH of 7.4 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037007 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006179 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006192 (5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036985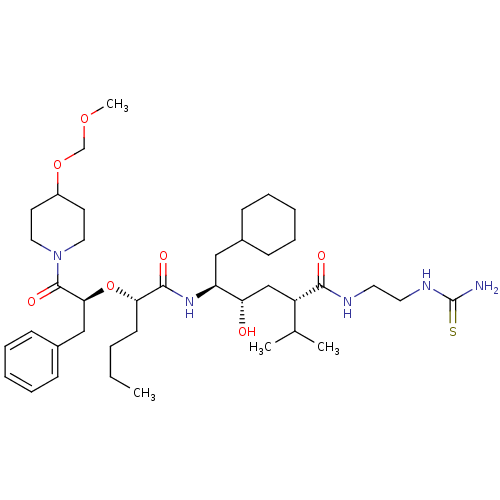 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006183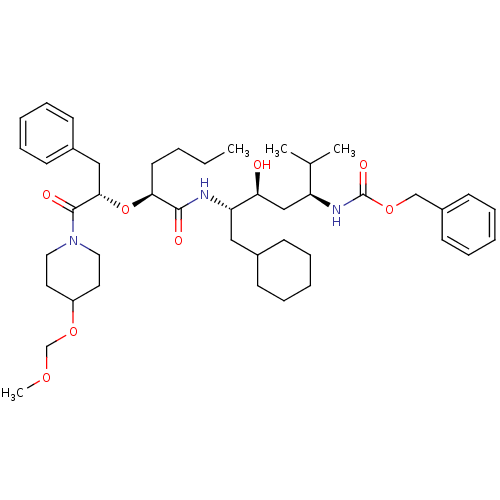 ((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro activity against human renin (pH 6.0) | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006202 (3-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against renin in monkey plasma at pH 7.7 | J Med Chem 35: 1735-46 (1992) BindingDB Entry DOI: 10.7270/Q27943MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 289 total ) | Next | Last >> |