

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50163748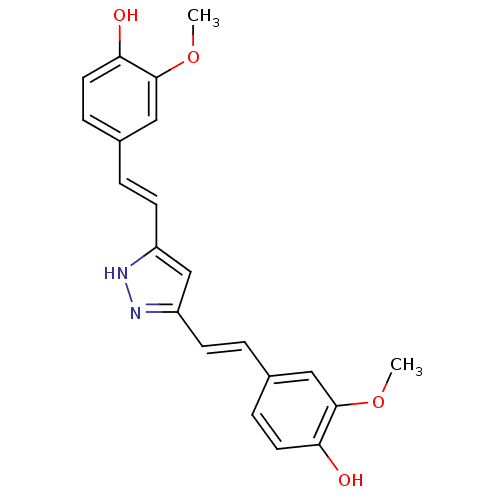 ((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated LTB4 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 15-LO-mediated 15-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition ... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of COX2-mediated PGD2 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of COX2-mediated PGF2alpha formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of COX1-mediated PGD2 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of COX1-mediated PGF2alpha formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES1-mediated PGE2 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5,12-DiHETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate additi... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5,12-DiHETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate additi... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020584 (CHEMBL2413471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5,12-DiHETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate additi... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50163748 ((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5,12-DiHETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate additi... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020584 (CHEMBL2413471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50163748 ((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated 5-HETE formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition me... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated LTB4 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated LTB4 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020584 (CHEMBL2413471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO-mediated LTB4 formation in LPS-stimulated human monocytes preincubated for 15 mins before arachidonic acid substrate addition meas... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50020588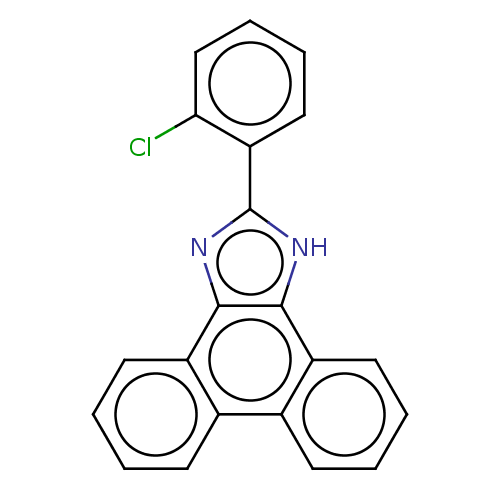 (CHEMBL270718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES1-mediated PGE2 production in microsomes of IL-1beta stimulated human A549 cells preincubated for 15 mins by RP-HPLC analysis | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli Bl21 (DE3) using arachidonic acid as substrate preincubated for 10 mins measured a... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078888 (CHEMBL3416359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078886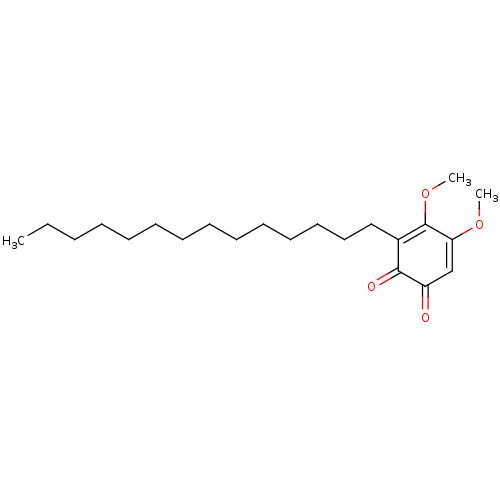 (CHEMBL3416361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078890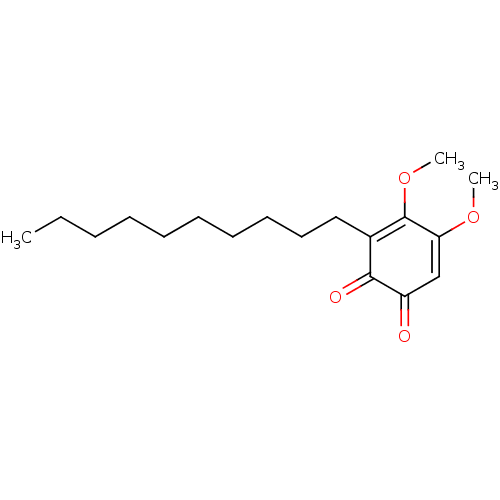 (CHEMBL3416357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078887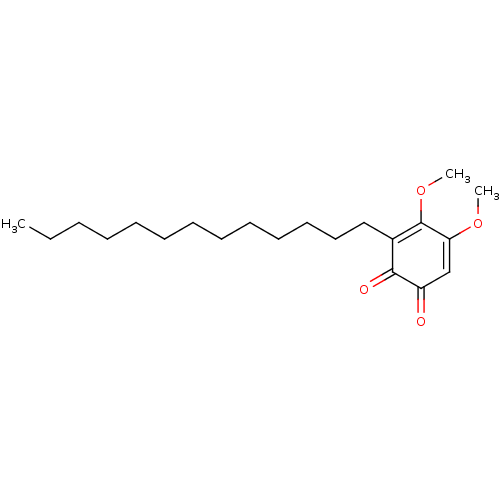 (CHEMBL3416360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation in presence of arachidonic acid pr... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in human neutrophils using arachidonic acid as substrate preincubated for 15 mins measured after 10 mins by HPLC analysis | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078850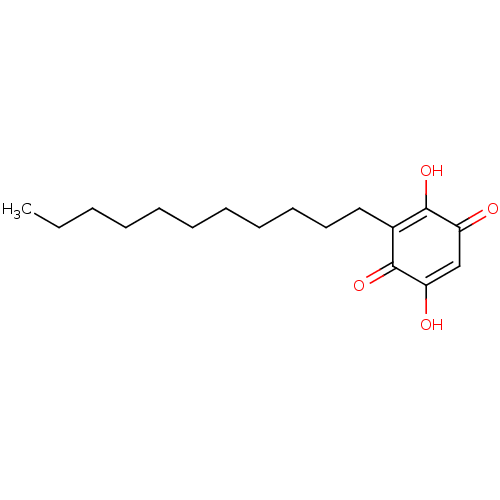 (CHEBI:4778 | Embelin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human 5-LOX by cell free assay | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078889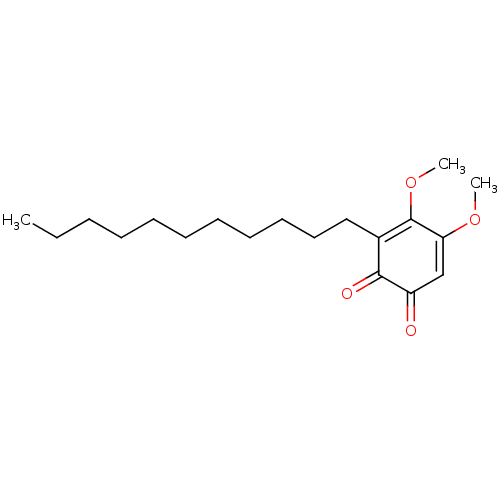 (CHEMBL3416358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078850 (CHEBI:4778 | Embelin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078890 (CHEMBL3416357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation in presence of arachidonic acid pr... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50163748 ((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli Bl21 (DE3) using arachidonic acid as substrate preincubated for 10 mins measured a... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078887 (CHEMBL3416360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020584 (CHEMBL2413471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli Bl21 (DE3) using arachidonic acid as substrate preincubated for 10 mins measured a... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078886 (CHEMBL3416361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation in presence of arachidonic acid pr... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078889 (CHEMBL3416358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078883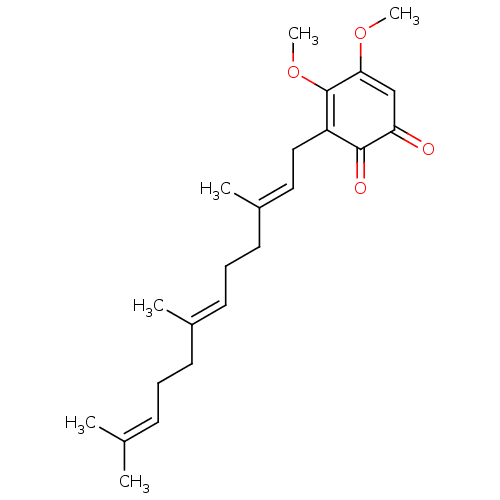 (CHEMBL3416365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation in presence of arachidonic acid pr... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50020589 (CHEMBL3290441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli Bl21 (DE3) using arachidonic acid as substrate preincubated for 10 mins measured a... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078889 (CHEMBL3416358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation in presence of arachidonic acid pr... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385376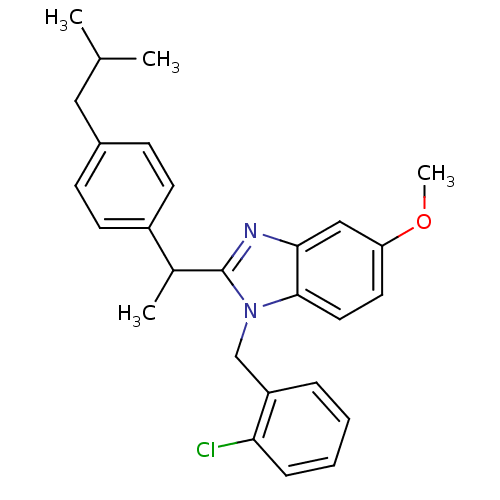 (CHEMBL2036377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078886 (CHEMBL3416361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078887 (CHEMBL3416360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078890 (CHEMBL3416357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078888 (CHEMBL3416359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078888 (CHEMBL3416359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation in presence of arachidonic acid pr... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078883 (CHEMBL3416365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-LOX in A23187-stimulated human blood PMNL assessed as reduction in lipoxygenase products formation pre-incubated for 15 mins followed... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50385380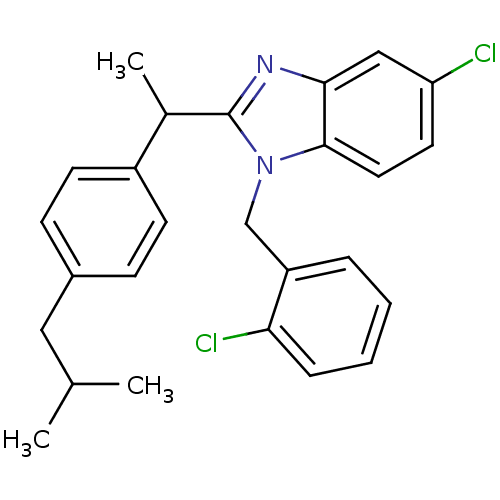 (CHEMBL2036382) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University Curated by ChEMBL | Assay Description Inhibition of FLAP in A23187-stimulated human neutrophils assessed as 5-LO product formation preincubated for 15 mins measured after 10 mins | Bioorg Med Chem 20: 3728-41 (2012) Article DOI: 10.1016/j.bmc.2012.04.048 BindingDB Entry DOI: 10.7270/Q26H4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078847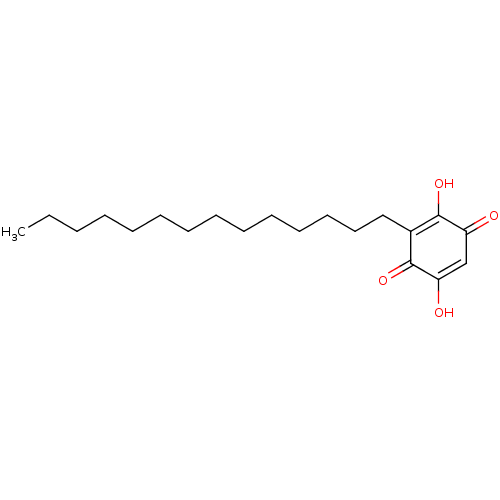 (CHEMBL3416165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50078849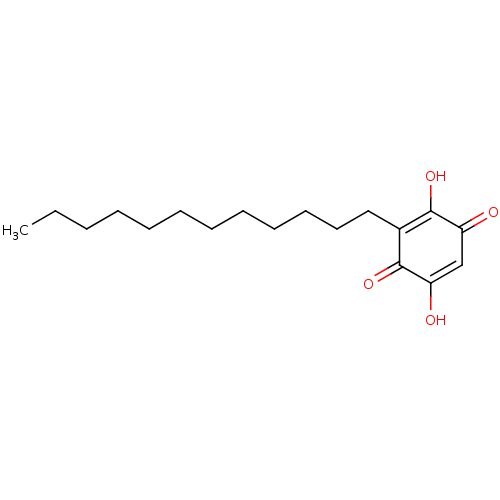 (CHEMBL3416164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in Escherichia coli BL21 incubated for 10 mins in presence of arachidonic acid by RP-HPLC based cell-... | Eur J Med Chem 94: 132-9 (2015) Article DOI: 10.1016/j.ejmech.2015.02.042 BindingDB Entry DOI: 10.7270/Q2T72K5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 522 total ) | Next | Last >> |