

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50338518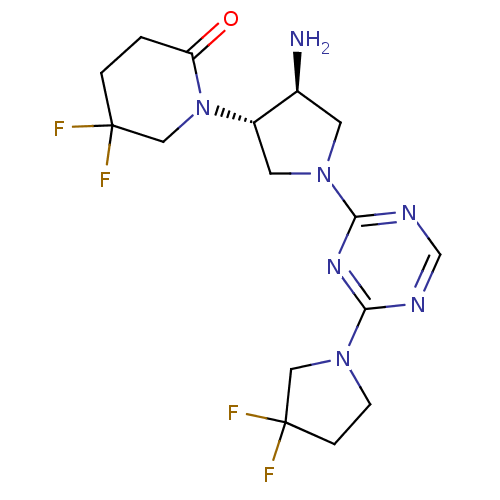 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119679 (2-[(S)-2-(2-{2-[4-(2-Bromo-acetyl)-phenoxy]-acetyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119683 (2-[(S)-2-(2-{[4'-(2-Bromo-acetyl)-biphenyl-2-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119685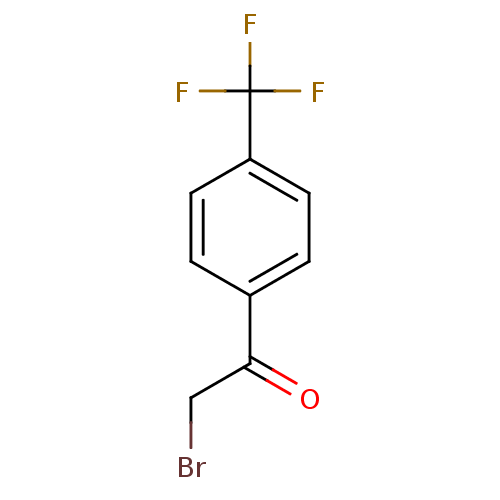 (2-Bromo-1-(4-trifluoromethyl-phenyl)-ethanone | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM7880 (2-bromo-1-(4-phenylphenyl)ethan-1-one | CHEMBL4130...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119682 (2-Bromo-1-naphthalen-2-yl-ethanone | CHEMBL101423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 (Homo sapiens (Human)) | BDBM50119679 (2-[(S)-2-(2-{2-[4-(2-Bromo-acetyl)-phenoxy]-acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards SHP 1 receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of FAP | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP9 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of POP | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP3 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP2 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119687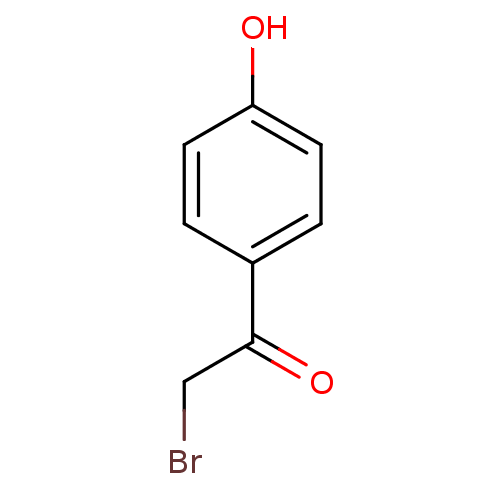 (2-Bromo-1-(4-hydroxy-phenyl)-ethanone | CHEMBL1029...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119681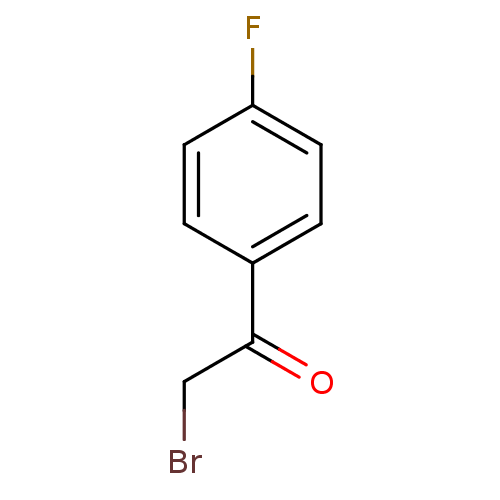 (2-Bromo-1-(4-fluoro-phenyl)-ethanone | CHEMBL31704...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM7875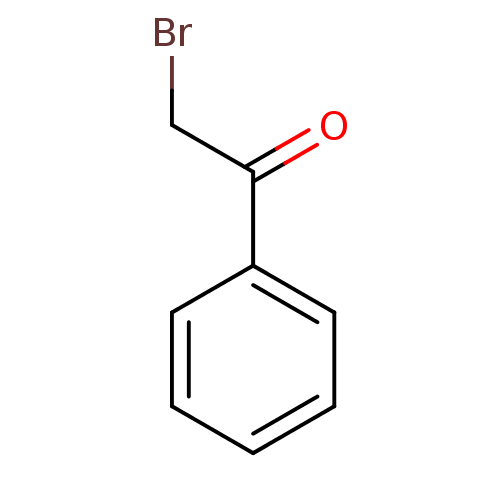 (2-bromo-1-phenylethan-1-one | CHEMBL102953 | Halom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119686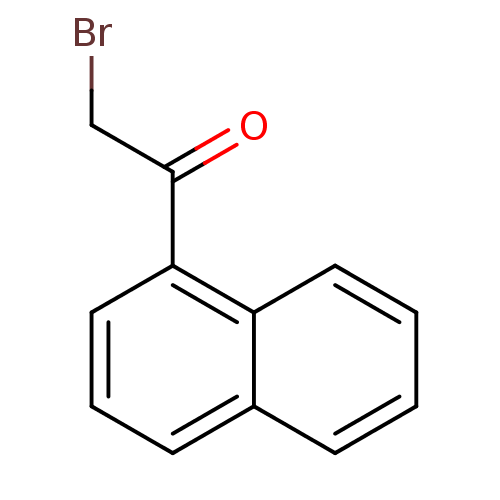 (2-Bromo-1-naphthalen-1-yl-ethanone | CHEMBL320836) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM7879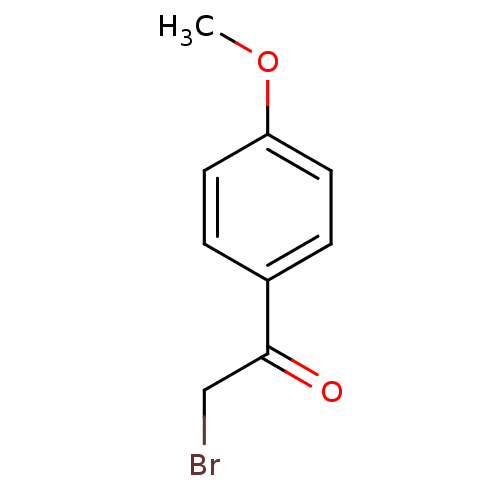 (2-bromo-1-(4-methoxyphenyl)ethan-1-one | CHEMBL103...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119689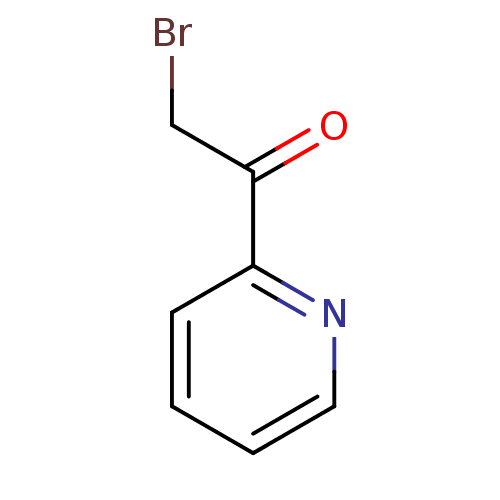 (2-Bromo-1-pyridin-2-yl-ethanone | 2-bromo-1-(pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119692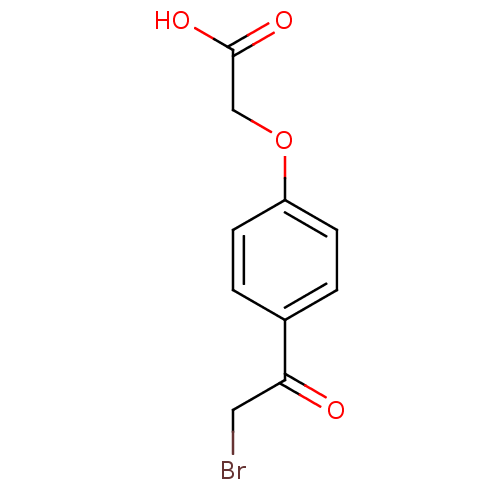 (CHEMBL104927 | [4-(2-Bromo-acetyl)-phenoxy]-acetic...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards SHP 1 receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119691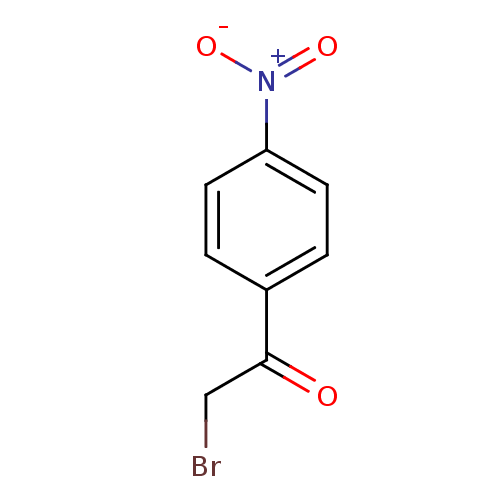 (2-Bromo-1-(4-nitro-phenyl)-ethanone | CHEMBL106072) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 (Homo sapiens (Human)) | BDBM50119683 (2-[(S)-2-(2-{[4'-(2-Bromo-acetyl)-biphenyl-2-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards SHP 1 receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119695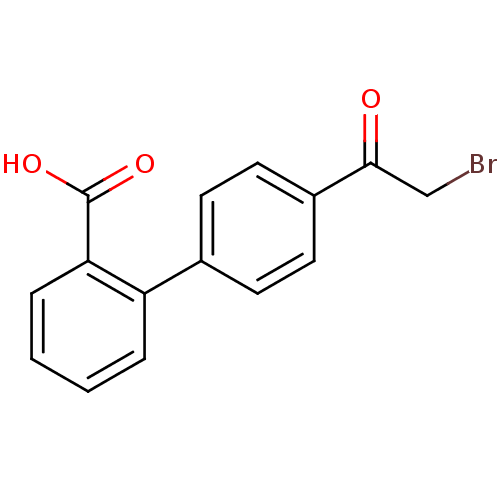 (4'-(2-Bromo-acetyl)-biphenyl-2-carboxylic acid | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards SHP 1 receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119680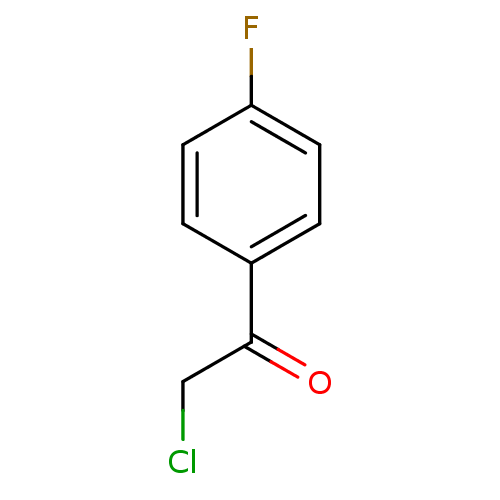 (2-Chloro-1-(4-fluoro-phenyl)-ethanone | CHEMBL1050...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM7873 (2-chloro-1-phenylethan-1-one | CHEMBL105712 | Halo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119694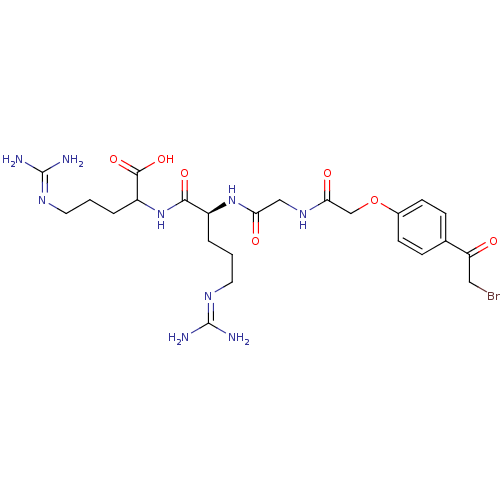 (2-[(S)-2-(2-{2-[4-(2-Bromo-acetyl)-phenoxy]-acetyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards SHP 1 receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119688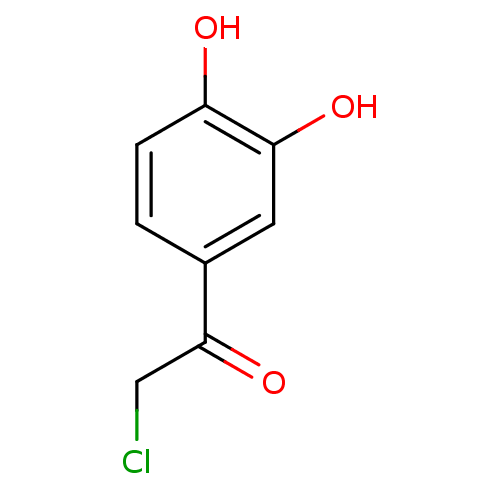 (2-chloro-1-(3,4-dihydroxyphenyl)ethanone | 2-chlor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119684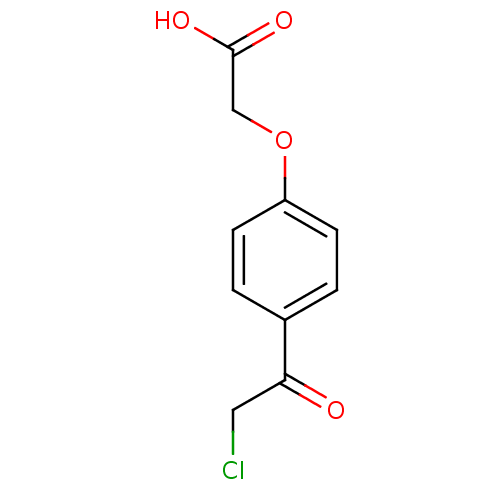 (CHEMBL319676 | [4-(2-Chloro-acetyl)-phenoxy]-aceti...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119690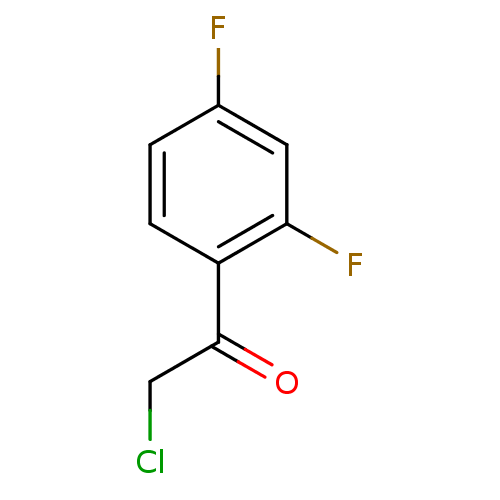 (2-Chloro-1-(2,4-difluoro-phenyl)-ethanone | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.75E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50119693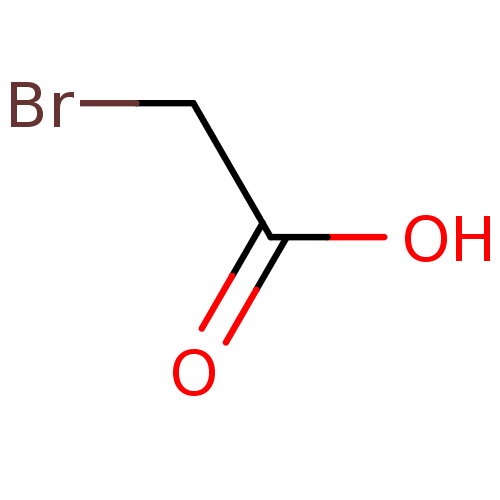 (2-BROMOACETYL GROUP | 2-bromoacetic acid | Bromo-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid UniChem Patents | PubMed | 7.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Dissociation constant of the compound towards Protein-tyrosine phosphatase 1B receptor-inhibitor complex was determined using PNP as substrate | Bioorg Med Chem Lett 12: 3047-50 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16315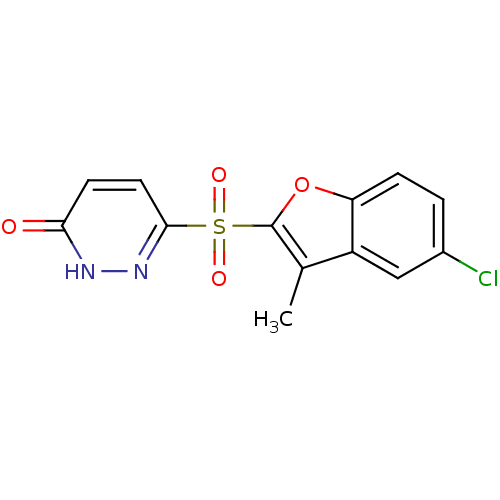 (6-[(5-chloro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | 7.0 | 24 |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 46: 2283-6 (2003) Article DOI: 10.1021/jm034065z BindingDB Entry DOI: 10.7270/Q2NV9GGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16315 (6-[(5-chloro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50338507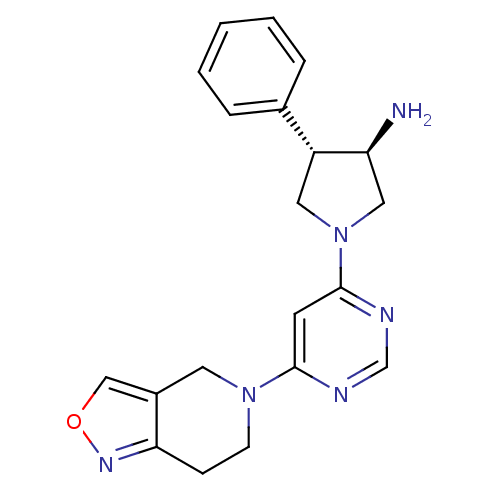 ((3R,4S)-1-(6-(6,7-dihydroisoxazolo[4,3-c]pyridin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439642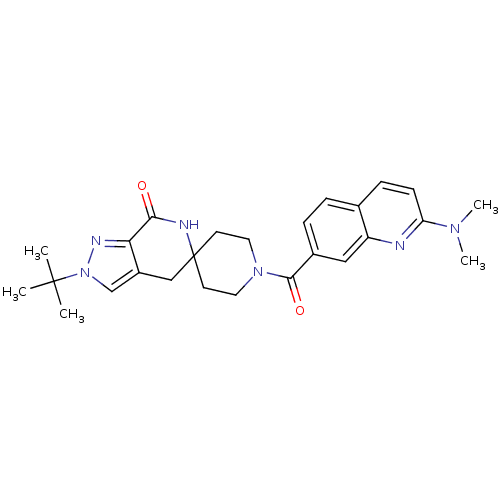 (CHEMBL2419589 | US8993586, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16633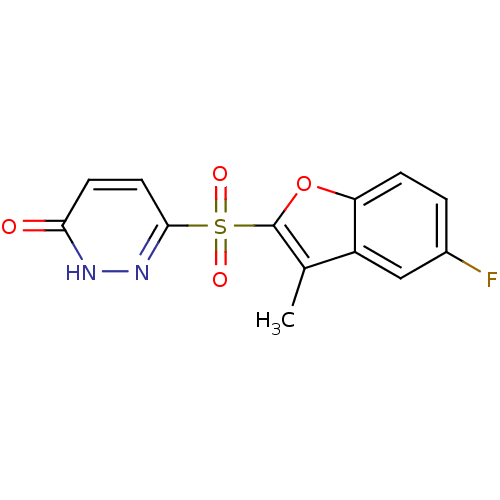 (6-[(5-fluoro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439644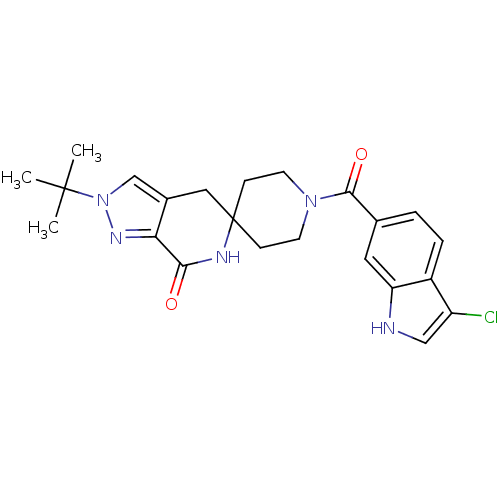 (CHEMBL2419593 | US8993586, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118710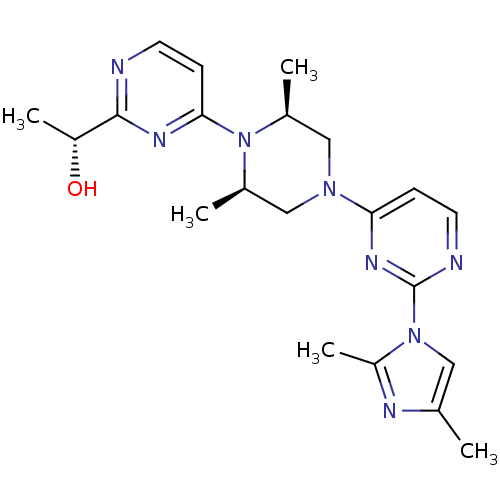 (1-(4-{4-[2-(2,4-Dimethyl-imidazol-1-yl)-pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118706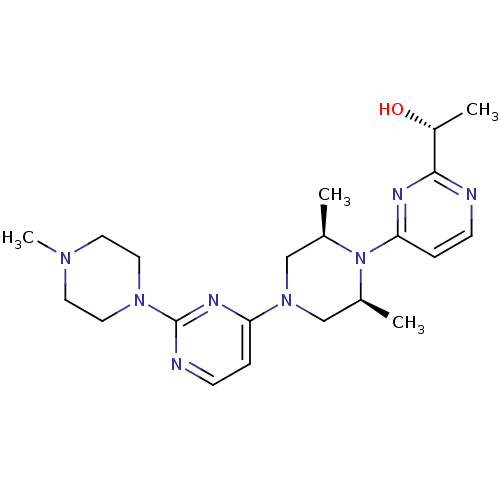 ((S)-1-(4-{2,6-Dimethyl-4-[2-(4-methyl-piperazin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452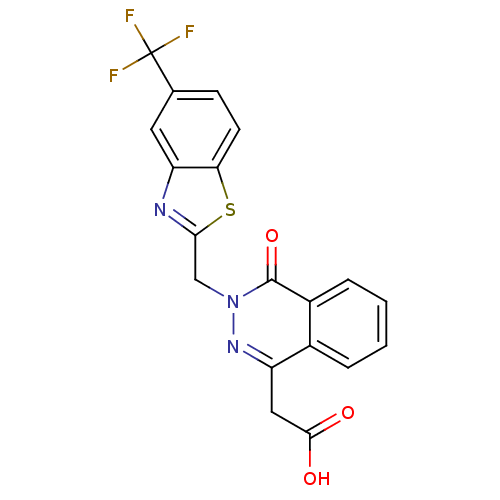 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | 24 |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 46: 2283-6 (2003) Article DOI: 10.1021/jm034065z BindingDB Entry DOI: 10.7270/Q2NV9GGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439634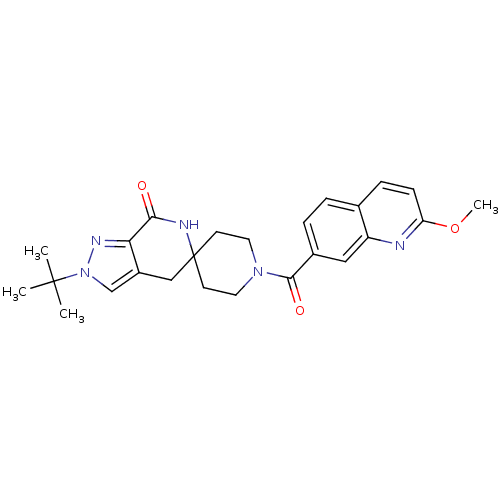 (CHEMBL2419596 | US8993586, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50278106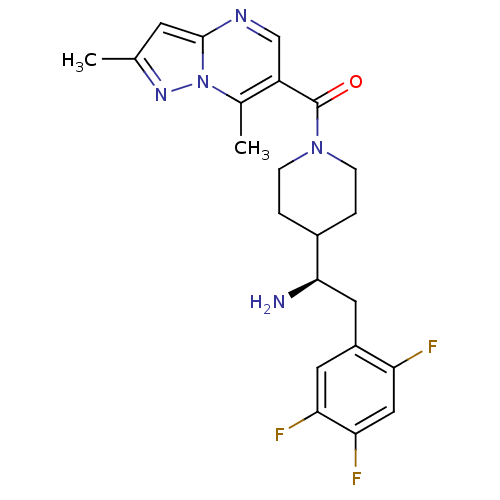 ((R)-(4-(1-amino-2-(2,4,5-trifluorophenyl)ethyl)pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 19: 2220-3 (2009) Article DOI: 10.1016/j.bmcl.2009.02.099 BindingDB Entry DOI: 10.7270/Q2RN37QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439643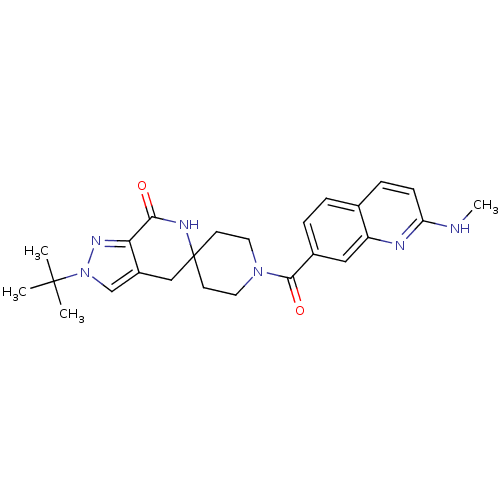 (CHEMBL2419598 | US8993586, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439641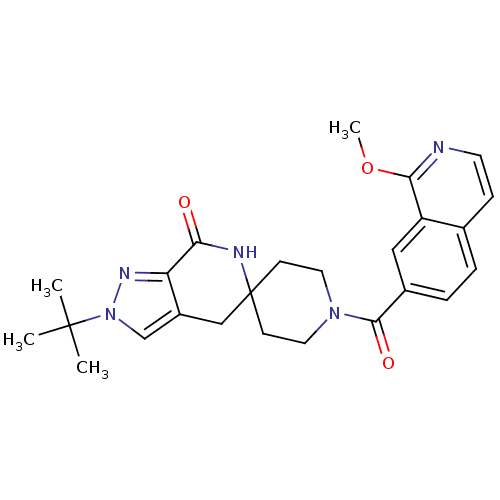 (CHEMBL2419597 | US8993586, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439645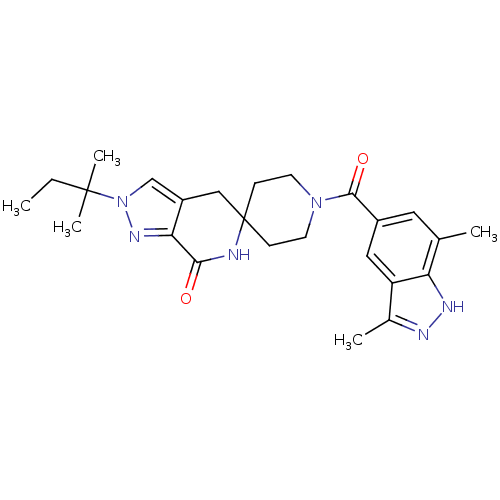 (CHEMBL2419607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50113497 (1-(4-{(2S,6R)-4-[2-((R)-1-Hydroxy-ethyl)-pyrimidin...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant rat sorbitol dehydrogenase | Bioorg Med Chem Lett 12: 1477-80 (2002) BindingDB Entry DOI: 10.7270/Q2XS5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439635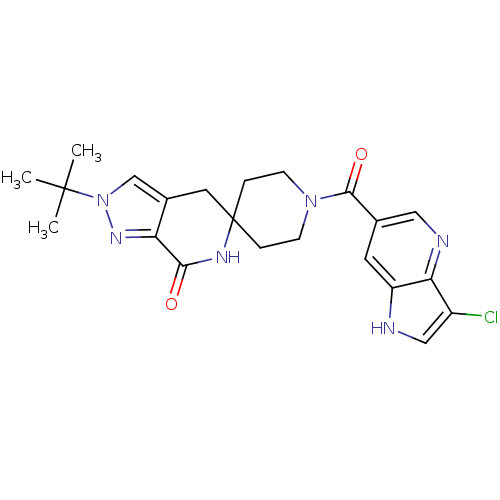 (CHEMBL2419594 | US8993586, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16634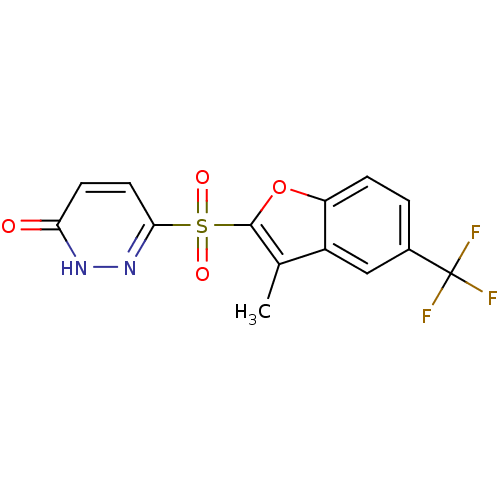 (6-{[3-methyl-5-(trifluoromethyl)-1-benzofuran-2-]s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118709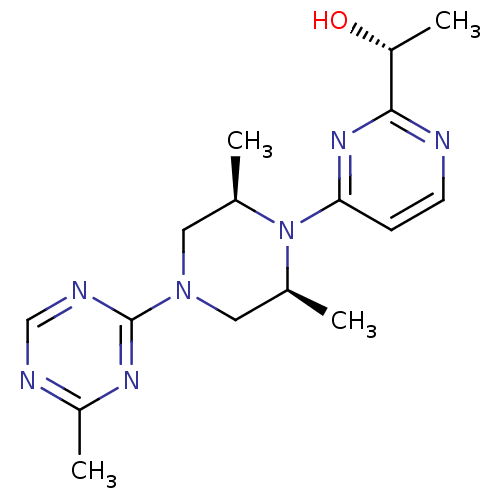 (1-{4-[2,6-Dimethyl-4-(4-methyl-[1,3,5]triazin-2-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 493 total ) | Next | Last >> |