 Found 153 hits with Last Name = 'belvedere' and Initial = 's'
Found 153 hits with Last Name = 'belvedere' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase
(Cryptosporidium parvum) | BDBM50135767
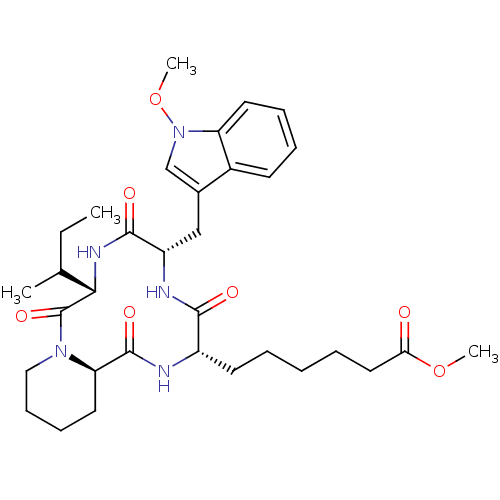
(6-[(6S,9S,12S,14aR)-6-sec-Butyl-9-(1-methoxy-1H-in...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)OC)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C33H47N5O7/c1-5-21(2)29-33(43)37-18-12-11-16-27(37)32(42)34-24(14-7-6-8-17-28(39)44-3)30(40)35-25(31(41)36-29)19-22-20-38(45-4)26-15-10-9-13-23(22)26/h9-10,13,15,20-21,24-25,27,29H,5-8,11-12,14,16-19H2,1-4H3,(H,34,42)(H,35,40)(H,36,41)/t21?,24-,25-,27+,29-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration determined against Histone deacetylase from Eimeria tenella |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50238632
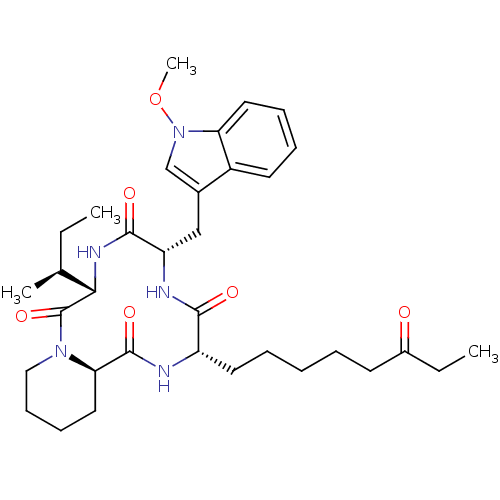
((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration determined against Histone deacetylase from Eimeria tenella |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50135749

((6S,9S,12S,14aR)-6-sec-Butyl-9-(1-methoxy-1H-indol...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)C2CO2)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C34H47N5O7/c1-4-21(2)30-34(44)38-17-11-10-15-27(38)33(43)35-24(13-6-5-7-16-28(40)29-20-46-29)31(41)36-25(32(42)37-30)18-22-19-39(45-3)26-14-9-8-12-23(22)26/h8-9,12,14,19,21,24-25,27,29-30H,4-7,10-11,13,15-18,20H2,1-3H3,(H,35,43)(H,36,41)(H,37,42)/t21?,24-,25-,27+,29?,30-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration determined against Histone deacetylase from Eimeria tenella |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213122
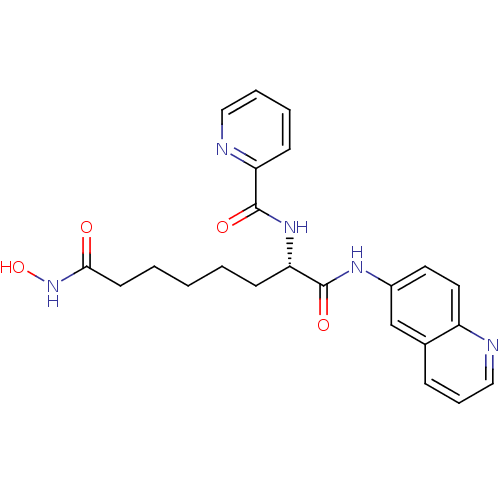
((S)-N-(8-(hydroxyamino)-1,8-dioxo-1-(quinolin-6-yl...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)c1ccccn1)C(=O)Nc1ccc2ncccc2c1 Show InChI InChI=1S/C23H25N5O4/c29-21(28-32)10-3-1-2-9-20(27-22(30)19-8-4-5-13-25-19)23(31)26-17-11-12-18-16(15-17)7-6-14-24-18/h4-8,11-15,20,32H,1-3,9-10H2,(H,26,31)(H,27,30)(H,28,29)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50135746
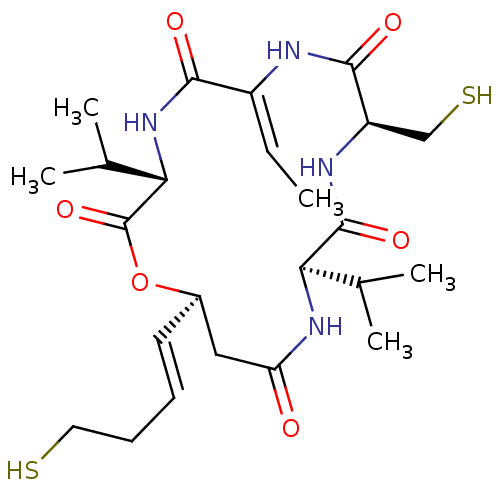
((3S,14S,15S)-6-Ethylidene-12-((R)-isopropyl)-3-iso...)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114813

(8-(4'-Bromo-biphenyl-4-yl)-8-oxo-octanoic acid hyd...)Show InChI InChI=1S/C20H22BrNO3/c21-18-13-11-16(12-14-18)15-7-9-17(10-8-15)19(23)5-3-1-2-4-6-20(24)22-25/h7-14,25H,1-6H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2b
(Zea mays) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against maize Histone deacetylase 2 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2b
(Zea mays) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against maize Histone deacetylase 2 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213132
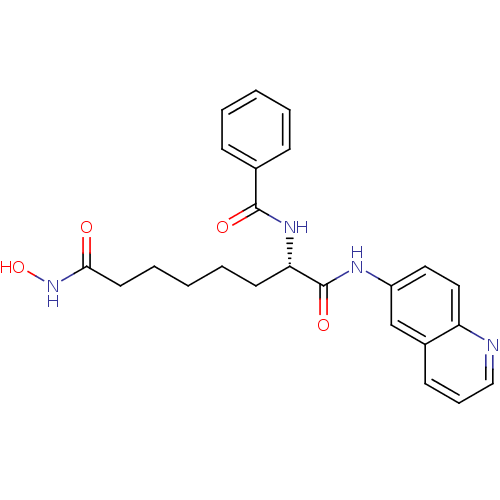
((S)-N-(8-(hydroxyamino)-1,8-dioxo-1-(quinolin-6-yl...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)c1ccccc1)C(=O)Nc1ccc2ncccc2c1 Show InChI InChI=1S/C24H26N4O4/c29-22(28-32)12-6-2-5-11-21(27-23(30)17-8-3-1-4-9-17)24(31)26-19-13-14-20-18(16-19)10-7-15-25-20/h1,3-4,7-10,13-16,21,32H,2,5-6,11-12H2,(H,26,31)(H,27,30)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50135746

((3S,14S,15S)-6-Ethylidene-12-((R)-isopropyl)-3-iso...)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Histone deacetylase 2 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50135751
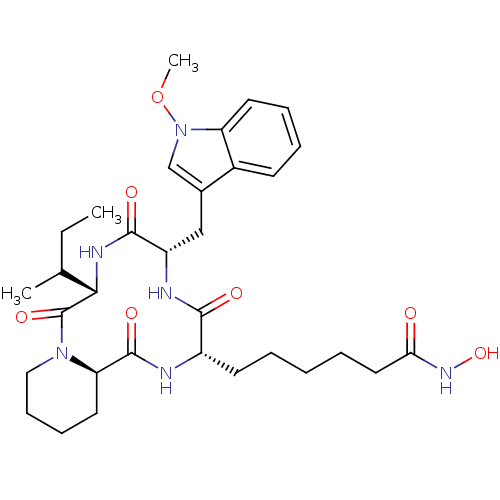
(6-[(6S,9S,12S,14aR)-6-sec-Butyl-9-(1-methoxy-1H-in...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)NO)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C32H46N6O7/c1-4-20(2)28-32(43)37-17-11-10-15-26(37)31(42)33-23(13-6-5-7-16-27(39)36-44)29(40)34-24(30(41)35-28)18-21-19-38(45-3)25-14-9-8-12-22(21)25/h8-9,12,14,19-20,23-24,26,28,44H,4-7,10-11,13,15-18H2,1-3H3,(H,33,42)(H,34,40)(H,35,41)(H,36,39)/t20?,23-,24-,26+,28-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration determined against Histone deacetylase from Eimeria tenella |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114835
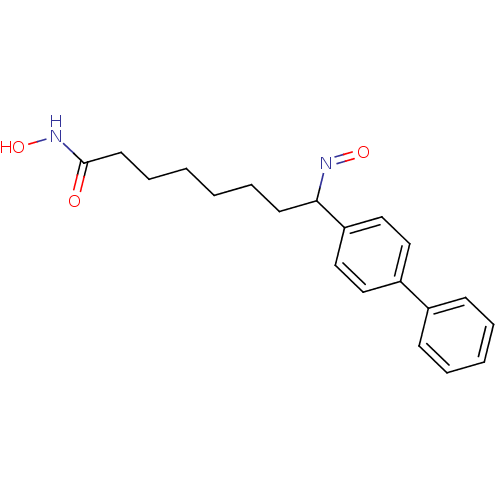
((E)-8-(biphenyl-4-yl)-N-hydroxy-8-(hydroxyimino)oc...)Show InChI InChI=1S/C20H24N2O3/c23-20(22-25)11-7-2-1-6-10-19(21-24)18-14-12-17(13-15-18)16-8-4-3-5-9-16/h3-5,8-9,12-15,19,25H,1-2,6-7,10-11H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213130

((S)-N-(8-(hydroxyamino)-1,8-dioxo-1-(quinolin-8-yl...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)c1ccccn1)C(=O)Nc1cccc2cccnc12 Show InChI InChI=1S/C23H25N5O4/c29-20(28-32)13-3-1-2-11-19(27-22(30)18-10-4-5-14-24-18)23(31)26-17-12-6-8-16-9-7-15-25-21(16)17/h4-10,12,14-15,19,32H,1-3,11,13H2,(H,26,31)(H,27,30)(H,28,29)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50135741
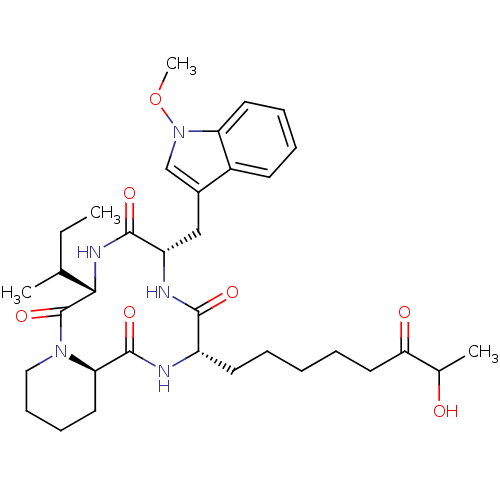
((6S,9S,12S,14aR)-6-sec-Butyl-12-(7-hydroxy-6-oxo-o...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)C(C)O)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C34H49N5O7/c1-5-21(2)30-34(45)38-18-12-11-16-28(38)33(44)35-25(14-7-6-8-17-29(41)22(3)40)31(42)36-26(32(43)37-30)19-23-20-39(46-4)27-15-10-9-13-24(23)27/h9-10,13,15,20-22,25-26,28,30,40H,5-8,11-12,14,16-19H2,1-4H3,(H,35,44)(H,36,42)(H,37,43)/t21?,22?,25-,26-,28+,30-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration determined against Histone deacetylase from Eimeria tenella |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114814
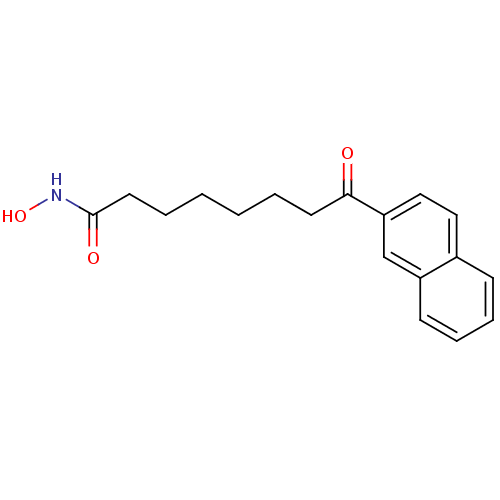
(8-Naphthalen-2-yl-8-oxo-octanoic acid hydroxyamide...)Show InChI InChI=1S/C18H21NO3/c20-17(9-3-1-2-4-10-18(21)19-22)16-12-11-14-7-5-6-8-15(14)13-16/h5-8,11-13,22H,1-4,9-10H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114816
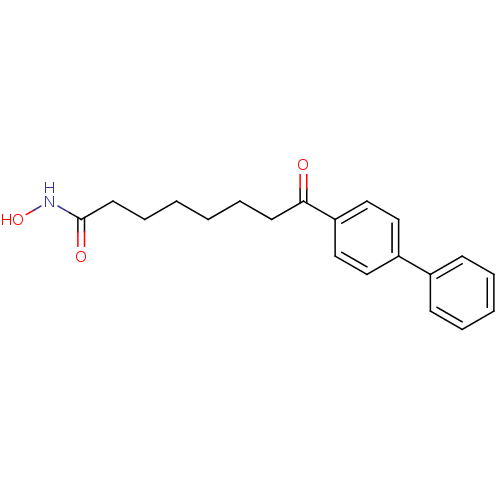
(8-(biphenyl-4-yl)-N-hydroxy-8-oxooctanamide | 8-Bi...)Show InChI InChI=1S/C20H23NO3/c22-19(10-6-1-2-7-11-20(23)21-24)18-14-12-17(13-15-18)16-8-4-3-5-9-16/h3-5,8-9,12-15,24H,1-2,6-7,10-11H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114829
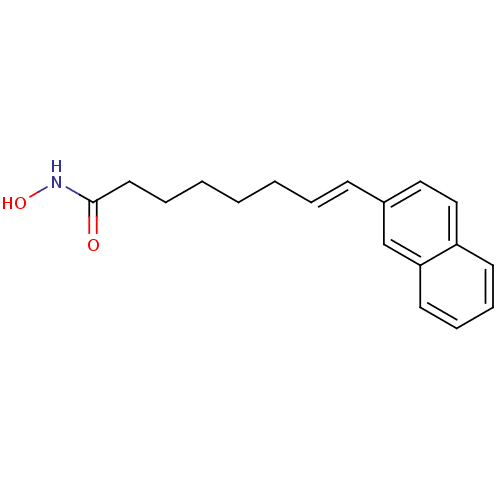
((E)-8-Naphthalen-2-yl-oct-7-enoic acid hydroxyamid...)Show InChI InChI=1S/C18H21NO2/c20-18(19-21)11-5-3-1-2-4-8-15-12-13-16-9-6-7-10-17(16)14-15/h4,6-10,12-14,21H,1-3,5,11H2,(H,19,20)/b8-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213124
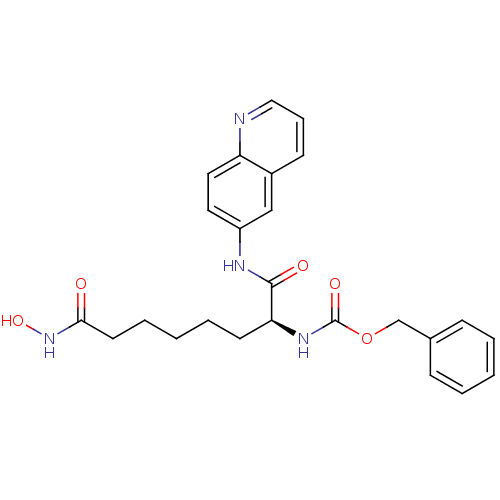
((S)-benzyl 8-(hydroxyamino)-1,8-dioxo-1-(quinolin-...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)Nc1ccc2ncccc2c1 Show InChI InChI=1S/C25H28N4O5/c30-23(29-33)12-6-2-5-11-22(28-25(32)34-17-18-8-3-1-4-9-18)24(31)27-20-13-14-21-19(16-20)10-7-15-26-21/h1,3-4,7-10,13-16,22,33H,2,5-6,11-12,17H2,(H,27,31)(H,28,32)(H,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114823

(8-Naphthalen-2-yl-non-8-enoic acid hydroxyamide | ...)Show InChI InChI=1S/C19H23NO2/c1-15(8-4-2-3-5-11-19(21)20-22)17-13-12-16-9-6-7-10-18(16)14-17/h6-7,9-10,12-14,22H,1-5,8,11H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114828

((E)-N-hydroxy-8-(hydroxyimino)-8-(naphthalen-2-yl)...)Show InChI InChI=1S/C18H22N2O3/c21-18(20-23)10-4-2-1-3-9-17(19-22)16-12-11-14-7-5-6-8-15(14)13-16/h5-8,11-13,17,23H,1-4,9-10H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213128

((S)-N-(8-(hydroxyamino)-1,8-dioxo-1-(quinolin-8-yl...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)c1ccccc1)C(=O)Nc1cccc2cccnc12 Show InChI InChI=1S/C24H26N4O4/c29-21(28-32)15-6-2-5-13-20(27-23(30)18-9-3-1-4-10-18)24(31)26-19-14-7-11-17-12-8-16-25-22(17)19/h1,3-4,7-12,14,16,20,32H,2,5-6,13,15H2,(H,26,31)(H,27,30)(H,28,29)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50123975
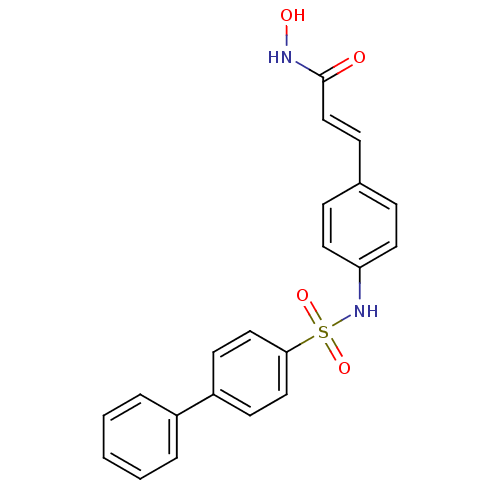
(3-[4-(biphenyl-4-sulfonylamino)-phenyl]-N-hydroxy-...)Show SMILES ONC(=O)\C=C\c1ccc(NS(=O)(=O)c2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C21H18N2O4S/c24-21(22-25)15-8-16-6-11-19(12-7-16)23-28(26,27)20-13-9-18(10-14-20)17-4-2-1-3-5-17/h1-15,23,25H,(H,22,24)/b15-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50216027
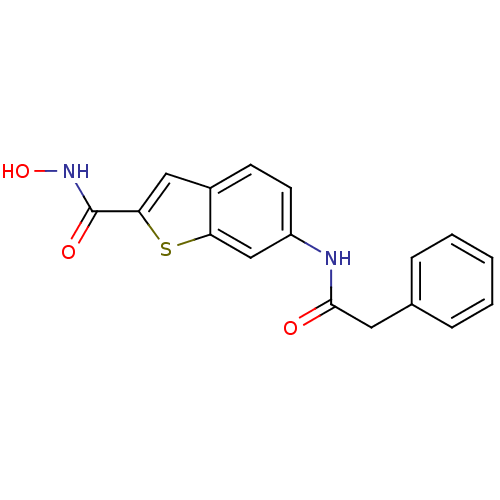
(CHEMBL247183 | N-hydroxy-6-(2-phenylacetamido)benz...)Show InChI InChI=1S/C17H14N2O3S/c20-16(8-11-4-2-1-3-5-11)18-13-7-6-12-9-15(17(21)19-22)23-14(12)10-13/h1-7,9-10,22H,8H2,(H,18,20)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 17: 4562-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.091
BindingDB Entry DOI: 10.7270/Q2CC10C4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50216014
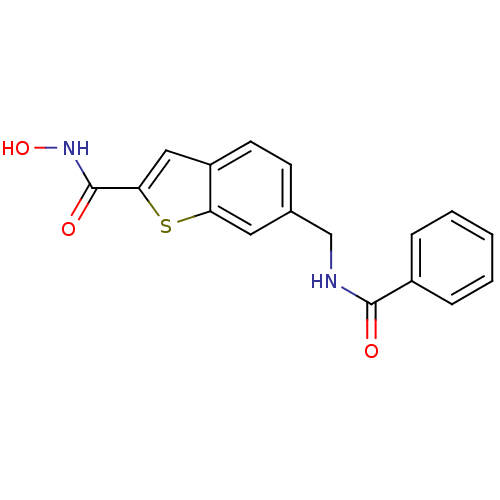
(6-(benzamidomethyl)-N-hydroxybenzo[b]thiophene-2-c...)Show InChI InChI=1S/C17H14N2O3S/c20-16(12-4-2-1-3-5-12)18-10-11-6-7-13-9-15(17(21)19-22)23-14(13)8-11/h1-9,22H,10H2,(H,18,20)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 17: 4562-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.091
BindingDB Entry DOI: 10.7270/Q2CC10C4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213135
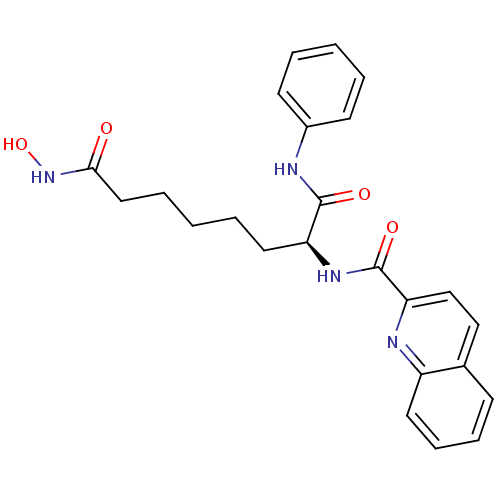
((S)-N-(8-(hydroxyamino)-1,8-dioxo-1-(phenylamino)o...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C24H26N4O4/c29-22(28-32)14-6-2-5-13-20(23(30)25-18-10-3-1-4-11-18)27-24(31)21-16-15-17-9-7-8-12-19(17)26-21/h1,3-4,7-12,15-16,20,32H,2,5-6,13-14H2,(H,25,30)(H,27,31)(H,28,29)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213126
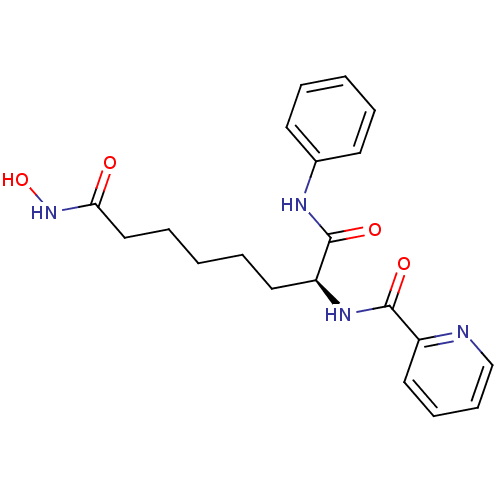
((S)-N-(8-(hydroxyamino)-1,8-dioxo-1-(phenylamino)o...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)c1ccccn1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H24N4O4/c25-18(24-28)13-6-2-5-12-17(20(27)22-15-9-3-1-4-10-15)23-19(26)16-11-7-8-14-21-16/h1,3-4,7-11,14,17,28H,2,5-6,12-13H2,(H,22,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213123
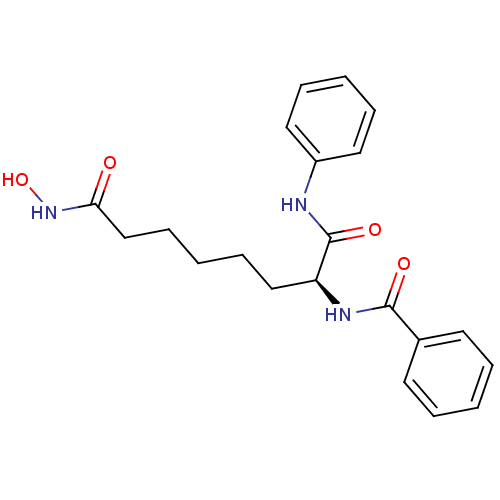
((S)-N-(8-(hydroxyamino)-1,8-dioxo-1-(phenylamino)o...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C21H25N3O4/c25-19(24-28)15-9-3-8-14-18(21(27)22-17-12-6-2-7-13-17)23-20(26)16-10-4-1-5-11-16/h1-2,4-7,10-13,18,28H,3,8-9,14-15H2,(H,22,27)(H,23,26)(H,24,25)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50200895
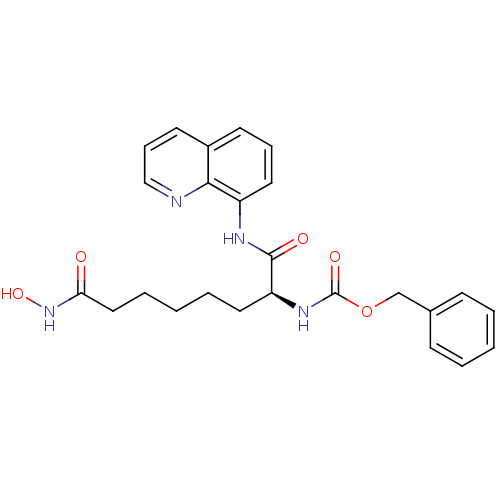
((S)-benzyl 8-(hydroxyamino)-1,8-dioxo-1-(quinolin-...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)Nc1cccc2cccnc12 |r| Show InChI InChI=1S/C25H28N4O5/c30-22(29-33)15-6-2-5-13-21(28-25(32)34-17-18-9-3-1-4-10-18)24(31)27-20-14-7-11-19-12-8-16-26-23(19)20/h1,3-4,7-12,14,16,21,33H,2,5-6,13,15,17H2,(H,27,31)(H,28,32)(H,29,30)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114826
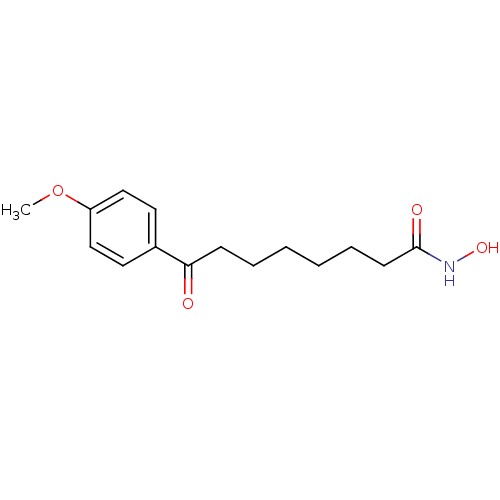
(8-(4-Methoxy-phenyl)-8-oxo-octanoic acid hydroxyam...)Show InChI InChI=1S/C15H21NO4/c1-20-13-10-8-12(9-11-13)14(17)6-4-2-3-5-7-15(18)16-19/h8-11,19H,2-7H2,1H3,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213131
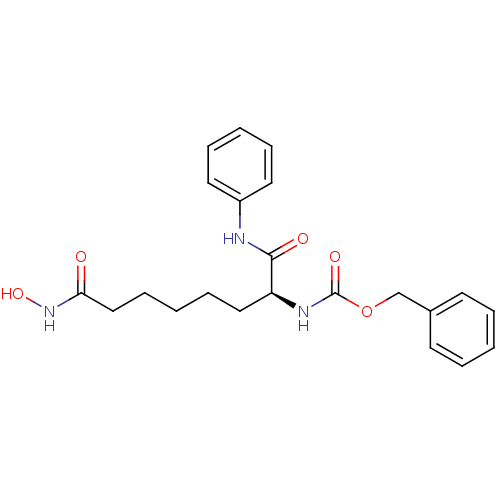
((S)-benzyl 8-(hydroxyamino)-1,8-dioxo-1-(phenylami...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C22H27N3O5/c26-20(25-29)15-9-3-8-14-19(21(27)23-18-12-6-2-7-13-18)24-22(28)30-16-17-10-4-1-5-11-17/h1-2,4-7,10-13,19,29H,3,8-9,14-16H2,(H,23,27)(H,24,28)(H,25,26)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50216021
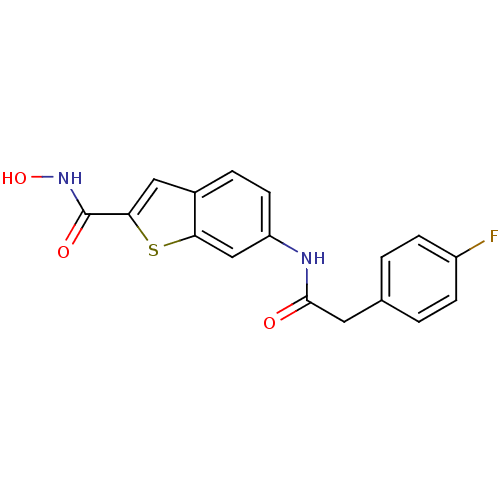
(6-(2-(4-fluorophenyl)acetamido)-N-hydroxybenzo[b]t...)Show InChI InChI=1S/C17H13FN2O3S/c18-12-4-1-10(2-5-12)7-16(21)19-13-6-3-11-8-15(17(22)20-23)24-14(11)9-13/h1-6,8-9,23H,7H2,(H,19,21)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 17: 4562-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.091
BindingDB Entry DOI: 10.7270/Q2CC10C4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50216038
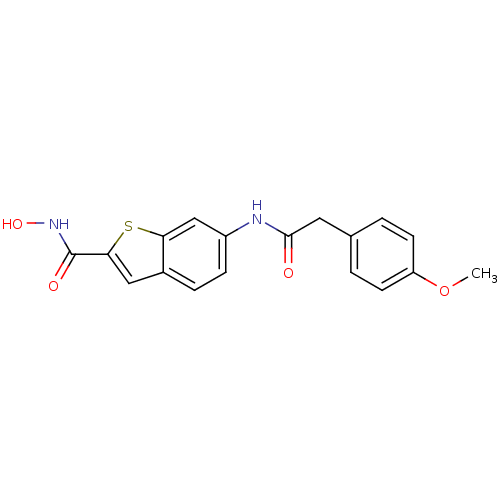
(CHEMBL247384 | N-hydroxy-6-(2-(4-methoxyphenyl)ace...)Show InChI InChI=1S/C18H16N2O4S/c1-24-14-6-2-11(3-7-14)8-17(21)19-13-5-4-12-9-16(18(22)20-23)25-15(12)10-13/h2-7,9-10,23H,8H2,1H3,(H,19,21)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 17: 4562-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.091
BindingDB Entry DOI: 10.7270/Q2CC10C4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50216016
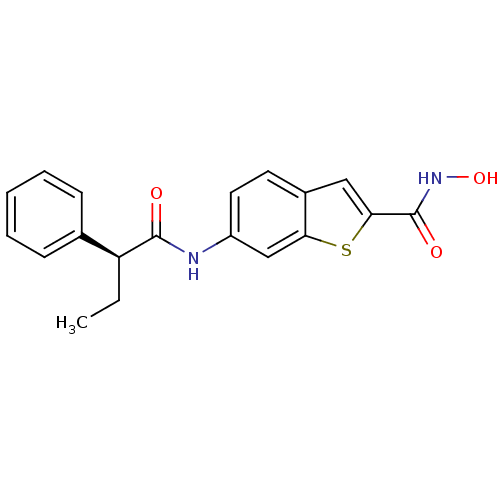
((S)-N-hydroxy-6-(2-phenylbutanamido)benzo[b]thioph...)Show SMILES CC[C@H](C(=O)Nc1ccc2cc(sc2c1)C(=O)NO)c1ccccc1 Show InChI InChI=1S/C19H18N2O3S/c1-2-15(12-6-4-3-5-7-12)18(22)20-14-9-8-13-10-17(19(23)21-24)25-16(13)11-14/h3-11,15,24H,2H2,1H3,(H,20,22)(H,21,23)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 17: 4562-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.091
BindingDB Entry DOI: 10.7270/Q2CC10C4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50135746

((3S,14S,15S)-6-Ethylidene-12-((R)-isopropyl)-3-iso...)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Histone deacetylase 4 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114833

(8-Naphthalen-2-yl-octanoic acid hydroxyamide | CHE...)Show InChI InChI=1S/C18H23NO2/c20-18(19-21)11-5-3-1-2-4-8-15-12-13-16-9-6-7-10-17(16)14-15/h6-7,9-10,12-14,21H,1-5,8,11H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50216032
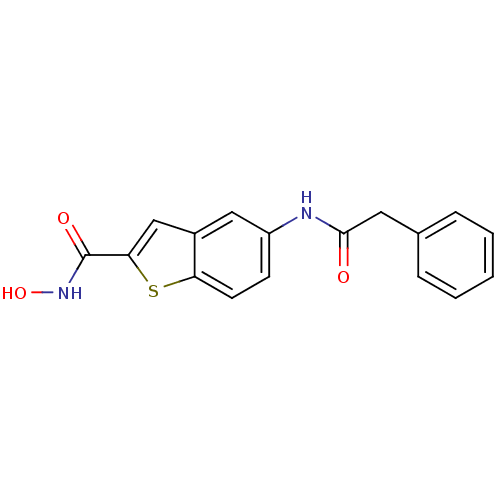
(CHEMBL247184 | N-hydroxy-5-(2-phenylacetamido)benz...)Show InChI InChI=1S/C17H14N2O3S/c20-16(8-11-4-2-1-3-5-11)18-13-6-7-14-12(9-13)10-15(23-14)17(21)19-22/h1-7,9-10,22H,8H2,(H,18,20)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 17: 4562-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.091
BindingDB Entry DOI: 10.7270/Q2CC10C4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50135750
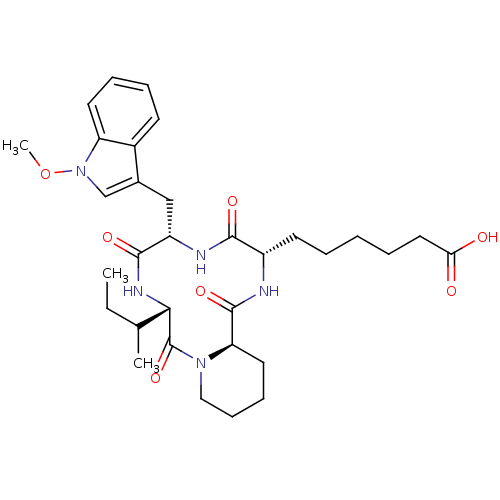
(6-[(6S,9S,12S,14aR)-6-sec-Butyl-9-(1-methoxy-1H-in...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(O)=O)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C32H45N5O7/c1-4-20(2)28-32(43)36-17-11-10-15-26(36)31(42)33-23(13-6-5-7-16-27(38)39)29(40)34-24(30(41)35-28)18-21-19-37(44-3)25-14-9-8-12-22(21)25/h8-9,12,14,19-20,23-24,26,28H,4-7,10-11,13,15-18H2,1-3H3,(H,33,42)(H,34,40)(H,35,41)(H,38,39)/t20?,23-,24-,26+,28-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration determined against Histone deacetylase from Eimeria tenella |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2b
(Zea mays) | BDBM50115654

(CHEMBL111993 | Octanedioic acid hydroxyamide ((S)-...)Show SMILES ONC(=O)CCCCCCC(=O)N[C@@H](Cc1cccc2ccccc12)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C29H35N3O4/c33-27(17-6-1-2-7-18-28(34)32-36)31-26(29(35)30-20-19-22-11-4-3-5-12-22)21-24-15-10-14-23-13-8-9-16-25(23)24/h3-5,8-16,26,36H,1-2,6-7,17-21H2,(H,30,35)(H,31,33)(H,32,34)/t26-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against maize Histone deacetylase 2 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114820
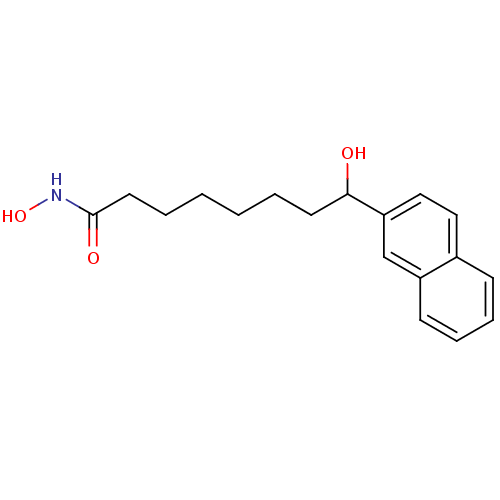
(8-Hydroxy-8-naphthalen-2-yl-octanoic acid hydroxya...)Show InChI InChI=1S/C18H23NO3/c20-17(9-3-1-2-4-10-18(21)19-22)16-12-11-14-7-5-6-8-15(14)13-16/h5-8,11-13,17,20,22H,1-4,9-10H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2b
(Zea mays) | BDBM50115657
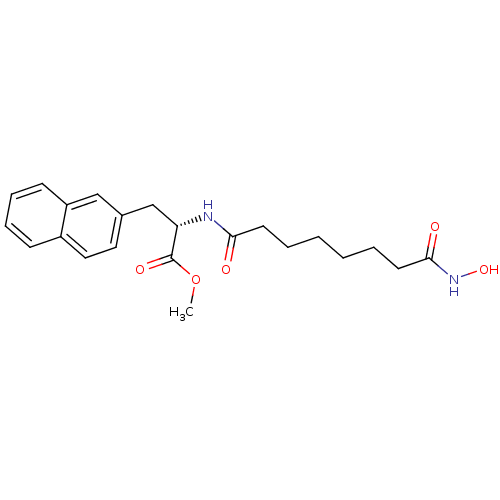
((S)-2-(7-Hydroxycarbamoyl-heptanoylamino)-3-naphth...)Show SMILES COC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)CCCCCCC(=O)NO Show InChI InChI=1S/C22H28N2O5/c1-29-22(27)19(15-16-12-13-17-8-6-7-9-18(17)14-16)23-20(25)10-4-2-3-5-11-21(26)24-28/h6-9,12-14,19,28H,2-5,10-11,15H2,1H3,(H,23,25)(H,24,26)/t19-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against maize Histone deacetylase 2 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50123967

(3-[4-(Biphenyl-4-ylsulfamoyl)-phenyl]-N-hydroxy-ac...)Show SMILES ONC(=O)\C=C\c1ccc(cc1)S(=O)(=O)Nc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H18N2O4S/c24-21(22-25)15-8-16-6-13-20(14-7-16)28(26,27)23-19-11-9-18(10-12-19)17-4-2-1-3-5-17/h1-15,23,25H,(H,22,24)/b15-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50216019
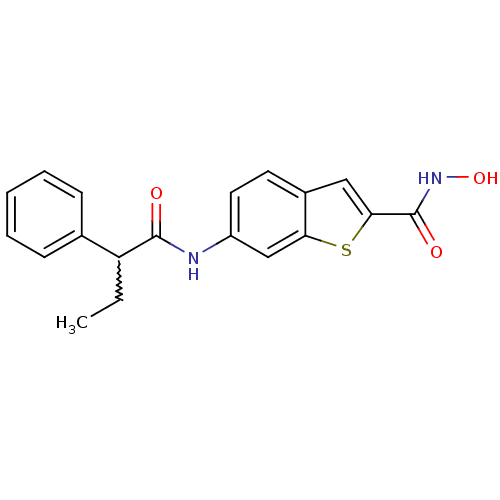
(CHEMBL246783 | N-hydroxy-6-(2-phenylbutanamido)ben...)Show SMILES CCC(C(=O)Nc1ccc2cc(sc2c1)C(=O)NO)c1ccccc1 |w:2.1| Show InChI InChI=1S/C19H18N2O3S/c1-2-15(12-6-4-3-5-7-12)18(22)20-14-9-8-13-10-17(19(23)21-24)25-16(13)11-14/h3-11,15,24H,2H2,1H3,(H,20,22)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 17: 4562-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.091
BindingDB Entry DOI: 10.7270/Q2CC10C4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50213133

((S)-N-(8-(hydroxyamino)-1,8-dioxo-1-(quinolin-8-yl...)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)Nc1cccc2cccnc12 Show InChI InChI=1S/C27H27N5O4/c33-24(32-36)14-3-1-2-12-22(26(34)30-21-13-6-9-19-10-7-17-28-25(19)21)31-27(35)23-16-15-18-8-4-5-11-20(18)29-23/h4-11,13,15-17,22,36H,1-3,12,14H2,(H,30,34)(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50216031

(CHEMBL247784 | N-hydroxy-6-((phenylamino)methyl)be...)Show InChI InChI=1S/C16H14N2O2S/c19-16(18-20)15-9-12-7-6-11(8-14(12)21-15)10-17-13-4-2-1-3-5-13/h1-9,17,20H,10H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 17: 4562-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.091
BindingDB Entry DOI: 10.7270/Q2CC10C4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114812
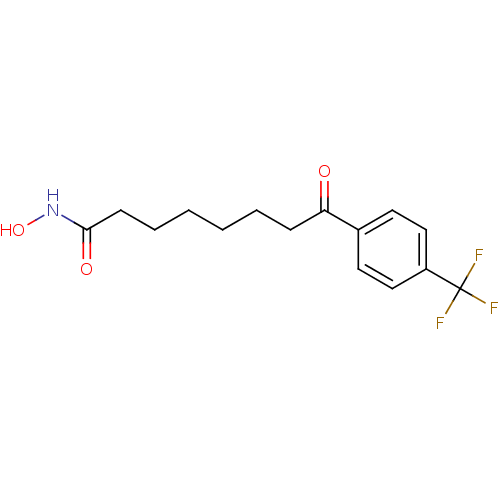
(8-Oxo-8-(4-trifluoromethyl-phenyl)-octanoic acid h...)Show InChI InChI=1S/C15H18F3NO3/c16-15(17,18)12-9-7-11(8-10-12)13(20)5-3-1-2-4-6-14(21)19-22/h7-10,22H,1-6H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50114817
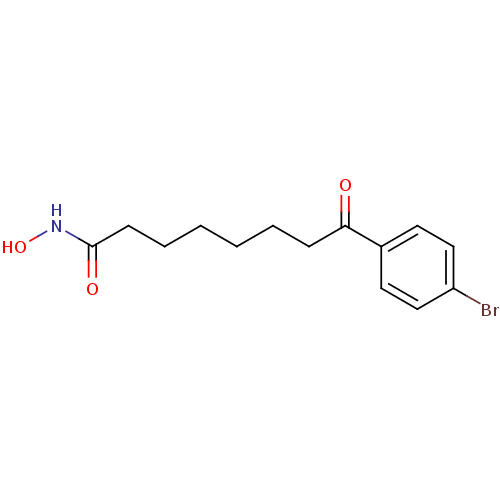
(8-(4-Bromo-phenyl)-8-oxo-octanoic acid hydroxyamid...)Show InChI InChI=1S/C14H18BrNO3/c15-12-9-7-11(8-10-12)13(17)5-3-1-2-4-6-14(18)16-19/h7-10,19H,1-6H2,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Histone deacetylase 1 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Histone deacetylase 2 |
J Med Chem 46: 5097-116 (2003)
Article DOI: 10.1021/jm0303094
BindingDB Entry DOI: 10.7270/Q2MP5413 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC 1 |
Bioorg Med Chem Lett 17: 3969-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.089
BindingDB Entry DOI: 10.7270/Q2M908CJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data