 Found 88 hits with Last Name = 'bianco' and Initial = 'a'
Found 88 hits with Last Name = 'bianco' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50339190
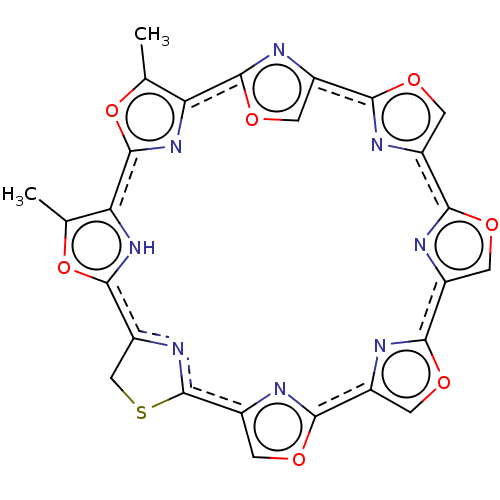
(CHEMBL443683 | telomestatin)Show SMILES Cc1oc2nc1c1nc(co1)c1nc(co1)c1nc(co1)c1nc(co1)c1nc(co1)c1SCc(n1)c1[nH]c2c(C)o1 Show InChI InChI=1S/C26H14N8O7S/c1-9-17-24-30-14(6-39-24)21-28-12(4-37-21)19-27-11(3-35-19)20-29-13(5-36-20)22-31-15(7-38-22)26-32-16(8-42-26)23-33-18(10(2)40-23)25(34-17)41-9/h3-7,33H,8H2,1-2H3/b20-11+,21-14+,26-15+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of human Telomerase activity in cell free system by TRAP assay |
J Med Chem 54: 1140-56 (2011)
Article DOI: 10.1021/jm1013665
BindingDB Entry DOI: 10.7270/Q2GB24CR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50397360

(CHEMBL2170177 | US10188756, Compound CN110)Show InChI InChI=1S/C17H16N2O3/c1-22-15-6-2-12(3-7-15)11-19-9-8-13-4-5-14(10-16(13)19)17(20)18-21/h2-10,21H,11H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor receptor superfamily member 5
(Homo sapiens (Human)) | BDBM50325995
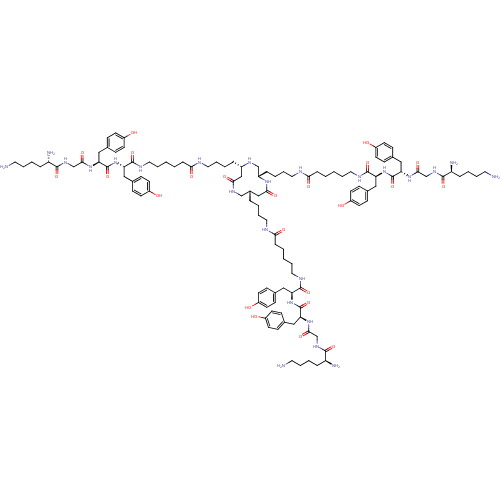
((2S,2'S,2''S)-N,N',N''-((4S,4'S,4''S,7S,7'S,7''S)-...)Show SMILES NCCCC[C@H](N)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCCCCCC(=O)NCCCC[C@@H]1CNC(=O)C[C@H](CCCCNC(=O)CCCCCNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CNC(=O)[C@@H](N)CCCCN)NC[C@H](CCCCNC(=O)CCCCCNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CNC(=O)[C@@H](N)CCCCN)NC(=O)C1 |r| Show InChI InChI=1S/C117H176N24O23/c118-55-13-7-25-92(121)109(156)132-74-106(153)136-98(67-80-37-49-89(145)50-38-80)115(162)139-95(64-77-31-43-86(142)44-32-77)112(159)127-61-16-1-4-28-101(148)124-58-19-10-22-83-70-105(152)135-85(24-12-21-60-126-103(150)30-6-3-18-63-129-114(161)97(66-79-35-47-88(144)48-36-79)141-117(164)100(69-82-41-53-91(147)54-42-82)138-108(155)76-134-111(158)94(123)27-9-15-57-120)73-130-84(71-104(151)131-72-83)23-11-20-59-125-102(149)29-5-2-17-62-128-113(160)96(65-78-33-45-87(143)46-34-78)140-116(163)99(68-81-39-51-90(146)52-40-81)137-107(154)75-133-110(157)93(122)26-8-14-56-119/h31-54,83-85,92-100,130,142-147H,1-30,55-76,118-123H2,(H,124,148)(H,125,149)(H,126,150)(H,127,159)(H,128,160)(H,129,161)(H,131,151)(H,132,156)(H,133,157)(H,134,158)(H,135,152)(H,136,153)(H,137,154)(H,138,155)(H,139,162)(H,140,163)(H,141,164)/t83-,84-,85-,92-,93-,94-,95-,96-,97-,98-,99-,100-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Biologie Moléculaire et Cellulaire
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CD40L:mouse CD8 tail binding to human CD40 by surface plasmon resonance method |
Nat Chem Biol 1: 377-82 (2005)
BindingDB Entry DOI: 10.7270/Q2WD40SN |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor receptor superfamily member 5
(Homo sapiens (Human)) | BDBM50325994
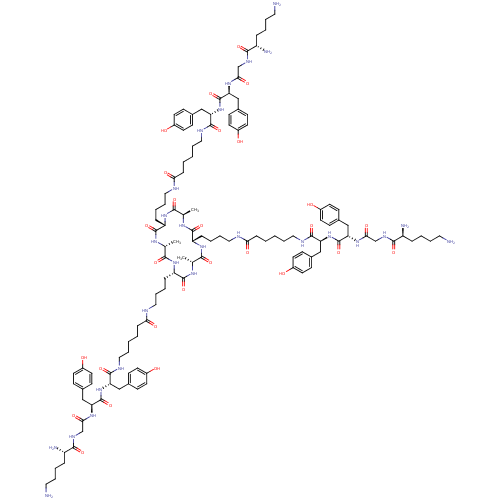
((2S,2'S,2''S)-N,N',N''-((4S,4'S,4''S,7S,7'S,7''S)-...)Show SMILES C[C@H]1NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CNC(=O)[C@@H](N)CCCCN)NC(=O)[C@@H](C)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CNC(=O)[C@@H](N)CCCCN)NC(=O)[C@@H](C)NC(=O)[C@H](CCCCNC(=O)CCCCCNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CNC(=O)[C@@H](N)CCCCN)NC1=O |r| Show InChI InChI=1S/C123H183N27O27/c1-76-109(163)145-95(29-14-23-62-131-104(158)32-8-5-20-65-134-116(170)98(68-80-36-48-86(152)49-37-80)149-122(176)101(71-83-42-54-89(155)55-43-83)143-107(161)74-137-113(167)92(128)26-11-17-59-125)119(173)140-78(3)111(165)147-96(30-15-24-63-132-105(159)33-9-6-21-66-135-117(171)99(69-81-38-50-87(153)51-39-81)150-123(177)102(72-84-44-56-90(156)57-45-84)144-108(162)75-138-114(168)93(129)27-12-18-60-126)120(174)141-77(2)110(164)146-94(118(172)139-76)28-13-22-61-130-103(157)31-7-4-19-64-133-115(169)97(67-79-34-46-85(151)47-35-79)148-121(175)100(70-82-40-52-88(154)53-41-82)142-106(160)73-136-112(166)91(127)25-10-16-58-124/h34-57,76-78,91-102,151-156H,4-33,58-75,124-129H2,1-3H3,(H,130,157)(H,131,158)(H,132,159)(H,133,169)(H,134,170)(H,135,171)(H,136,166)(H,137,167)(H,138,168)(H,139,172)(H,140,173)(H,141,174)(H,142,160)(H,143,161)(H,144,162)(H,145,163)(H,146,164)(H,147,165)(H,148,175)(H,149,176)(H,150,177)/t76-,77-,78-,91+,92+,93+,94+,95+,96+,97+,98+,99+,100+,101+,102+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Biologie Moléculaire et Cellulaire
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CD40L:mouse CD8 tail binding to human CD40 by surface plasmon resonance method |
Nat Chem Biol 1: 377-82 (2005)
BindingDB Entry DOI: 10.7270/Q2WD40SN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50207561
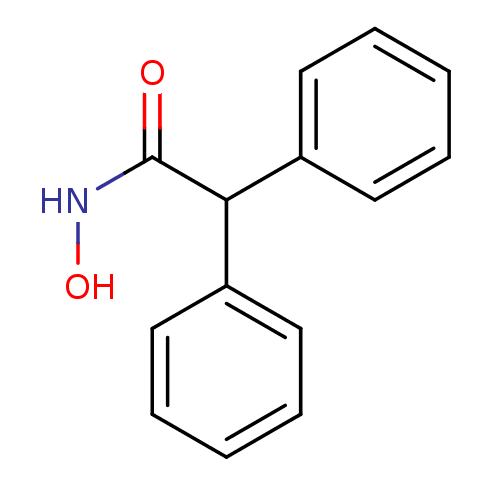
(CHEMBL396097 | Class IIa HDAC inhibitor, Compound ...)Show InChI InChI=1S/C14H13NO2/c16-14(15-17)13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,13,17H,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229834
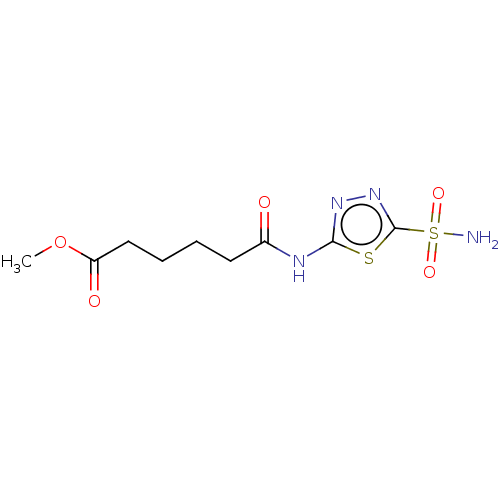
(CHEMBL328270)Show InChI InChI=1S/C9H14N4O5S2/c1-18-7(15)5-3-2-4-6(14)11-8-12-13-9(19-8)20(10,16)17/h2-5H2,1H3,(H2,10,16,17)(H,11,12,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229838
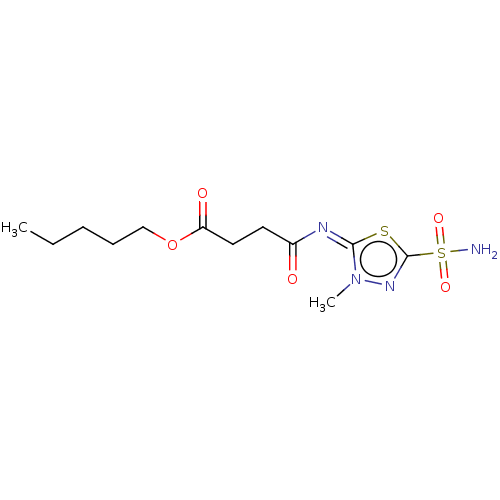
(CHEMBL88183)Show InChI InChI=1S/C12H20N4O5S2/c1-3-4-5-8-21-10(18)7-6-9(17)14-11-16(2)15-12(22-11)23(13,19)20/h3-8H2,1-2H3,(H2,13,19,20)/b14-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229841
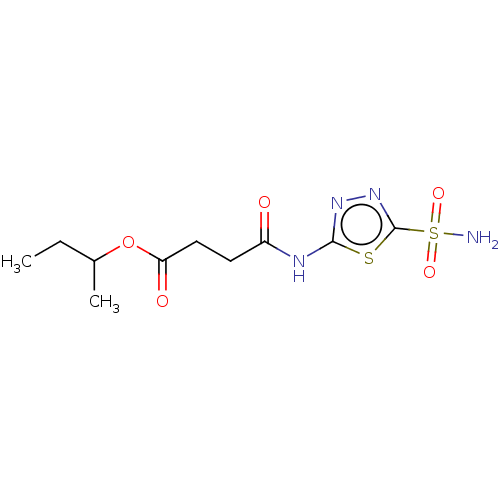
(CHEMBL420799)Show InChI InChI=1S/C10H16N4O5S2/c1-3-6(2)19-8(16)5-4-7(15)12-9-13-14-10(20-9)21(11,17)18/h6H,3-5H2,1-2H3,(H2,11,17,18)(H,12,13,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229842
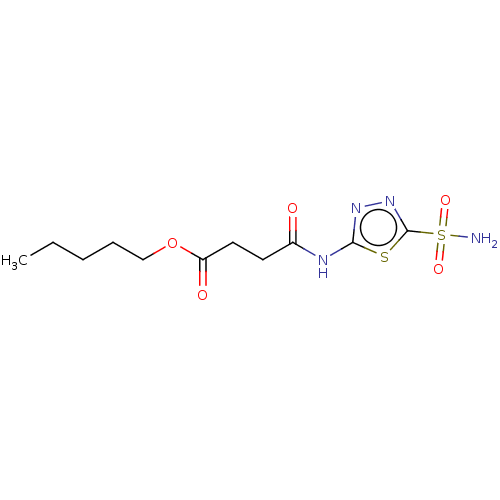
(CHEMBL90119)Show InChI InChI=1S/C11H18N4O5S2/c1-2-3-4-7-20-9(17)6-5-8(16)13-10-14-15-11(21-10)22(12,18)19/h2-7H2,1H3,(H2,12,18,19)(H,13,14,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582546
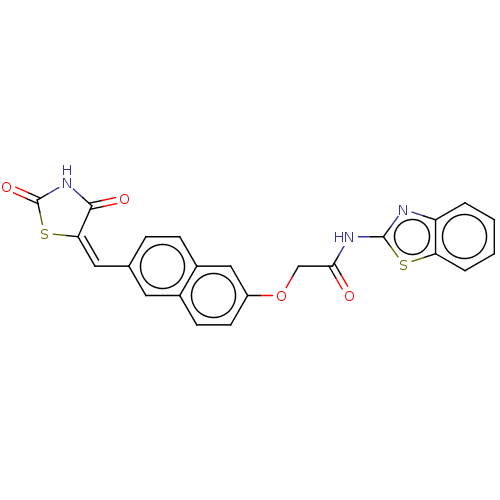
(CHEMBL5083726)Show SMILES O=C(COc1ccc2cc(\C=C3\SC(=O)NC3=O)ccc2c1)Nc1nc2ccccc2s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50212309
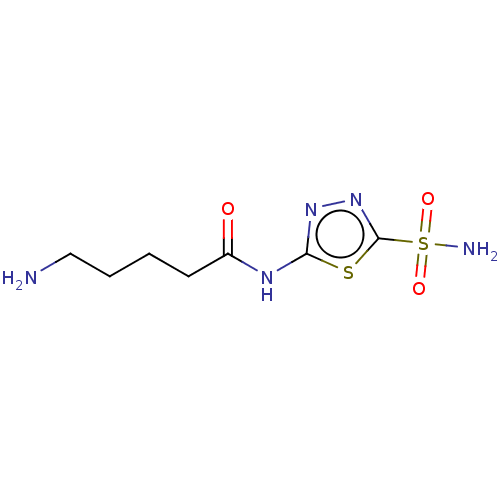
(CHEMBL91302)Show InChI InChI=1S/C7H13N5O3S2/c8-4-2-1-3-5(13)10-6-11-12-7(16-6)17(9,14)15/h1-4,8H2,(H2,9,14,15)(H,10,11,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229847
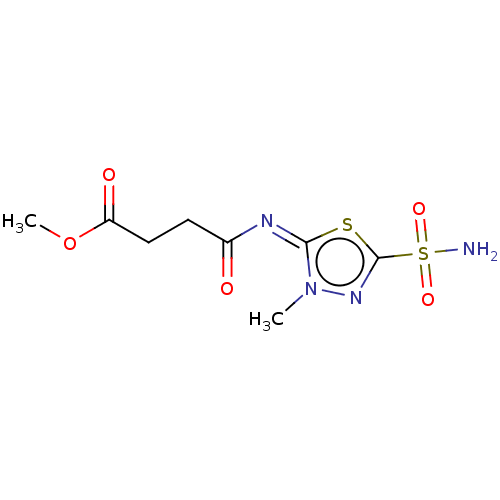
(CHEMBL91512)Show InChI InChI=1S/C8H12N4O5S2/c1-12-7(18-8(11-12)19(9,15)16)10-5(13)3-4-6(14)17-2/h3-4H2,1-2H3,(H2,9,15,16)/b10-7+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229846
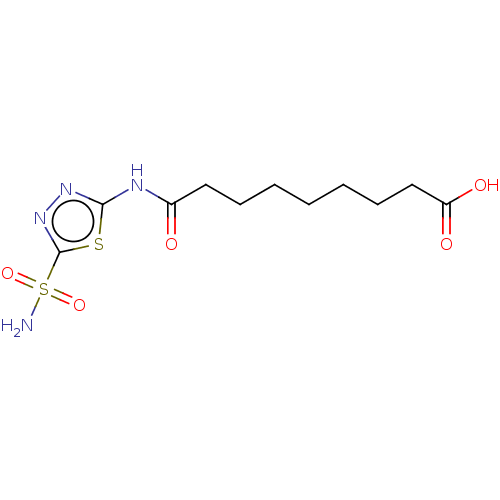
(CHEMBL264307)Show InChI InChI=1S/C11H18N4O5S2/c12-22(19,20)11-15-14-10(21-11)13-8(16)6-4-2-1-3-5-7-9(17)18/h1-7H2,(H,17,18)(H2,12,19,20)(H,13,14,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229839
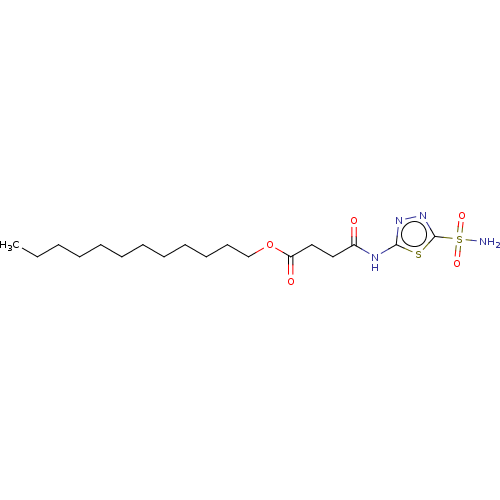
(CHEMBL91622)Show SMILES CCCCCCCCCCCCOC(=O)CCC(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C18H32N4O5S2/c1-2-3-4-5-6-7-8-9-10-11-14-27-16(24)13-12-15(23)20-17-21-22-18(28-17)29(19,25)26/h2-14H2,1H3,(H2,19,25,26)(H,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229845

(CHEMBL91517)Show InChI InChI=1S/C7H10N4O5S2/c8-18(15,16)7-11-10-6(17-7)9-4(12)2-1-3-5(13)14/h1-3H2,(H,13,14)(H2,8,15,16)(H,9,10,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229835
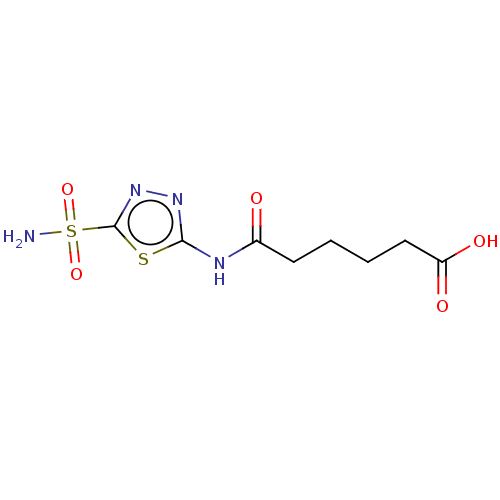
(CHEMBL90857)Show InChI InChI=1S/C8H12N4O5S2/c9-19(16,17)8-12-11-7(18-8)10-5(13)3-1-2-4-6(14)15/h1-4H2,(H,14,15)(H2,9,16,17)(H,10,11,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50185303
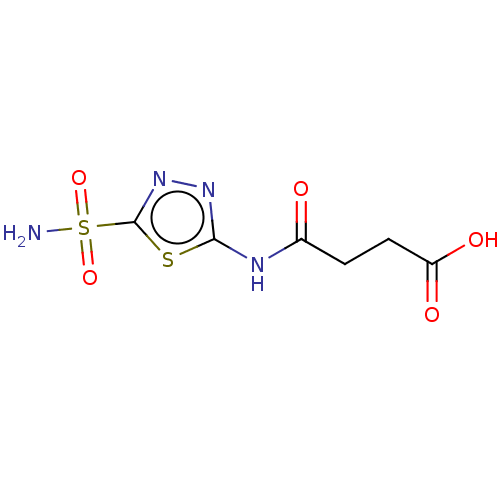
(CHEMBL88115)Show InChI InChI=1S/C6H8N4O5S2/c7-17(14,15)6-10-9-5(16-6)8-3(11)1-2-4(12)13/h1-2H2,(H,12,13)(H2,7,14,15)(H,8,9,11) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582535
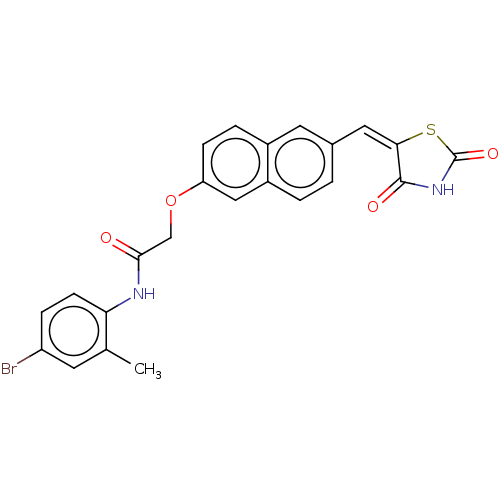
(CHEMBL5087514)Show SMILES Cc1cc(Br)ccc1NC(=O)COc1ccc2cc(\C=C3\SC(=O)NC3=O)ccc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229836
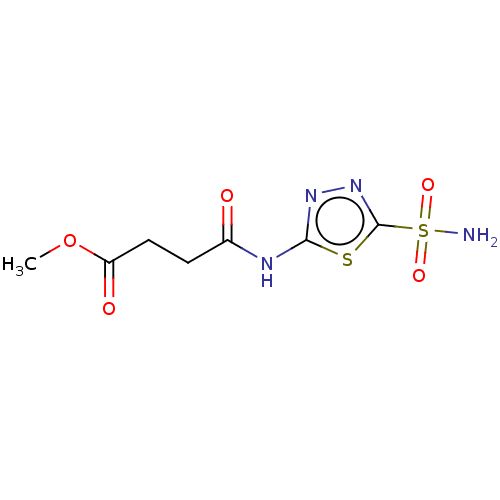
(CHEMBL92500)Show InChI InChI=1S/C7H10N4O5S2/c1-16-5(13)3-2-4(12)9-6-10-11-7(17-6)18(8,14)15/h2-3H2,1H3,(H2,8,14,15)(H,9,10,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229843
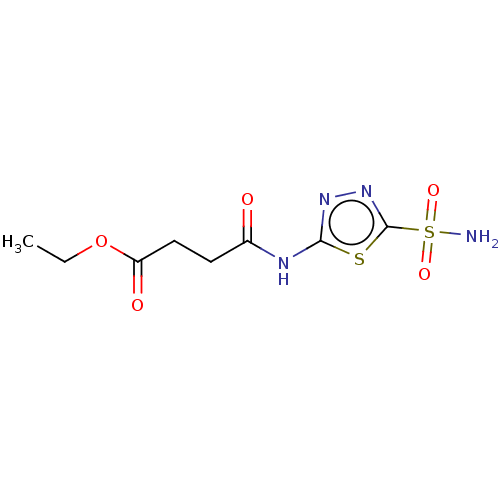
(CHEMBL91131)Show InChI InChI=1S/C8H12N4O5S2/c1-2-17-6(14)4-3-5(13)10-7-11-12-8(18-7)19(9,15)16/h2-4H2,1H3,(H2,9,15,16)(H,10,11,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582542
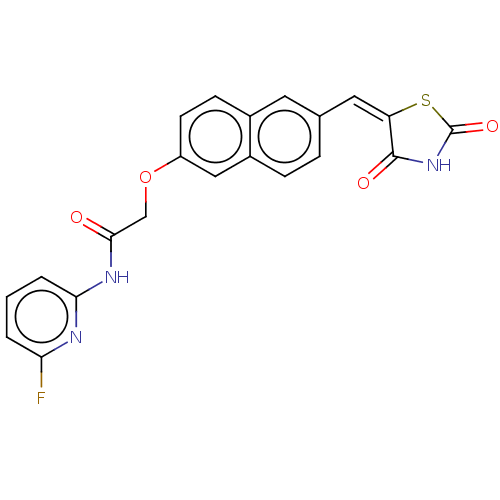
(CHEMBL5094339)Show SMILES Fc1cccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229844
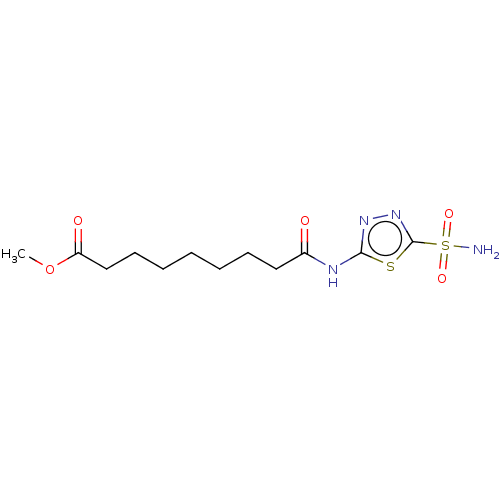
(CHEMBL328566)Show InChI InChI=1S/C12H20N4O5S2/c1-21-10(18)8-6-4-2-3-5-7-9(17)14-11-15-16-12(22-11)23(13,19)20/h2-8H2,1H3,(H2,13,19,20)(H,14,15,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229837
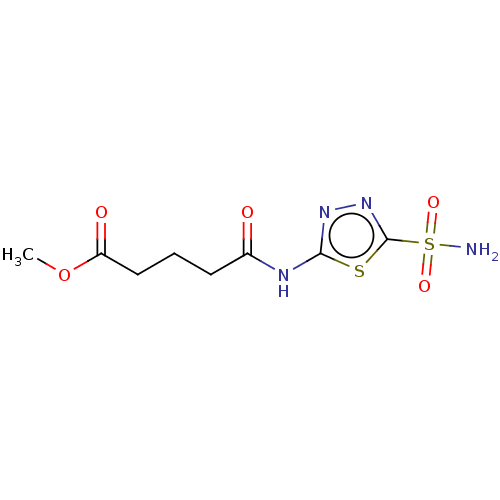
(CHEMBL89489)Show InChI InChI=1S/C8H12N4O5S2/c1-17-6(14)4-2-3-5(13)10-7-11-12-8(18-7)19(9,15)16/h2-4H2,1H3,(H2,9,15,16)(H,10,11,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229840
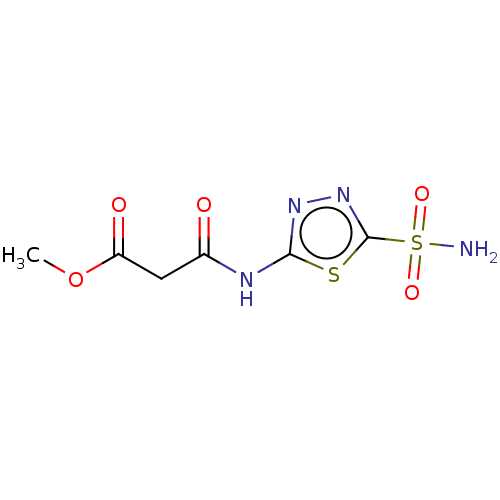
(CHEMBL329625)Show InChI InChI=1S/C6H8N4O5S2/c1-15-4(12)2-3(11)8-5-9-10-6(16-5)17(7,13)14/h2H2,1H3,(H2,7,13,14)(H,8,9,11) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582532
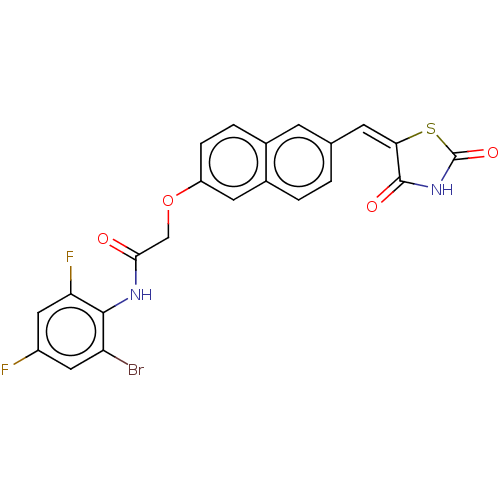
(CHEMBL5085545)Show SMILES Fc1cc(F)c(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)c(Br)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582537
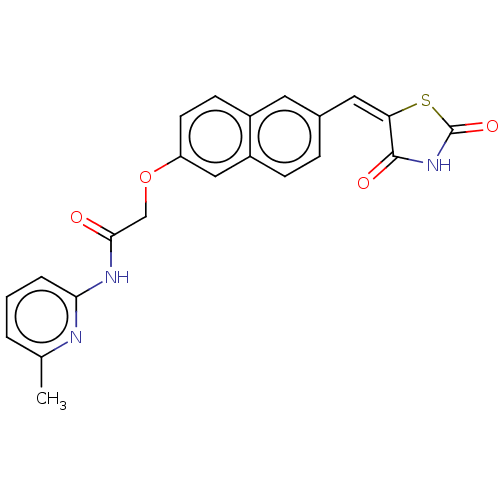
(CHEMBL5073824)Show SMILES Cc1cccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582544
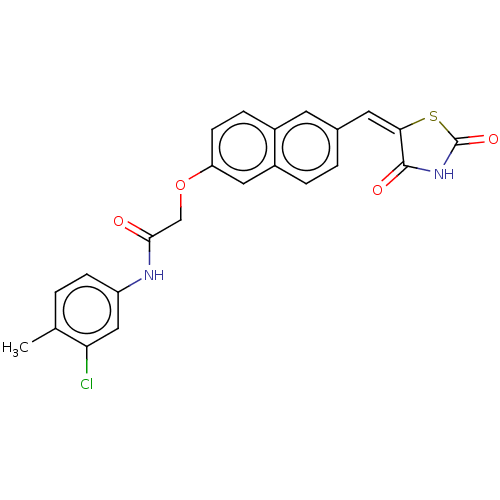
(CHEMBL5073547)Show SMILES Cc1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)cc1Cl | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582547
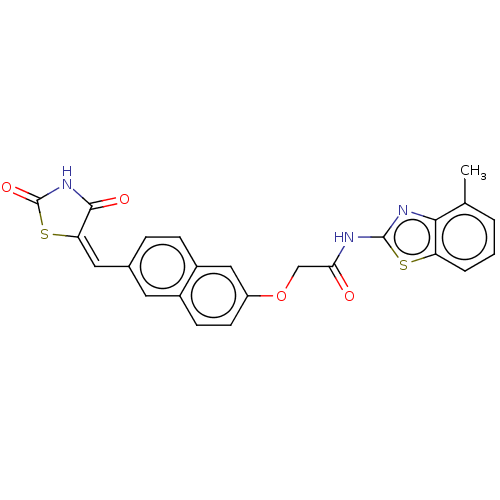
(CHEMBL5082258)Show SMILES Cc1cccc2sc(NC(=O)COc3ccc4cc(\C=C5\SC(=O)NC5=O)ccc4c3)nc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582530
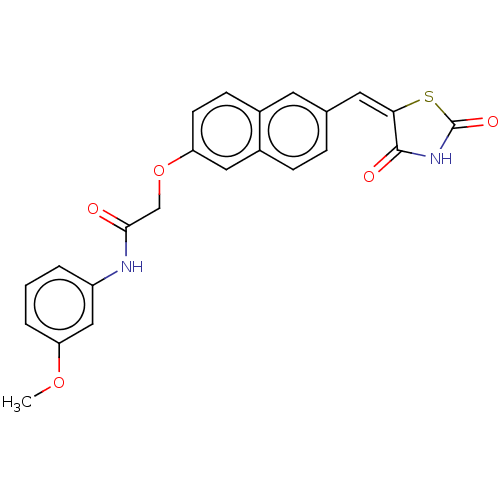
(CHEMBL5090794)Show SMILES COc1cccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582525
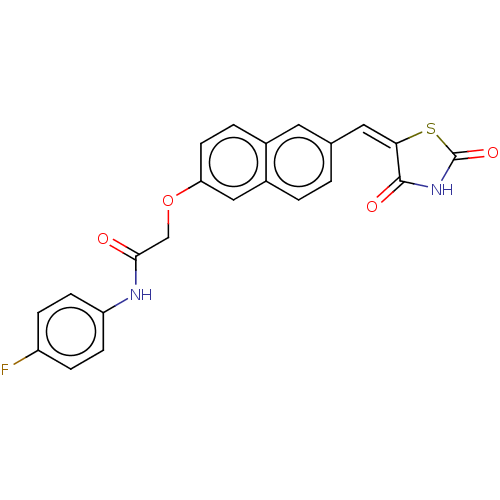
(CHEMBL5072376)Show SMILES Fc1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582540
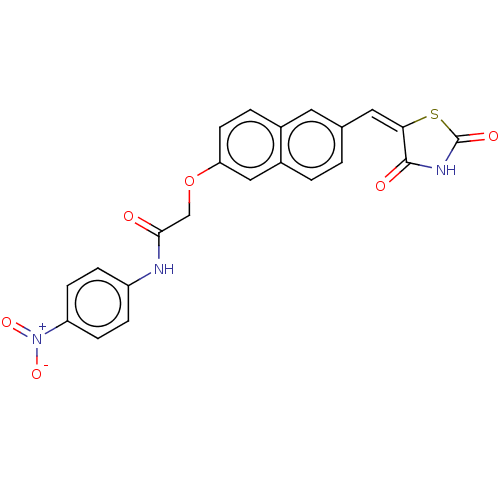
(CHEMBL5085291)Show SMILES [O-][N+](=O)c1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582543
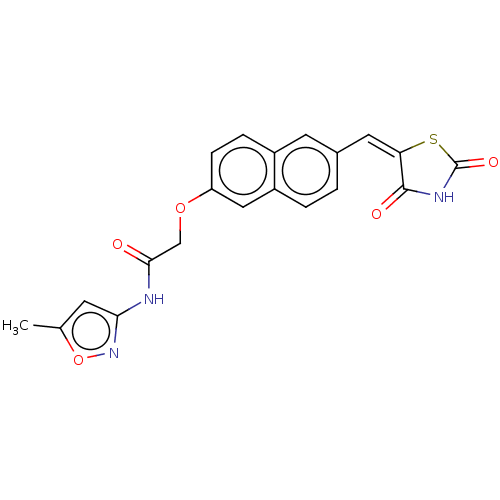
(CHEMBL5092097)Show SMILES Cc1cc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)no1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582526
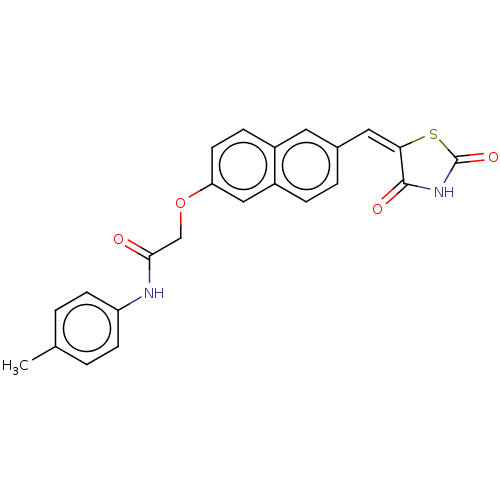
(CHEMBL5089555)Show SMILES Cc1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582541
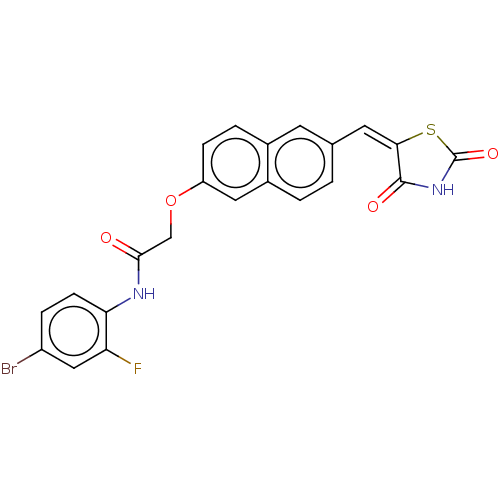
(CHEMBL5084422)Show SMILES Fc1cc(Br)ccc1NC(=O)COc1ccc2cc(\C=C3\SC(=O)NC3=O)ccc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582524
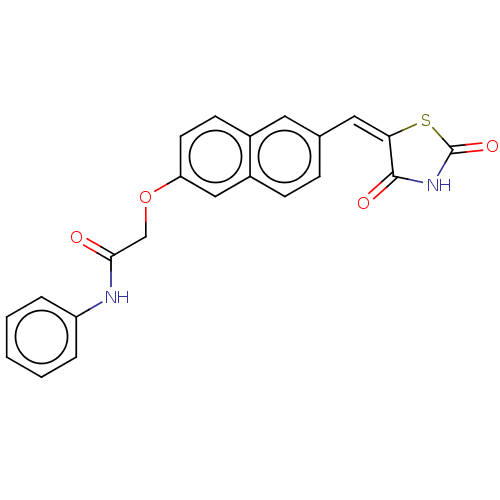
(CHEMBL5089186)Show SMILES O=C(COc1ccc2cc(\C=C3\SC(=O)NC3=O)ccc2c1)Nc1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50582550
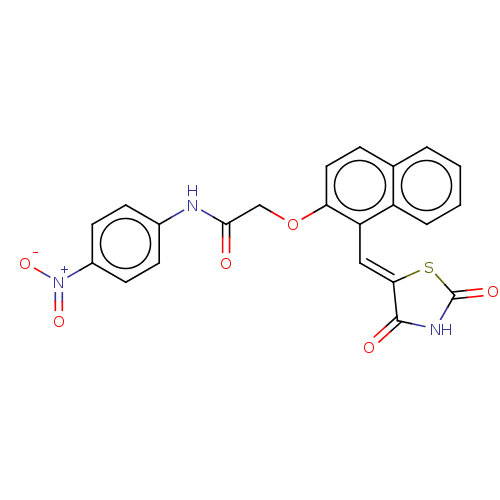
(CHEMBL5085184)Show SMILES [O-][N+](=O)c1ccc(NC(=O)COc2ccc3ccccc3c2\C=C2/SC(=O)NC2=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582528
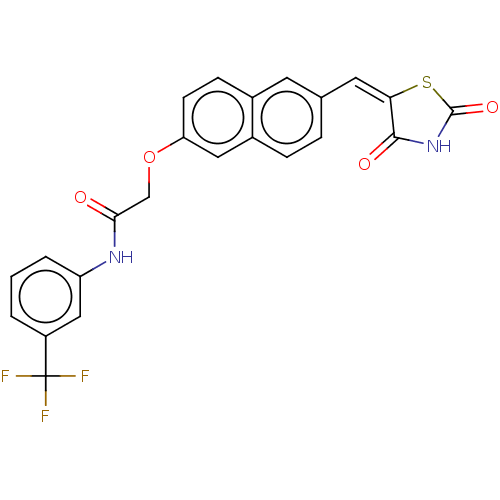
(CHEMBL5086747)Show SMILES FC(F)(F)c1cccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50582546

(CHEMBL5083726)Show SMILES O=C(COc1ccc2cc(\C=C3\SC(=O)NC3=O)ccc2c1)Nc1nc2ccccc2s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582538
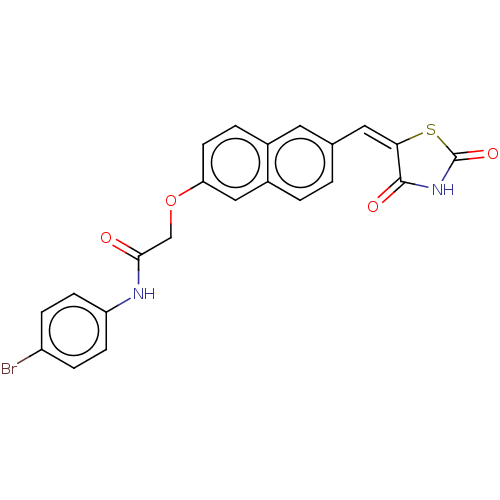
(CHEMBL5091419)Show SMILES Brc1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50339188
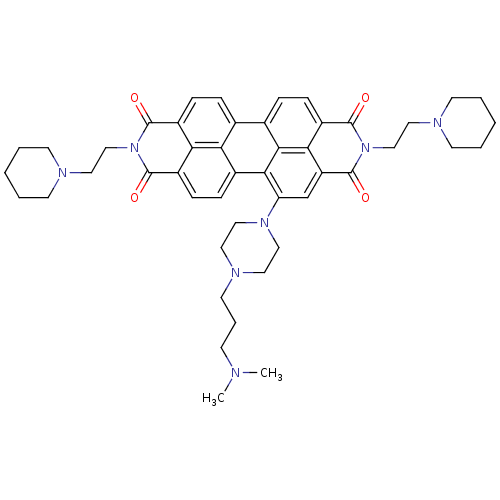
(CHEMBL1689442 | N,N'-Bis[2-(1-piperidino)-ethyl]-1...)Show SMILES CN(C)CCCN1CCN(CC1)c1cc2c3c(ccc4c5ccc6c7c(ccc(c1c34)c57)c(=O)n(CCN1CCCCC1)c6=O)c(=O)n(CCN1CCCCC1)c2=O Show InChI InChI=1S/C47H55N7O4/c1-48(2)16-9-21-51-22-26-52(27-23-51)38-30-37-41-36(46(57)54(47(37)58)29-25-50-19-7-4-8-20-50)14-11-32-31-10-13-34-40-35(15-12-33(39(31)40)42(38)43(32)41)45(56)53(44(34)55)28-24-49-17-5-3-6-18-49/h10-15,30H,3-9,16-29H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of human Telomerase activity in cell free system by TRAP assay |
J Med Chem 54: 1140-56 (2011)
Article DOI: 10.1021/jm1013665
BindingDB Entry DOI: 10.7270/Q2GB24CR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582548
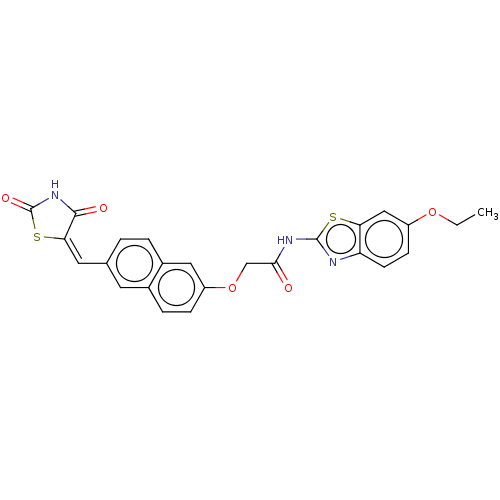
(CHEMBL5078917)Show SMILES CCOc1ccc2nc(NC(=O)COc3ccc4cc(\C=C5\SC(=O)NC5=O)ccc4c3)sc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582529
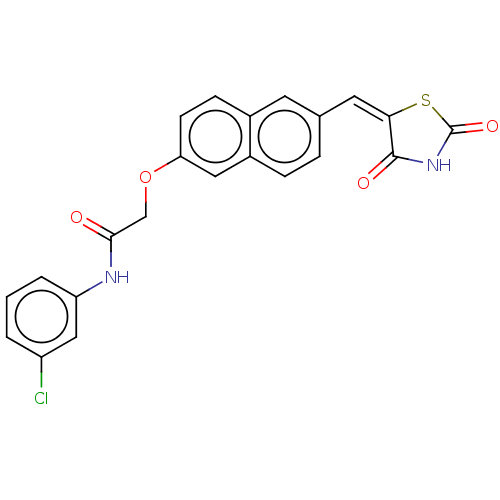
(CHEMBL5082106)Show SMILES Clc1cccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582534
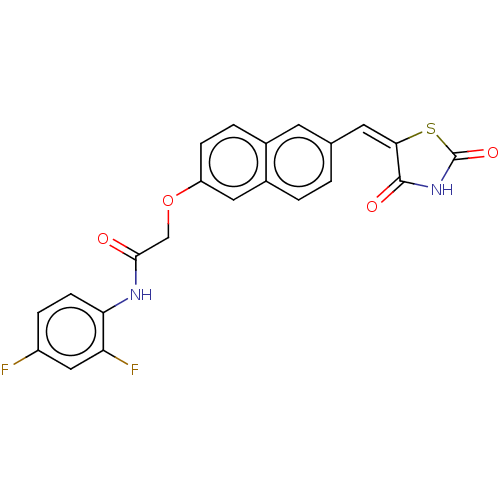
(CHEMBL5093916)Show SMILES Fc1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)c(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582539
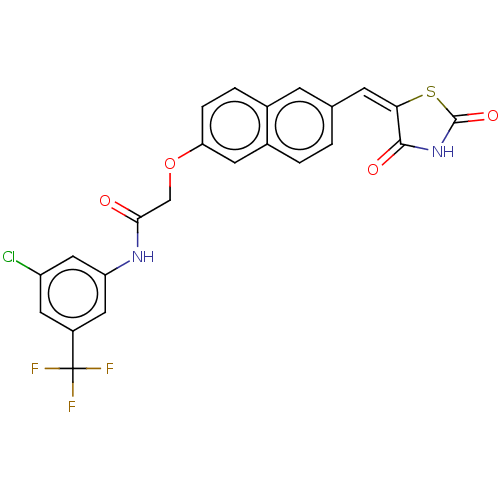
(CHEMBL5081178)Show SMILES FC(F)(F)c1cc(Cl)cc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50582540

(CHEMBL5085291)Show SMILES [O-][N+](=O)c1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50582524

(CHEMBL5089186)Show SMILES O=C(COc1ccc2cc(\C=C3\SC(=O)NC3=O)ccc2c1)Nc1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582527
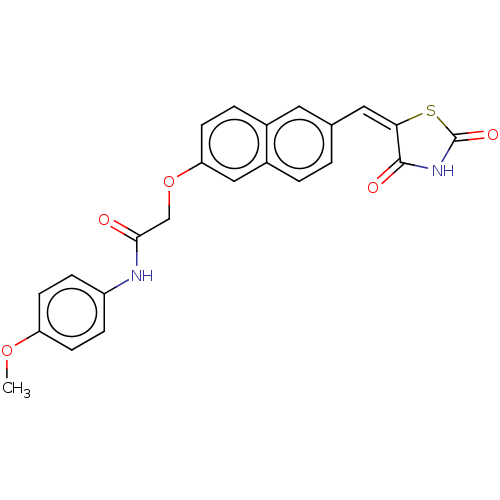
(CHEMBL5082287)Show SMILES COc1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582545
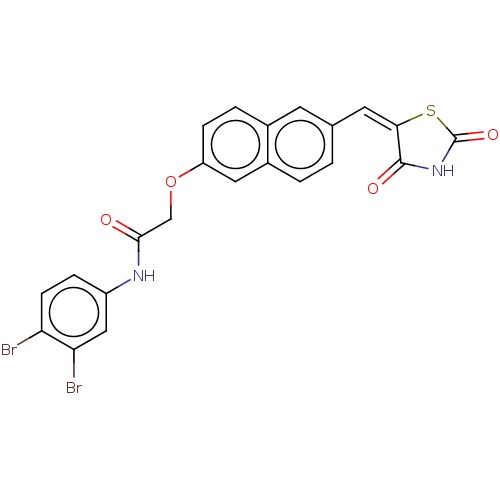
(CHEMBL5076630)Show SMILES Brc1ccc(NC(=O)COc2ccc3cc(\C=C4\SC(=O)NC4=O)ccc3c2)cc1Br | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50582533
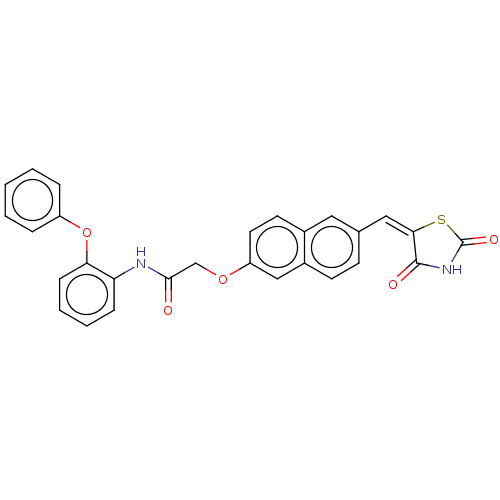
(CHEMBL5091605)Show SMILES O=C(COc1ccc2cc(\C=C3\SC(=O)NC3=O)ccc2c1)Nc1ccccc1Oc1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys (trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00491
BindingDB Entry DOI: 10.7270/Q2X352B7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data