

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22158 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | 1.40 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22160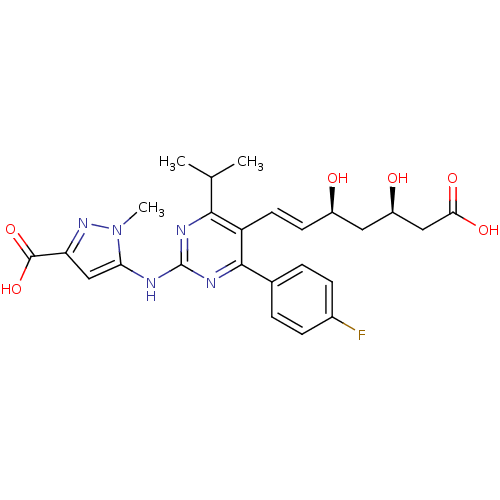 (pyrazole compound, 14a | sodium 5-({5-[(1E,3S,5R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | 13.7 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22157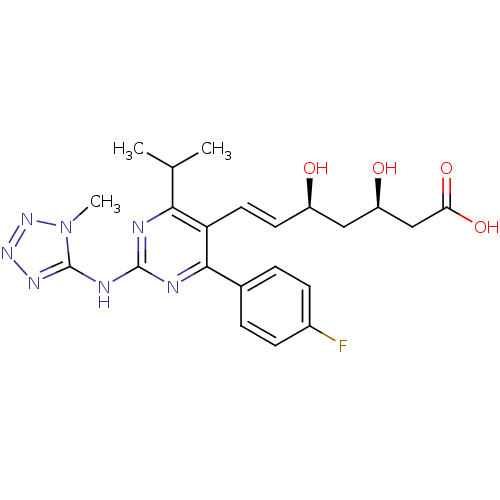 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(1-methyl-1H-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | 1.5 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22163 (BMS-644950 | sodium (3R,5S,6E)-7-[4-(4-fluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 3.95 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22154 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(1-methyl-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | 3.70 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22152 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[N-(1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | 5.40 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22147 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(1,2-oxazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | 1.40 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22150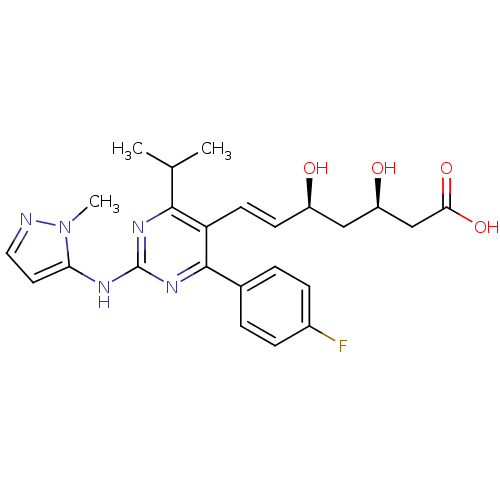 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(1-methyl-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 1.30 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22155 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | 1.20 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22151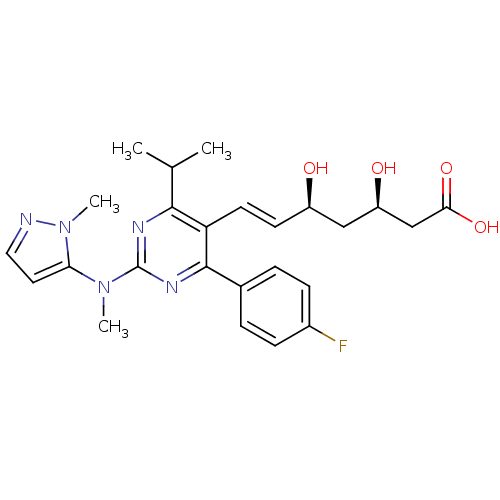 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | 2.5 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22162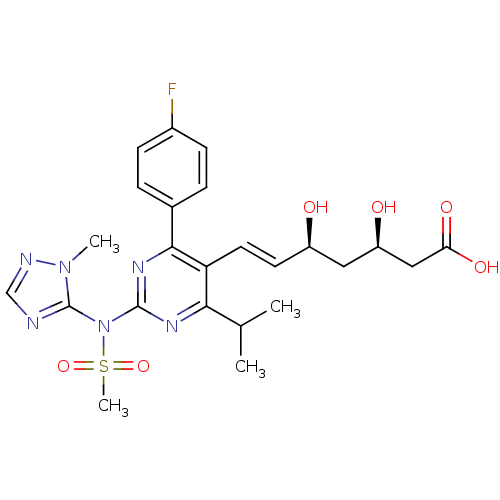 (sodium (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[N-(1-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | 7.5 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22161 (sodium (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | 3.70 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18372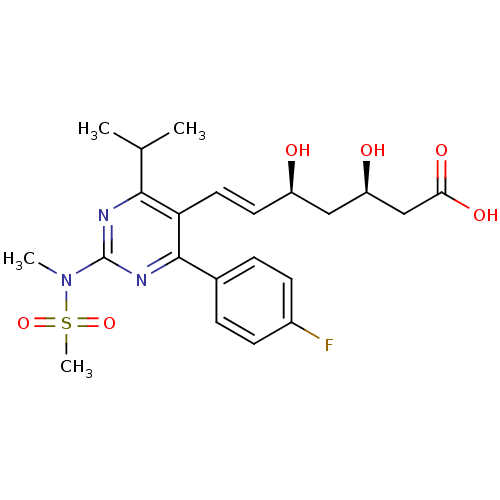 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3.10 | n/a | 0.600 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22156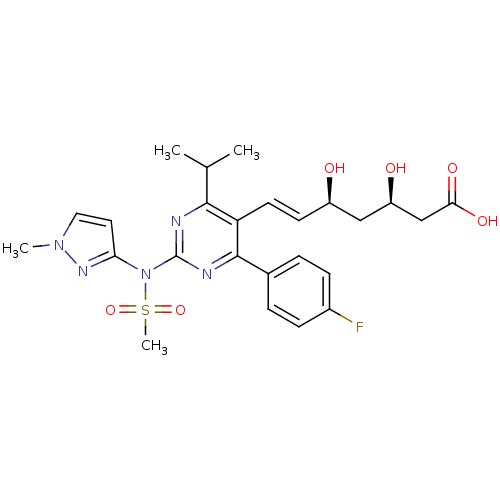 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[N-(1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | 7.70 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22159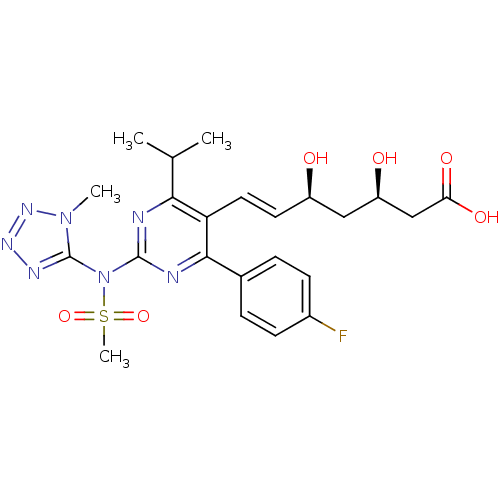 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[N-(1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | 1 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18375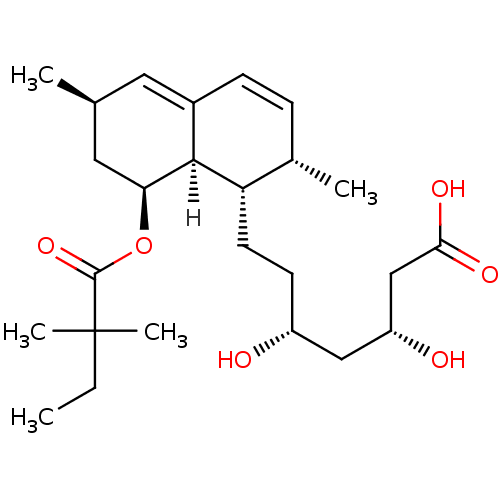 ((3R,5R)-7-[(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.30 | n/a | 6.20 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22164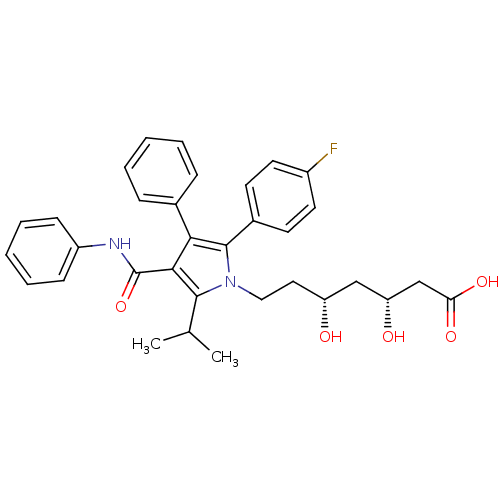 ((3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 6.20 | n/a | 2.5 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22146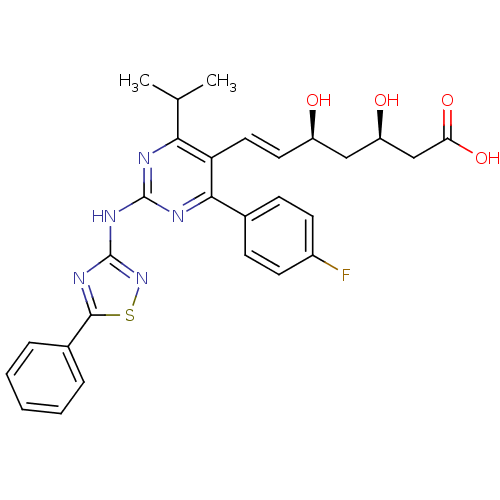 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(5-phenyl-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.90 | n/a | 7.70 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22148 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1,2-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | 9 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18376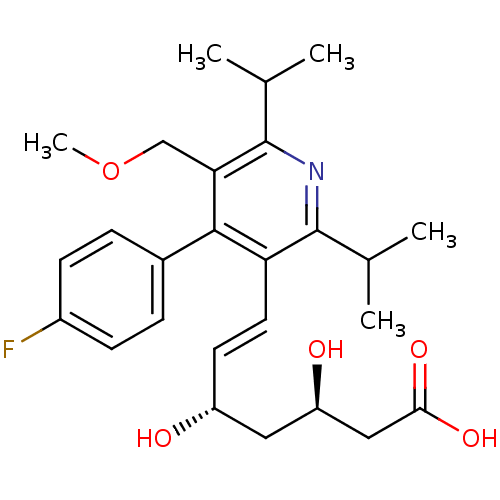 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-5-(methoxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 9.80 | n/a | 2.30 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22153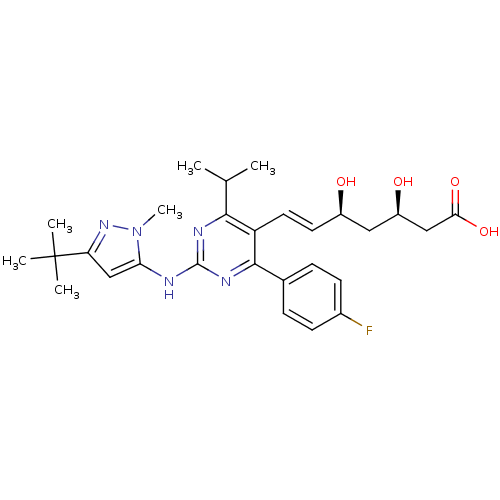 ((3R,5S,6E)-7-{2-[(3-tert-butyl-1-methyl-1H-pyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12.4 | n/a | 1.90 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM20688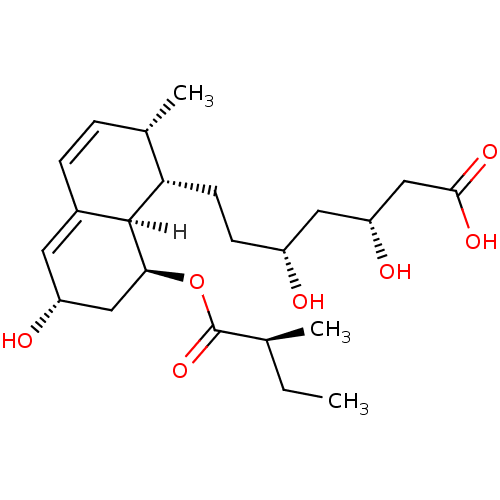 ((3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31.6 | n/a | 29 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM22149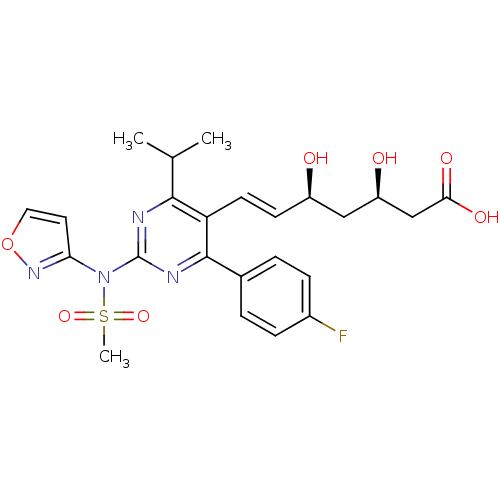 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[N-(1,2-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | 5.5 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company | Assay Description Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... | J Med Chem 51: 2722-33 (2008) Article DOI: 10.1021/jm800001n BindingDB Entry DOI: 10.7270/Q2FT8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430217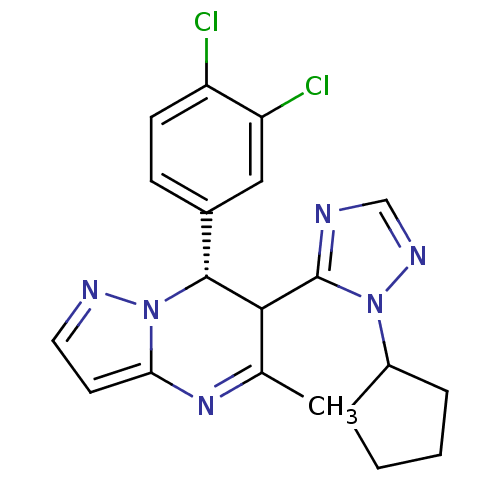 (CHEMBL2331989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430216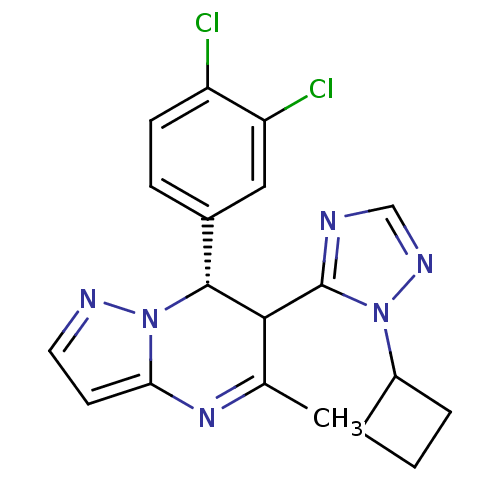 (CHEMBL2331990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430219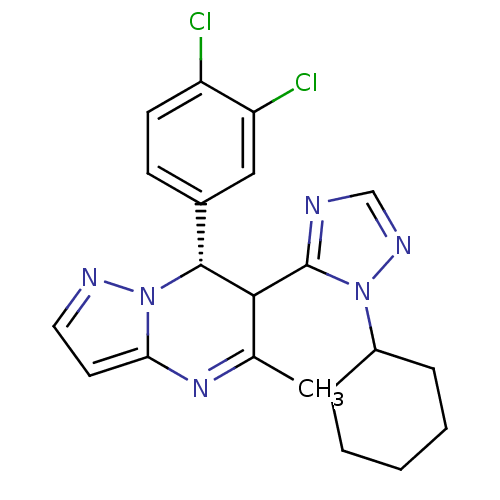 (CHEMBL2331987) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50385935 (CHEMBL2042152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human KV1.5 ion channel expressed in mouse L929 cells by whole cell patch clamp assay | J Med Chem 55: 3036-48 (2012) Article DOI: 10.1021/jm201386u BindingDB Entry DOI: 10.7270/Q2KK9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50385942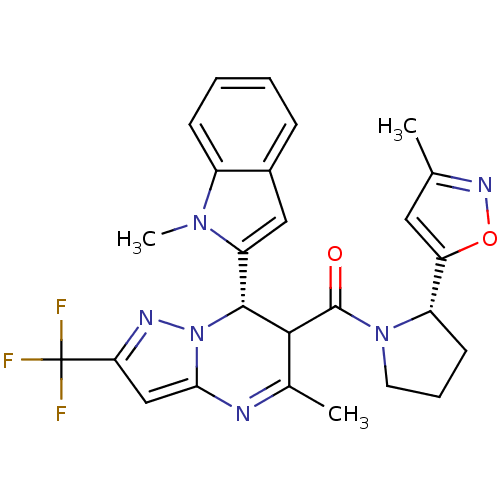 (CHEMBL2042162) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human KV1.5 ion channel expressed in mouse L929 cells by whole cell patch clamp assay | J Med Chem 55: 3036-48 (2012) Article DOI: 10.1021/jm201386u BindingDB Entry DOI: 10.7270/Q2KK9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430227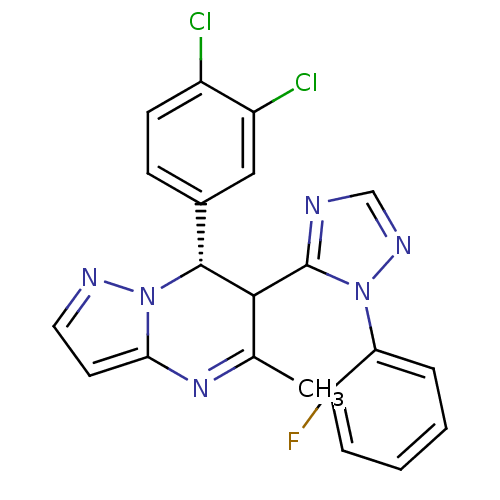 (CHEMBL2332455) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430225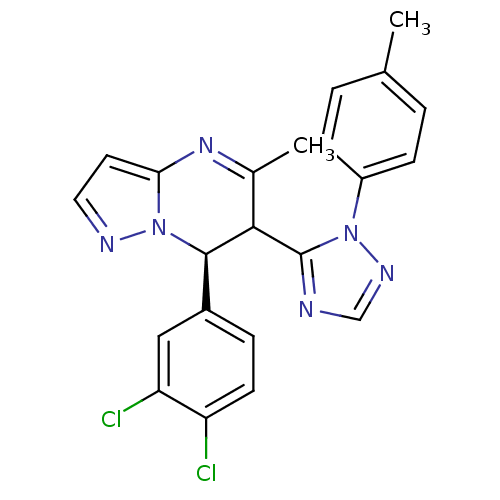 (CHEMBL2332457) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50385937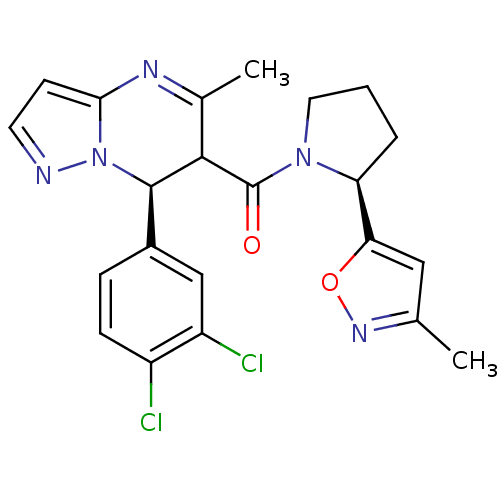 (CHEMBL2042156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human KV1.5 ion channel expressed in mouse L929 cells by whole cell patch clamp assay | J Med Chem 55: 3036-48 (2012) Article DOI: 10.1021/jm201386u BindingDB Entry DOI: 10.7270/Q2KK9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430201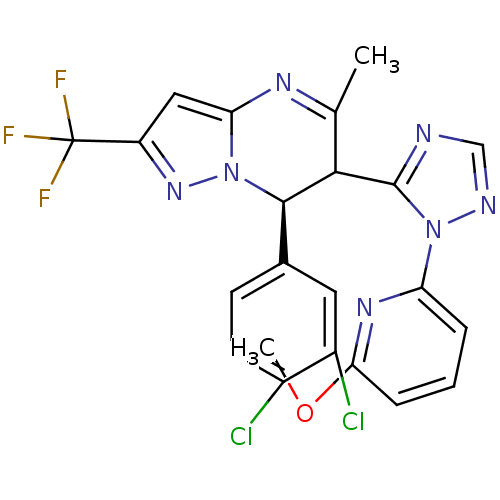 (CHEMBL2332448) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430220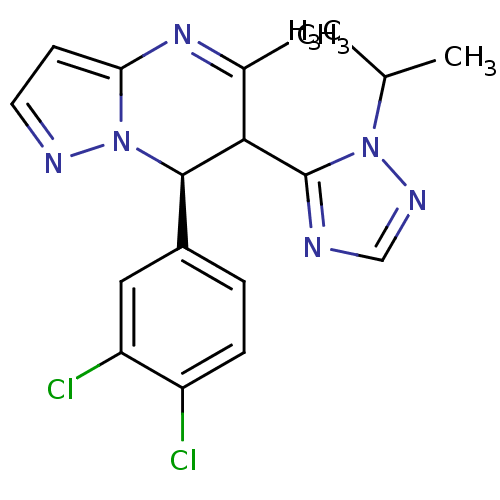 (CHEMBL2331986) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430211 (CHEMBL2331996) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430206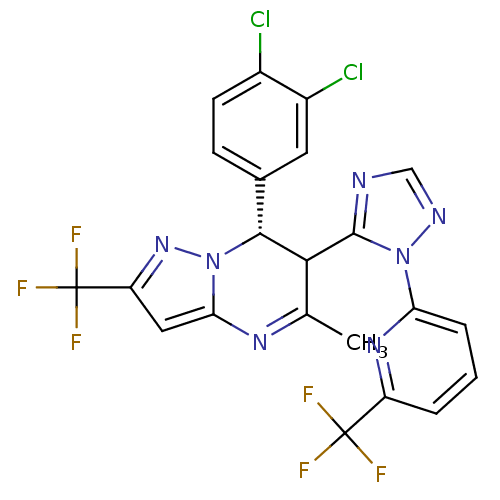 (CHEMBL2332443) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430228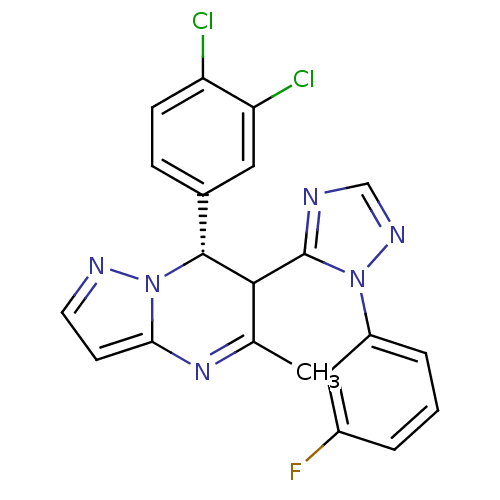 (CHEMBL2332454) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged PPAR-alpha LBD expressed in Escherichia coli BL21 (DE3) PlysS after 30 mins in presence of fluorescein ligand FL... | ACS Med Chem Lett 7: 590-4 (2016) Article DOI: 10.1021/acsmedchemlett.6b00033 BindingDB Entry DOI: 10.7270/Q2CR5W99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | 4 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For hPPAR alpha, percentage inhibition was calculated relative to unlabeled GW2331, which was used as the active site-specific competitive binder. Fl... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430197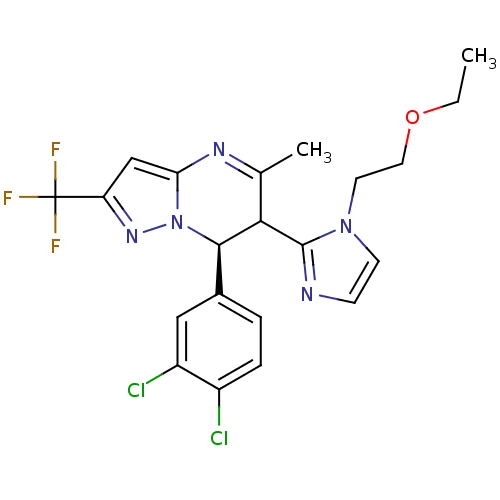 (CHEMBL2332452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50385936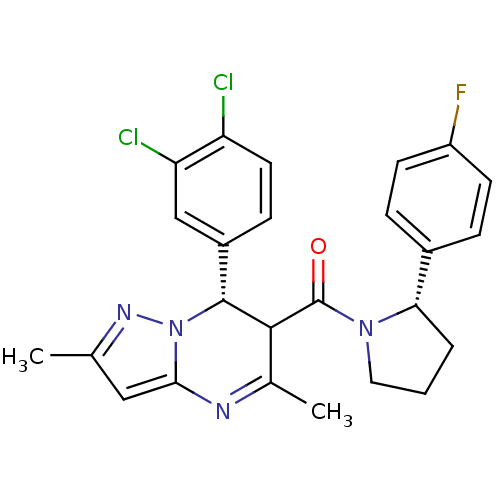 (CHEMBL2042154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human KV1.5 ion channel expressed in mouse L929 cells by whole cell patch clamp assay | J Med Chem 55: 3036-48 (2012) Article DOI: 10.1021/jm201386u BindingDB Entry DOI: 10.7270/Q2KK9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50385939 (CHEMBL2042158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human KV1.5 ion channel expressed in mouse L929 cells by whole cell patch clamp assay | J Med Chem 55: 3036-48 (2012) Article DOI: 10.1021/jm201386u BindingDB Entry DOI: 10.7270/Q2KK9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430221 (CHEMBL2331985) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430212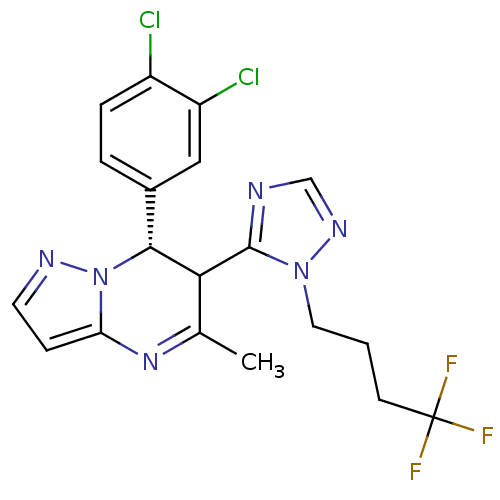 (CHEMBL2331994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430229 (CHEMBL2332453) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430226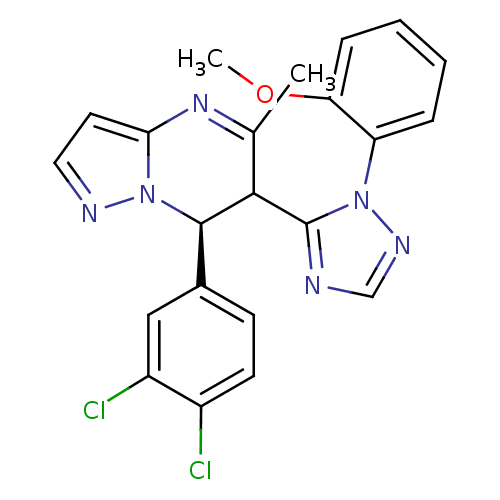 (CHEMBL2332456) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50385931 (CHEMBL2042147) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human KV1.5 ion channel expressed in mouse L929 cells by whole cell patch clamp assay | J Med Chem 55: 3036-48 (2012) Article DOI: 10.1021/jm201386u BindingDB Entry DOI: 10.7270/Q2KK9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430210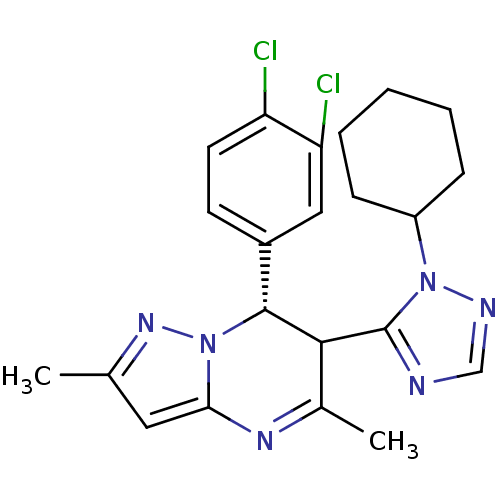 (CHEMBL2331997) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430199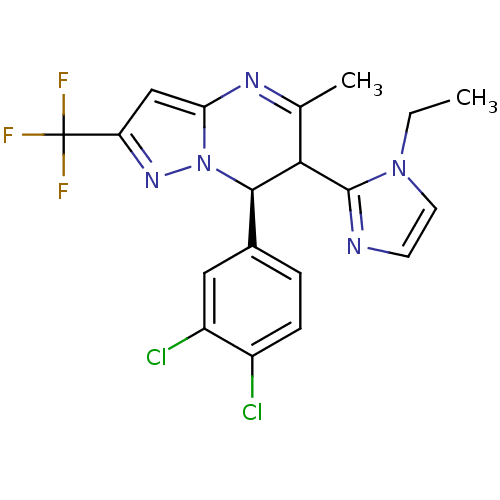 (CHEMBL2332450) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430223 (CHEMBL2332465) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314811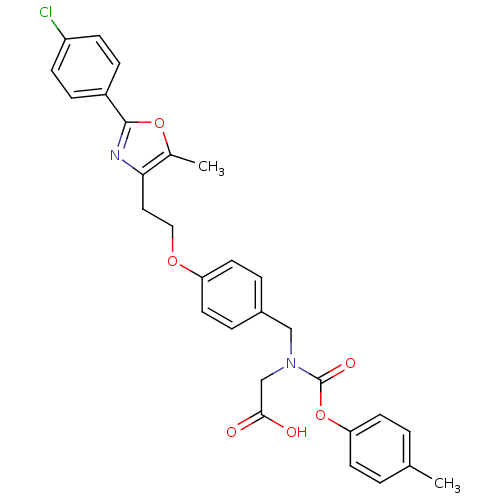 (2-((4-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 287 total ) | Next | Last >> |