 Found 60 hits with Last Name = 'bowman' and Initial = 'mj'
Found 60 hits with Last Name = 'bowman' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59224
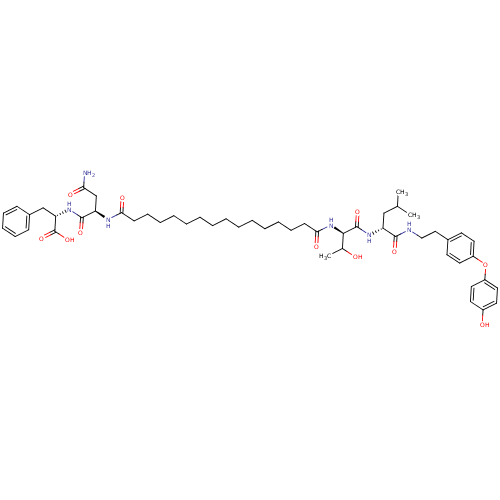
(Pepstatin analog, 12)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(C)O)C(=O)NCCc1ccc(Oc2ccc(O)cc2)cc1 |r| Show InChI InChI=1S/C53H76N6O11/c1-36(2)33-43(50(65)55-32-31-38-23-27-41(28-24-38)70-42-29-25-40(61)26-30-42)57-52(67)49(37(3)60)59-48(64)22-18-13-11-9-7-5-4-6-8-10-12-17-21-47(63)56-44(35-46(54)62)51(66)58-45(53(68)69)34-39-19-15-14-16-20-39/h14-16,19-20,23-30,36-37,43-45,49,60-61H,4-13,17-18,21-22,31-35H2,1-3H3,(H2,54,62)(H,55,65)(H,56,63)(H,57,67)(H,58,66)(H,59,64)(H,68,69)/t37?,43-,44-,45+,49-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | 360 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59223
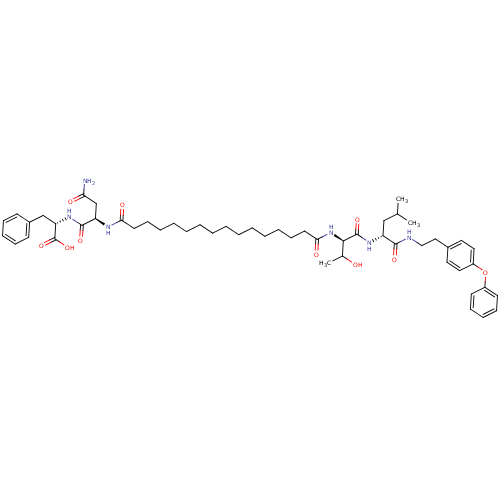
(Pepstatin analog, 11)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(C)O)C(=O)NCCc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C53H76N6O10/c1-37(2)34-43(50(64)55-33-32-39-28-30-42(31-29-39)69-41-24-18-15-19-25-41)57-52(66)49(38(3)60)59-48(63)27-21-13-11-9-7-5-4-6-8-10-12-20-26-47(62)56-44(36-46(54)61)51(65)58-45(53(67)68)35-40-22-16-14-17-23-40/h14-19,22-25,28-31,37-38,43-45,49,60H,4-13,20-21,26-27,32-36H2,1-3H3,(H2,54,61)(H,55,64)(H,56,62)(H,57,66)(H,58,65)(H,59,63)(H,67,68)/t38?,43-,44-,45+,49-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 175 | n/a | 730 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59213
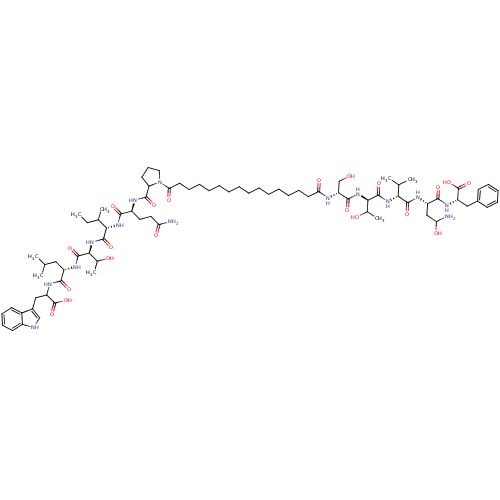
(Pepstatin analog, 1)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)C1CCCN1C(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CO)C(=O)N[C@H](C(C)O)C(=O)N[C@H](C(C)C)C(=O)N[C@@H](CC(N)O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](CC(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C78H122N14O20/c1-9-46(6)65(74(106)91-66(47(7)94)75(107)84-54(38-44(2)3)69(101)87-57(78(111)112)40-50-42-81-52-31-26-25-30-51(50)52)89-68(100)53(35-36-60(79)96)83-72(104)59-32-27-37-92(59)63(99)34-24-19-17-15-13-11-10-12-14-16-18-23-33-62(98)82-58(43-93)71(103)90-67(48(8)95)76(108)88-64(45(4)5)73(105)85-55(41-61(80)97)70(102)86-56(77(109)110)39-49-28-21-20-22-29-49/h20-22,25-26,28-31,42,44-48,53-59,61,64-67,81,93-95,97H,9-19,23-24,27,32-41,43,80H2,1-8H3,(H2,79,96)(H,82,98)(H,83,104)(H,84,107)(H,85,105)(H,86,102)(H,87,101)(H,88,108)(H,89,100)(H,90,103)(H,91,106)(H,109,110)(H,111,112)/t46?,47?,48?,53-,54-,55-,56-,57?,58+,59?,61?,64+,65-,66-,67+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | 350 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59220
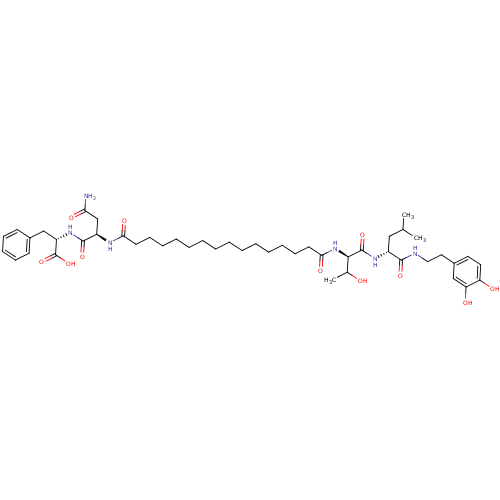
(Pepstatin analog, 8)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(C)O)C(=O)NCCc1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C47H72N6O11/c1-31(2)27-35(44(60)49-26-25-34-23-24-38(55)39(56)29-34)51-46(62)43(32(3)54)53-42(59)22-18-13-11-9-7-5-4-6-8-10-12-17-21-41(58)50-36(30-40(48)57)45(61)52-37(47(63)64)28-33-19-15-14-16-20-33/h14-16,19-20,23-24,29,31-32,35-37,43,54-56H,4-13,17-18,21-22,25-28,30H2,1-3H3,(H2,48,57)(H,49,60)(H,50,58)(H,51,62)(H,52,61)(H,53,59)(H,63,64)/t32?,35-,36-,37+,43-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59222
(Pepstatin analog, 10)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(C)O)C(=O)NCCc1ccc(N)cc1 |r| Show InChI InChI=1S/C47H73N7O9/c1-32(2)29-37(44(59)50-28-27-34-23-25-36(48)26-24-34)52-46(61)43(33(3)55)54-42(58)22-18-13-11-9-7-5-4-6-8-10-12-17-21-41(57)51-38(31-40(49)56)45(60)53-39(47(62)63)30-35-19-15-14-16-20-35/h14-16,19-20,23-26,32-33,37-39,43,55H,4-13,17-18,21-22,27-31,48H2,1-3H3,(H2,49,56)(H,50,59)(H,51,57)(H,52,61)(H,53,60)(H,54,58)(H,62,63)/t33?,37-,38-,39+,43-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59219
(Pepstatin analog, 7)Show SMILES COc1ccc(CCNC(=O)[C@@H](CC(C)C)NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)C(O)=O)C(C)O)cc1 |r| Show InChI InChI=1S/C48H74N6O10/c1-33(2)30-38(45(59)50-29-28-35-24-26-37(64-4)27-25-35)52-47(61)44(34(3)55)54-43(58)23-19-14-12-10-8-6-5-7-9-11-13-18-22-42(57)51-39(32-41(49)56)46(60)53-40(48(62)63)31-36-20-16-15-17-21-36/h15-17,20-21,24-27,33-34,38-40,44,55H,5-14,18-19,22-23,28-32H2,1-4H3,(H2,49,56)(H,50,59)(H,51,57)(H,52,61)(H,53,60)(H,54,58)(H,62,63)/t34?,38-,39-,40+,44-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 720 | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59216
(Pepstatin analog, 4)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(C)O)C(=O)NCCc1cccc(O)c1 |r| Show InChI InChI=1S/C47H72N6O10/c1-32(2)28-37(44(59)49-27-26-35-22-19-23-36(55)29-35)51-46(61)43(33(3)54)53-42(58)25-18-13-11-9-7-5-4-6-8-10-12-17-24-41(57)50-38(31-40(48)56)45(60)52-39(47(62)63)30-34-20-15-14-16-21-34/h14-16,19-23,29,32-33,37-39,43,54-55H,4-13,17-18,24-28,30-31H2,1-3H3,(H2,48,56)(H,49,59)(H,50,57)(H,51,61)(H,52,60)(H,53,58)(H,62,63)/t33?,37-,38-,39+,43-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.13E+3 | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479598
(CHEMBL478672)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CSCCOCCOCCSC[C@H](NC(=O)COc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)COc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C52H66N6O12S2/c1-36(2)29-42(49(62)56-41(48(53)61)30-37-15-7-3-8-16-37)57-50(63)44(54-46(59)32-69-39-19-11-5-12-20-39)34-71-27-25-67-23-24-68-26-28-72-35-45(55-47(60)33-70-40-21-13-6-14-22-40)51(64)58-43(52(65)66)31-38-17-9-4-10-18-38/h3-22,36,41-45H,23-35H2,1-2H3,(H2,53,61)(H,54,59)(H,55,60)(H,56,62)(H,57,63)(H,58,64)(H,65,66)/t41-,42-,43-,44-,45-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59217
(Pepstatin analog, 5)Show SMILES COc1cccc(CCNC(=O)[C@@H](CC(C)C)NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)C(O)=O)C(C)O)c1 |r| Show InChI InChI=1S/C48H74N6O10/c1-33(2)29-38(45(59)50-28-27-36-23-20-24-37(30-36)64-4)52-47(61)44(34(3)55)54-43(58)26-19-14-12-10-8-6-5-7-9-11-13-18-25-42(57)51-39(32-41(49)56)46(60)53-40(48(62)63)31-35-21-16-15-17-22-35/h15-17,20-24,30,33-34,38-40,44,55H,5-14,18-19,25-29,31-32H2,1-4H3,(H2,49,56)(H,50,59)(H,51,57)(H,52,61)(H,53,60)(H,54,58)(H,62,63)/t34?,38-,39-,40+,44-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+3 | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479600
(CHEMBL504512)Show SMILES CCCCCCC(=O)N[C@@H](CSCCOCCOCCSC[C@H](NC(=O)CCCCCC)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C50H78N6O10S2/c1-5-7-9-17-23-44(57)52-42(48(61)55-40(31-36(3)4)47(60)54-39(46(51)59)32-37-19-13-11-14-20-37)34-67-29-27-65-25-26-66-28-30-68-35-43(53-45(58)24-18-10-8-6-2)49(62)56-41(50(63)64)33-38-21-15-12-16-22-38/h11-16,19-22,36,39-43H,5-10,17-18,23-35H2,1-4H3,(H2,51,59)(H,52,57)(H,53,58)(H,54,60)(H,55,61)(H,56,62)(H,63,64)/t39-,40-,41-,42-,43-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479594
(CHEMBL510483)Show SMILES CCCCCC(=O)N[C@@H](CSCCOCCOCCSC[C@H](NC(=O)CCCCC)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C48H74N6O10S2/c1-5-7-11-21-42(55)50-40(46(59)53-38(29-34(3)4)45(58)52-37(44(49)57)30-35-17-13-9-14-18-35)32-65-27-25-63-23-24-64-26-28-66-33-41(51-43(56)22-12-8-6-2)47(60)54-39(48(61)62)31-36-19-15-10-16-20-36/h9-10,13-20,34,37-41H,5-8,11-12,21-33H2,1-4H3,(H2,49,57)(H,50,55)(H,51,56)(H,52,58)(H,53,59)(H,54,60)(H,61,62)/t37-,38-,39-,40-,41-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59214
(Pepstatin analog, 2)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@H](Cc1ccccc1)C(O)=O)C(C)O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C48H73N7O10/c1-32(2)28-37(45(61)52-36(44(50)60)29-34-22-16-14-17-23-34)53-47(63)43(33(3)56)55-42(59)27-21-13-11-9-7-5-4-6-8-10-12-20-26-41(58)51-38(31-40(49)57)46(62)54-39(48(64)65)30-35-24-18-15-19-25-35/h14-19,22-25,32-33,36-39,43,56H,4-13,20-21,26-31H2,1-3H3,(H2,49,57)(H2,50,60)(H,51,58)(H,52,61)(H,53,63)(H,54,62)(H,55,59)(H,64,65)/t33?,36-,37-,38+,39+,43-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50370412
(CHEMBL449777)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)[C@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C48H73N7O10/c1-32(2)28-37(45(61)52-36(44(50)60)29-34-22-16-14-17-23-34)53-47(63)43(33(3)56)55-42(59)27-21-13-11-9-7-5-4-6-8-10-12-20-26-41(58)51-38(31-40(49)57)46(62)54-39(48(64)65)30-35-24-18-15-19-25-35/h14-19,22-25,32-33,36-39,43,56H,4-13,20-21,26-31H2,1-3H3,(H2,49,57)(H2,50,60)(H,51,58)(H,52,61)(H,53,63)(H,54,62)(H,55,59)(H,64,65)/t33-,36-,37-,38-,39-,43-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479603
(CHEMBL446001)Show SMILES CCCCC(=O)N[C@@H](CSCCOCCOCCSC[C@H](NC(=O)CCCC)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C46H70N6O10S2/c1-5-7-19-40(53)48-38(44(57)51-36(27-32(3)4)43(56)50-35(42(47)55)28-33-15-11-9-12-16-33)30-63-25-23-61-21-22-62-24-26-64-31-39(49-41(54)20-8-6-2)45(58)52-37(46(59)60)29-34-17-13-10-14-18-34/h9-18,32,35-39H,5-8,19-31H2,1-4H3,(H2,47,55)(H,48,53)(H,49,54)(H,50,56)(H,51,57)(H,52,58)(H,59,60)/t35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479593
(CHEMBL498854)Show SMILES CCCC(=O)N[C@@H](CSCCOCCOCCSC[C@H](NC(=O)CCC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C44H66N6O10S2/c1-5-13-38(51)46-36(42(55)49-34(25-30(3)4)41(54)48-33(40(45)53)26-31-15-9-7-10-16-31)28-61-23-21-59-19-20-60-22-24-62-29-37(47-39(52)14-6-2)43(56)50-35(44(57)58)27-32-17-11-8-12-18-32/h7-12,15-18,30,33-37H,5-6,13-14,19-29H2,1-4H3,(H2,45,53)(H,46,51)(H,47,52)(H,48,54)(H,49,55)(H,50,56)(H,57,58)/t33-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479592
(CHEMBL450301)Show SMILES CCC(=O)N[C@@H](CSCCOCCOCCSC[C@H](NC(=O)CC)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C42H62N6O10S2/c1-5-36(49)44-34(40(53)47-32(23-28(3)4)39(52)46-31(38(43)51)24-29-13-9-7-10-14-29)26-59-21-19-57-17-18-58-20-22-60-27-35(45-37(50)6-2)41(54)48-33(42(55)56)25-30-15-11-8-12-16-30/h7-16,28,31-35H,5-6,17-27H2,1-4H3,(H2,43,51)(H,44,49)(H,45,50)(H,46,52)(H,47,53)(H,48,54)(H,55,56)/t31-,32-,33-,34-,35-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by competitive inhibition assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479599
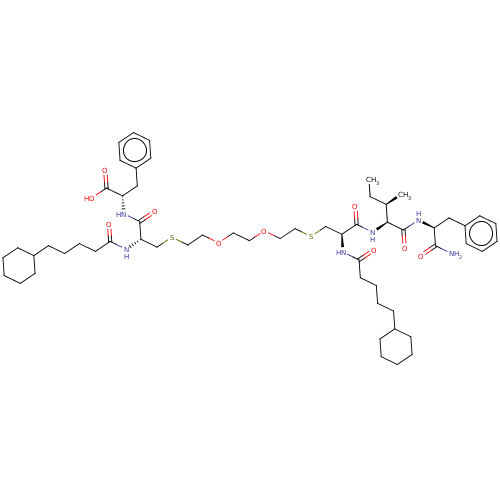
(CHEMBL508493)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CSCCOCCOCCSC[C@H](NC(=O)CCCCC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)CCCCC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C58H90N6O10S2/c1-3-42(2)53(57(70)62-47(54(59)67)38-45-26-12-6-13-27-45)64-56(69)50(61-52(66)31-19-17-25-44-22-10-5-11-23-44)41-76-37-35-74-33-32-73-34-36-75-40-49(60-51(65)30-18-16-24-43-20-8-4-9-21-43)55(68)63-48(58(71)72)39-46-28-14-7-15-29-46/h6-7,12-15,26-29,42-44,47-50,53H,3-5,8-11,16-25,30-41H2,1-2H3,(H2,59,67)(H,60,65)(H,61,66)(H,62,70)(H,63,68)(H,64,69)(H,71,72)/t42-,47+,48+,49+,50+,53+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479596
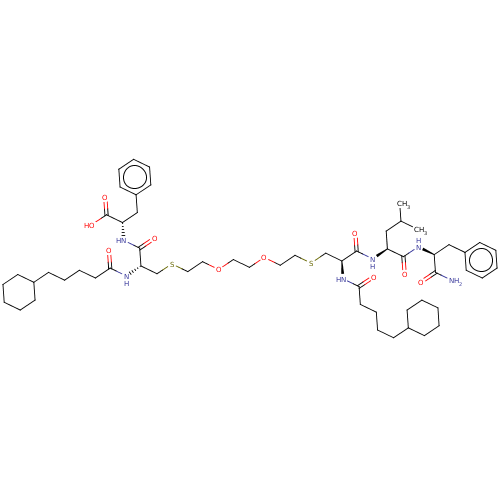
(CHEMBL454643)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CSCCOCCOCCSC[C@H](NC(=O)CCCCC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)CCCCC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C58H90N6O10S2/c1-42(2)37-48(55(68)62-47(54(59)67)38-45-25-11-5-12-26-45)63-56(69)50(60-52(65)29-17-15-23-43-19-7-3-8-20-43)40-75-35-33-73-31-32-74-34-36-76-41-51(61-53(66)30-18-16-24-44-21-9-4-10-22-44)57(70)64-49(58(71)72)39-46-27-13-6-14-28-46/h5-6,11-14,25-28,42-44,47-51H,3-4,7-10,15-24,29-41H2,1-2H3,(H2,59,67)(H,60,65)(H,61,66)(H,62,68)(H,63,69)(H,64,70)(H,71,72)/t47-,48-,49-,50-,51-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479601
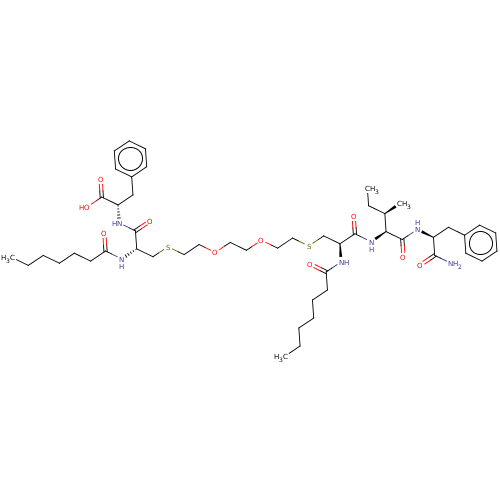
(CHEMBL507784)Show SMILES CCCCCCC(=O)N[C@@H](CSCCOCCOCCSC[C@H](NC(=O)CCCCCC)C(=O)N[C@@H]([C@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H78N6O10S2/c1-5-8-10-18-24-43(57)52-41(47(60)55-40(50(63)64)33-38-22-16-13-17-23-38)34-67-30-28-65-26-27-66-29-31-68-35-42(53-44(58)25-19-11-9-6-2)48(61)56-45(36(4)7-3)49(62)54-39(46(51)59)32-37-20-14-12-15-21-37/h12-17,20-23,36,39-42,45H,5-11,18-19,24-35H2,1-4H3,(H2,51,59)(H,52,57)(H,53,58)(H,54,62)(H,55,60)(H,56,61)(H,63,64)/t36-,39+,40+,41+,42+,45+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50410179

(CHEMBL2096800)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@H](C(C)C)C(=O)N[C@H]([C@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C72H123N13O17/c1-28-42(13)52(76-56(88)48(38(5)6)74-57(89)49(39(7)8)78-63(95)69(19,20)82-46(86)35-73-55(87)47(37(3)4)81-67(99)102-68(16,17)18)61(93)84-71(23,24)65(97)80-54(44(15)101-36-45-33-31-30-32-34-45)59(91)75-51(41(11)12)60(92)83-70(21,22)64(96)79-50(40(9)10)58(90)77-53(43(14)29-2)62(94)85-72(25,26)66(98)100-27/h30-34,37-44,47-54H,28-29,35-36H2,1-27H3,(H,73,87)(H,74,89)(H,75,91)(H,76,88)(H,77,90)(H,78,95)(H,79,96)(H,80,97)(H,81,99)(H,82,86)(H,83,92)(H,84,93)(H,85,94)/t42-,43-,44+,47-,48-,49-,50-,51-,52-,53-,54-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50410179

(CHEMBL2096800)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@H](C(C)C)C(=O)N[C@H]([C@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C72H123N13O17/c1-28-42(13)52(76-56(88)48(38(5)6)74-57(89)49(39(7)8)78-63(95)69(19,20)82-46(86)35-73-55(87)47(37(3)4)81-67(99)102-68(16,17)18)61(93)84-71(23,24)65(97)80-54(44(15)101-36-45-33-31-30-32-34-45)59(91)75-51(41(11)12)60(92)83-70(21,22)64(96)79-50(40(9)10)58(90)77-53(43(14)29-2)62(94)85-72(25,26)66(98)100-27/h30-34,37-44,47-54H,28-29,35-36H2,1-27H3,(H,73,87)(H,74,89)(H,75,91)(H,76,88)(H,77,90)(H,78,95)(H,79,96)(H,80,97)(H,81,99)(H,82,86)(H,83,92)(H,84,93)(H,85,94)/t42-,43-,44+,47-,48-,49-,50-,51-,52-,53-,54-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase from APP-transfected CHO cells |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50410182
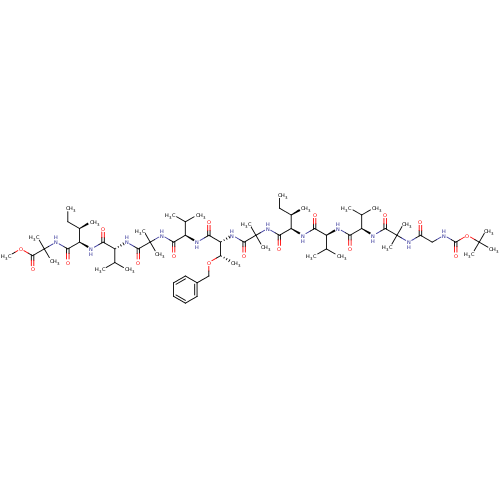
(CHEMBL2096799)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)CNC(=O)OC(C)(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@H](C(C)C)C(=O)N[C@H]([C@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C67H114N12O16/c1-26-39(11)48(71-51(81)44(35(3)4)69-52(82)45(36(5)6)73-58(88)64(17,18)76-43(80)33-68-62(92)95-63(14,15)16)56(86)78-66(21,22)60(90)75-50(41(13)94-34-42-31-29-28-30-32-42)54(84)70-47(38(9)10)55(85)77-65(19,20)59(89)74-46(37(7)8)53(83)72-49(40(12)27-2)57(87)79-67(23,24)61(91)93-25/h28-32,35-41,44-50H,26-27,33-34H2,1-25H3,(H,68,92)(H,69,82)(H,70,84)(H,71,81)(H,72,83)(H,73,88)(H,74,89)(H,75,90)(H,76,80)(H,77,85)(H,78,86)(H,79,87)/t39-,40-,41+,44+,45-,46-,47-,48-,49-,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50410183
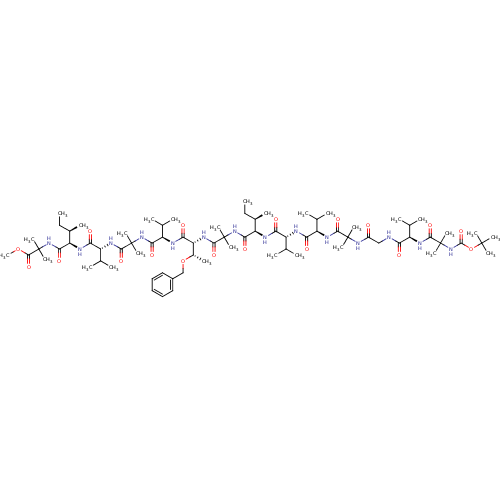
(CHEMBL2096801)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@H](NC(=O)C(C)(C)NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@H](C(C)C)C(=O)N[C@H]([C@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C76H130N14O18/c1-30-44(13)54(80-58(93)50(40(5)6)78-59(94)51(41(7)8)83-65(100)72(19,20)86-48(91)37-77-57(92)49(39(3)4)82-68(103)75(25,26)90-70(105)108-71(16,17)18)63(98)88-74(23,24)67(102)85-56(46(15)107-38-47-35-33-32-34-36-47)61(96)79-53(43(11)12)62(97)87-73(21,22)66(101)84-52(42(9)10)60(95)81-55(45(14)31-2)64(99)89-76(27,28)69(104)106-29/h32-36,39-46,49-56H,30-31,37-38H2,1-29H3,(H,77,92)(H,78,94)(H,79,96)(H,80,93)(H,81,95)(H,82,103)(H,83,100)(H,84,101)(H,85,102)(H,86,91)(H,87,97)(H,88,98)(H,89,99)(H,90,105)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150691
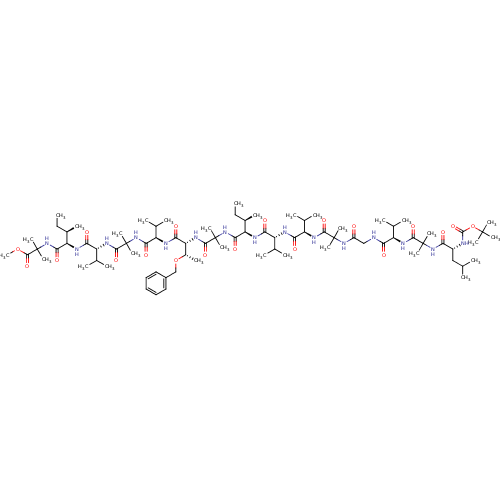
(311951 | CHEMBL437635)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@H](NC(=O)C(C)(C)NC(=O)[C@@H](CC(C)C)NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@H](C(C)C)C(=O)N[C@H]([C@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C82H141N15O19/c1-32-48(15)59(87-64(101)55(44(7)8)85-65(102)56(45(9)10)90-71(108)78(21,22)93-53(98)40-83-63(100)54(43(5)6)89-72(109)79(23,24)94-62(99)52(39-42(3)4)84-76(113)116-77(18,19)20)69(106)96-81(27,28)74(111)92-61(50(17)115-41-51-37-35-34-36-38-51)67(104)86-58(47(13)14)68(105)95-80(25,26)73(110)91-57(46(11)12)66(103)88-60(49(16)33-2)70(107)97-82(29,30)75(112)114-31/h34-38,42-50,52,54-61H,32-33,39-41H2,1-31H3,(H,83,100)(H,84,113)(H,85,102)(H,86,104)(H,87,101)(H,88,103)(H,89,109)(H,90,108)(H,91,110)(H,92,111)(H,93,98)(H,94,99)(H,95,105)(H,96,106)(H,97,107)/t48-,49-,50+,52-,54-,55-,56-,57-,58-,59-,60-,61-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150696
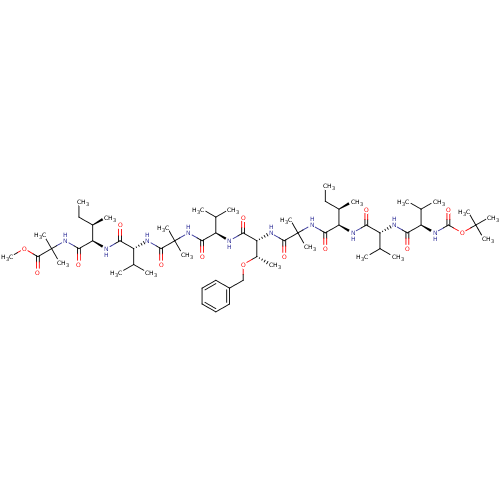
(CHEMBL436787 | methyl 2-[(2R,3R)-2-[(2R)-2-{2-[(2R...)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@H](C(C)C)C(=O)N[C@H]([C@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C61H104N10O14/c1-24-36(11)44(64-47(72)40(32(3)4)62-49(74)42(34(7)8)68-57(82)85-58(14,15)16)52(77)70-60(19,20)55(80)67-46(38(13)84-31-39-29-27-26-28-30-39)50(75)63-43(35(9)10)51(76)69-59(17,18)54(79)66-41(33(5)6)48(73)65-45(37(12)25-2)53(78)71-61(21,22)56(81)83-23/h26-30,32-38,40-46H,24-25,31H2,1-23H3,(H,62,74)(H,63,75)(H,64,72)(H,65,73)(H,66,79)(H,67,80)(H,68,82)(H,69,76)(H,70,77)(H,71,78)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150689
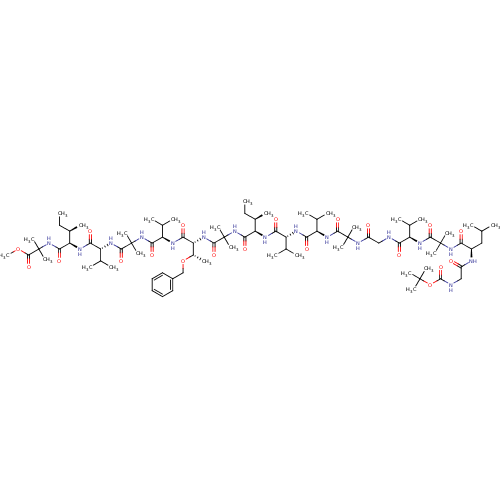
(311952 | CHEMBL411599)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@H](NC(=O)C(C)(C)NC(=O)[C@@H](CC(C)C)NC(=O)CNC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@H](C(C)C)C(=O)N[C@H]([C@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C84H144N16O20/c1-32-49(15)61(90-66(105)57(45(7)8)88-67(106)58(46(9)10)93-73(112)80(21,22)96-55(102)41-85-65(104)56(44(5)6)92-74(113)81(23,24)97-64(103)53(39-43(3)4)87-54(101)40-86-78(117)120-79(18,19)20)71(110)99-83(27,28)76(115)95-63(51(17)119-42-52-37-35-34-36-38-52)69(108)89-60(48(13)14)70(109)98-82(25,26)75(114)94-59(47(11)12)68(107)91-62(50(16)33-2)72(111)100-84(29,30)77(116)118-31/h34-38,43-51,53,56-63H,32-33,39-42H2,1-31H3,(H,85,104)(H,86,117)(H,87,101)(H,88,106)(H,89,108)(H,90,105)(H,91,107)(H,92,113)(H,93,112)(H,94,114)(H,95,115)(H,96,102)(H,97,103)(H,98,109)(H,99,110)(H,100,111)/t49-,50-,51+,53-,56-,57-,58-,59-,60-,61-,62-,63-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50410178
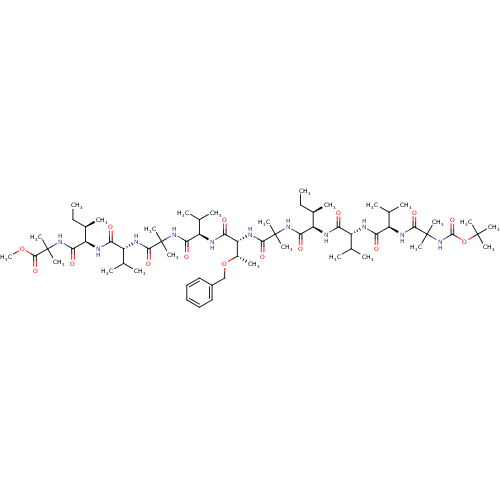
(CHEMBL2096802)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@H](C(C)C)C(=O)N[C@H]([C@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C65H111N11O15/c1-26-38(11)46(68-49(77)42(34(3)4)66-50(78)43(35(5)6)71-58(86)64(21,22)76-60(88)91-61(14,15)16)54(82)74-63(19,20)57(85)72-48(40(13)90-33-41-31-29-28-30-32-41)52(80)67-45(37(9)10)53(81)73-62(17,18)56(84)70-44(36(7)8)51(79)69-47(39(12)27-2)55(83)75-65(23,24)59(87)89-25/h28-32,34-40,42-48H,26-27,33H2,1-25H3,(H,66,78)(H,67,80)(H,68,77)(H,69,79)(H,70,84)(H,71,86)(H,72,85)(H,73,81)(H,74,82)(H,75,83)(H,76,88)/t38-,39-,40+,42-,43-,44-,45-,46-,47-,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150693
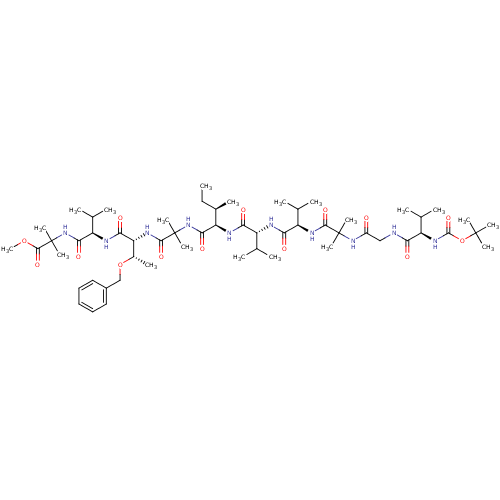
(CHEMBL415576 | methyl 2-[(2R)-2-[(2R,3S)-3-(benzyl...)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](NC(=O)[C@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@H]([C@H](C)OCc1ccccc1)C(=O)N[C@H](C(C)C)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C57H96N10O14/c1-22-34(10)42(49(74)66-56(17,18)51(76)63-43(35(11)80-29-36-26-24-23-25-27-36)47(72)60-41(33(8)9)48(73)67-57(19,20)52(77)79-21)61-45(70)39(31(4)5)59-46(71)40(32(6)7)62-50(75)55(15,16)65-37(68)28-58-44(69)38(30(2)3)64-53(78)81-54(12,13)14/h23-27,30-35,38-43H,22,28-29H2,1-21H3,(H,58,69)(H,59,71)(H,60,72)(H,61,70)(H,62,75)(H,63,76)(H,64,78)(H,65,68)(H,66,74)(H,67,73)/t34-,35+,38-,39-,40-,41-,42-,43-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150690

(CHEMBL409305 | methyl 2-[(2S,3S)-2-[(2S)-2-{2-[(2S...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)CNC(=O)OC(C)(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H]([C@@H](C)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C67H114N12O16/c1-26-39(11)48(71-51(81)44(35(3)4)69-52(82)45(36(5)6)73-58(88)64(17,18)76-43(80)33-68-62(92)95-63(14,15)16)56(86)78-66(21,22)60(90)75-50(41(13)94-34-42-31-29-28-30-32-42)54(84)70-47(38(9)10)55(85)77-65(19,20)59(89)74-46(37(7)8)53(83)72-49(40(12)27-2)57(87)79-67(23,24)61(91)93-25/h28-32,35-41,44-50H,26-27,33-34H2,1-25H3,(H,68,92)(H,69,82)(H,70,84)(H,71,81)(H,72,83)(H,73,88)(H,74,89)(H,75,90)(H,76,80)(H,77,85)(H,78,86)(H,79,87)/t39-,40-,41+,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150688
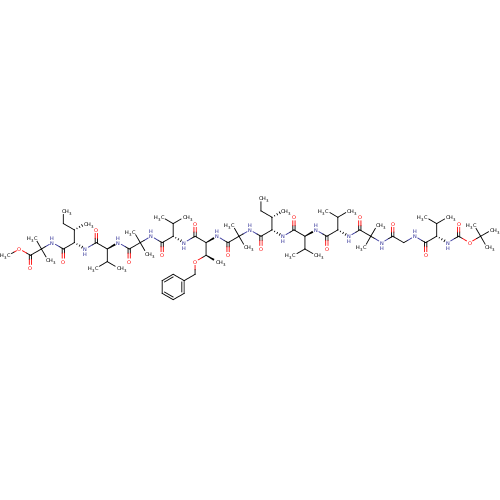
(311440 | CHEMBL413823)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H]([C@@H](C)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C72H123N13O17/c1-28-42(13)52(76-56(88)48(38(5)6)74-57(89)49(39(7)8)78-63(95)69(19,20)82-46(86)35-73-55(87)47(37(3)4)81-67(99)102-68(16,17)18)61(93)84-71(23,24)65(97)80-54(44(15)101-36-45-33-31-30-32-34-45)59(91)75-51(41(11)12)60(92)83-70(21,22)64(96)79-50(40(9)10)58(90)77-53(43(14)29-2)62(94)85-72(25,26)66(98)100-27/h30-34,37-44,47-54H,28-29,35-36H2,1-27H3,(H,73,87)(H,74,89)(H,75,91)(H,76,88)(H,77,90)(H,78,95)(H,79,96)(H,80,97)(H,81,99)(H,82,86)(H,83,92)(H,84,93)(H,85,94)/t42-,43-,44+,47-,48-,49-,50-,51-,52-,53-,54-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150694
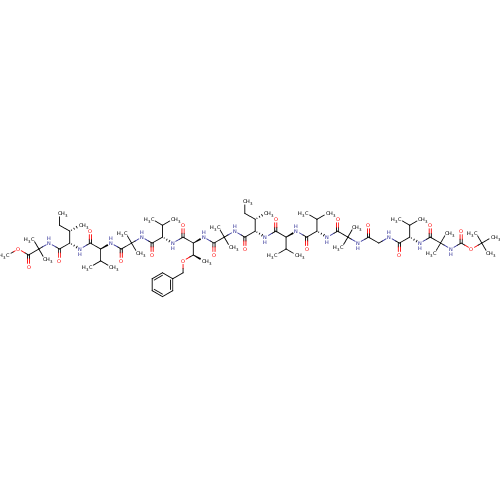
(311383 | CHEMBL437440)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H]([C@@H](C)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C76H130N14O18/c1-30-44(13)54(80-58(93)50(40(5)6)78-59(94)51(41(7)8)83-65(100)72(19,20)86-48(91)37-77-57(92)49(39(3)4)82-68(103)75(25,26)90-70(105)108-71(16,17)18)63(98)88-74(23,24)67(102)85-56(46(15)107-38-47-35-33-32-34-36-47)61(96)79-53(43(11)12)62(97)87-73(21,22)66(101)84-52(42(9)10)60(95)81-55(45(14)31-2)64(99)89-76(27,28)69(104)106-29/h32-36,39-46,49-56H,30-31,37-38H2,1-29H3,(H,77,92)(H,78,94)(H,79,96)(H,80,93)(H,81,95)(H,82,103)(H,83,100)(H,84,101)(H,85,102)(H,86,91)(H,87,97)(H,88,98)(H,89,99)(H,90,105)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479601

(CHEMBL507784)Show SMILES CCCCCCC(=O)N[C@@H](CSCCOCCOCCSC[C@H](NC(=O)CCCCCC)C(=O)N[C@@H]([C@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H78N6O10S2/c1-5-8-10-18-24-43(57)52-41(47(60)55-40(50(63)64)33-38-22-16-13-17-23-38)34-67-30-28-65-26-27-66-29-31-68-35-42(53-44(58)25-19-11-9-6-2)48(61)56-45(36(4)7-3)49(62)54-39(46(51)59)32-37-20-14-12-15-21-37/h12-17,20-23,36,39-42,45H,5-11,18-19,24-35H2,1-4H3,(H2,51,59)(H,52,57)(H,53,58)(H,54,62)(H,55,60)(H,56,61)(H,63,64)/t36-,39+,40+,41+,42+,45+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by continuous fluorometric assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479597
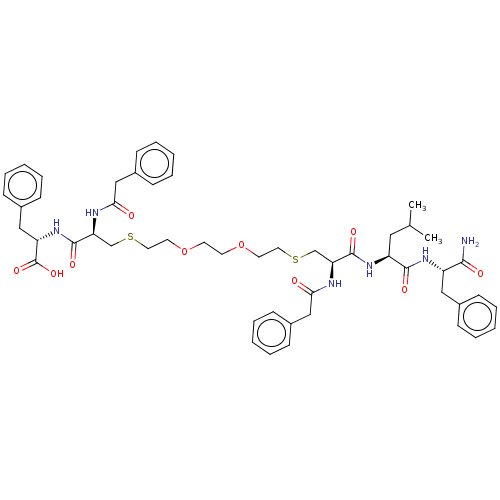
(CHEMBL466288)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CSCCOCCOCCSC[C@H](NC(=O)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C52H66N6O10S2/c1-36(2)29-42(49(62)56-41(48(53)61)30-37-15-7-3-8-16-37)57-50(63)44(54-46(59)32-39-19-11-5-12-20-39)34-69-27-25-67-23-24-68-26-28-70-35-45(55-47(60)33-40-21-13-6-14-22-40)51(64)58-43(52(65)66)31-38-17-9-4-10-18-38/h3-22,36,41-45H,23-35H2,1-2H3,(H2,53,61)(H,54,59)(H,55,60)(H,56,62)(H,57,63)(H,58,64)(H,65,66)/t41-,42-,43-,44-,45-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by continuous fluorometric assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479604
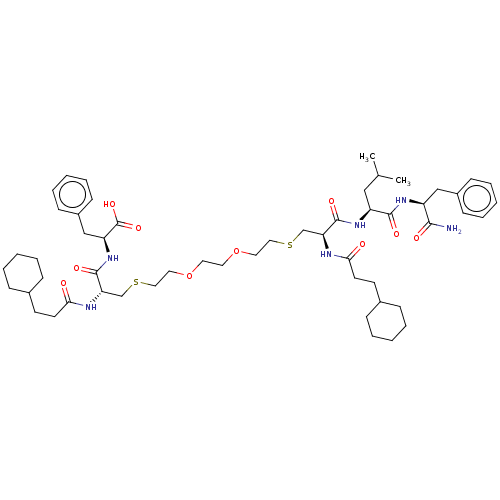
(CHEMBL449860)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CSCCOCCOCCSC[C@H](NC(=O)CCC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)CCC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C54H82N6O10S2/c1-38(2)33-44(51(64)58-43(50(55)63)34-41-19-11-5-12-20-41)59-52(65)46(56-48(61)25-23-39-15-7-3-8-16-39)36-71-31-29-69-27-28-70-30-32-72-37-47(57-49(62)26-24-40-17-9-4-10-18-40)53(66)60-45(54(67)68)35-42-21-13-6-14-22-42/h5-6,11-14,19-22,38-40,43-47H,3-4,7-10,15-18,23-37H2,1-2H3,(H2,55,63)(H,56,61)(H,57,62)(H,58,64)(H,59,65)(H,60,66)(H,67,68)/t43-,44-,45-,46-,47-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by continuous fluorometric assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479598

(CHEMBL478672)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CSCCOCCOCCSC[C@H](NC(=O)COc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)COc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C52H66N6O12S2/c1-36(2)29-42(49(62)56-41(48(53)61)30-37-15-7-3-8-16-37)57-50(63)44(54-46(59)32-69-39-19-11-5-12-20-39)34-71-27-25-67-23-24-68-26-28-72-35-45(55-47(60)33-70-40-21-13-6-14-22-40)51(64)58-43(52(65)66)31-38-17-9-4-10-18-38/h3-22,36,41-45H,23-35H2,1-2H3,(H2,53,61)(H,54,59)(H,55,60)(H,56,62)(H,57,63)(H,58,64)(H,65,66)/t41-,42-,43-,44-,45-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by continuous fluorometric assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150687
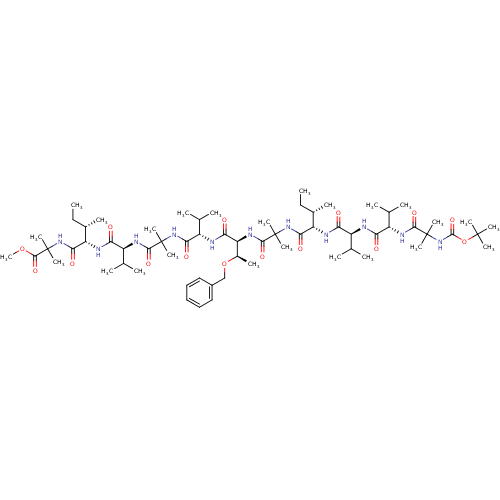
(CHEMBL410969 | methyl 2-[(2S,3S)-2-[(2S)-2-{2-[(2S...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H]([C@@H](C)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C65H111N11O15/c1-26-38(11)46(68-49(77)42(34(3)4)66-50(78)43(35(5)6)71-58(86)64(21,22)76-60(88)91-61(14,15)16)54(82)74-63(19,20)57(85)72-48(40(13)90-33-41-31-29-28-30-32-41)52(80)67-45(37(9)10)53(81)73-62(17,18)56(84)70-44(36(7)8)51(79)69-47(39(12)27-2)55(83)75-65(23,24)59(87)89-25/h28-32,34-40,42-48H,26-27,33H2,1-25H3,(H,66,78)(H,67,80)(H,68,77)(H,69,79)(H,70,84)(H,71,86)(H,72,85)(H,73,81)(H,74,82)(H,75,83)(H,76,88)/t38-,39-,40+,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479600

(CHEMBL504512)Show SMILES CCCCCCC(=O)N[C@@H](CSCCOCCOCCSC[C@H](NC(=O)CCCCCC)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C50H78N6O10S2/c1-5-7-9-17-23-44(57)52-42(48(61)55-40(31-36(3)4)47(60)54-39(46(51)59)32-37-19-13-11-14-20-37)34-67-29-27-65-25-26-66-28-30-68-35-43(53-45(58)24-18-10-8-6-2)49(62)56-41(50(63)64)33-38-21-15-12-16-22-38/h11-16,19-22,36,39-43H,5-10,17-18,23-35H2,1-4H3,(H2,51,59)(H,52,57)(H,53,58)(H,54,60)(H,55,61)(H,56,62)(H,63,64)/t39-,40-,41-,42-,43-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by continuous fluorometric assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59221
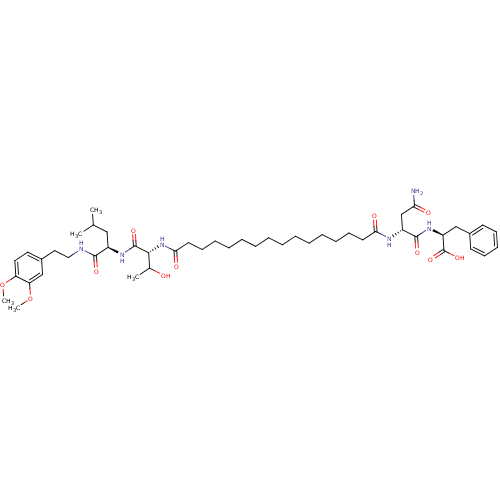
(Pepstatin analog, 9)Show SMILES COc1ccc(CCNC(=O)[C@@H](CC(C)C)NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)C(O)=O)C(C)O)cc1OC |r| Show InChI InChI=1S/C49H76N6O11/c1-33(2)29-37(46(60)51-28-27-36-25-26-40(65-4)41(31-36)66-5)53-48(62)45(34(3)56)55-44(59)24-20-15-13-11-9-7-6-8-10-12-14-19-23-43(58)52-38(32-42(50)57)47(61)54-39(49(63)64)30-35-21-17-16-18-22-35/h16-18,21-22,25-26,31,33-34,37-39,45,56H,6-15,19-20,23-24,27-30,32H2,1-5H3,(H2,50,57)(H,51,60)(H,52,58)(H,53,62)(H,54,61)(H,55,59)(H,63,64)/t34?,37-,38-,39+,45-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366941

(CHEMBL1790139)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC(=O)N(C)[C@@H](C(N)=O)C(=O)N[C@H](Cc1ccccc1)C(O)=O)[C@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H73N7O10/c1-32(2)29-37(45(61)51-36(43(49)59)30-34-23-17-15-18-24-34)52-46(62)41(33(3)56)54-39(57)27-21-13-11-9-7-5-6-8-10-12-14-22-28-40(58)55(4)42(44(50)60)47(63)53-38(48(64)65)31-35-25-19-16-20-26-35/h15-20,23-26,32-33,36-38,41-42,56H,5-14,21-22,27-31H2,1-4H3,(H2,49,59)(H2,50,60)(H,51,61)(H,52,62)(H,53,63)(H,54,57)(H,64,65)/t33-,36-,37-,38+,41-,42-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protease |
Bioorg Med Chem Lett 14: 1395-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.099
BindingDB Entry DOI: 10.7270/Q2KS6S4D |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50410181
(CHEMBL2096796)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)CNC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)[C@H](CC(C)C)NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H]([C@@H](C)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C82H141N15O19/c1-32-48(15)59(87-64(101)55(44(7)8)85-65(102)56(45(9)10)90-71(108)78(21,22)93-53(98)40-83-63(100)54(43(5)6)89-72(109)79(23,24)94-62(99)52(39-42(3)4)84-76(113)116-77(18,19)20)69(106)96-81(27,28)74(111)92-61(50(17)115-41-51-37-35-34-36-38-51)67(104)86-58(47(13)14)68(105)95-80(25,26)73(110)91-57(46(11)12)66(103)88-60(49(16)33-2)70(107)97-82(29,30)75(112)114-31/h34-38,42-50,52,54-61H,32-33,39-41H2,1-31H3,(H,83,100)(H,84,113)(H,85,102)(H,86,104)(H,87,101)(H,88,103)(H,89,109)(H,90,108)(H,91,110)(H,92,111)(H,93,98)(H,94,99)(H,95,105)(H,96,106)(H,97,107)/t48-,49-,50+,52-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366939
(CHEMBL1790126)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@@H](C(N)=O)C(=O)N(C)[C@H](Cc1ccccc1)C(O)=O)[C@H](C)O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C47H72N6O9/c1-33(2)31-37(44(58)49-30-29-35-23-17-15-18-24-35)50-45(59)41(34(3)54)51-39(55)27-21-13-11-9-7-5-6-8-10-12-14-22-28-40(56)52-42(43(48)57)46(60)53(4)38(47(61)62)32-36-25-19-16-20-26-36/h15-20,23-26,33-34,37-38,41-42,54H,5-14,21-22,27-32H2,1-4H3,(H2,48,57)(H,49,58)(H,50,59)(H,51,55)(H,52,56)(H,61,62)/t34-,37-,38+,41-,42-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protease |
Bioorg Med Chem Lett 14: 1395-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.099
BindingDB Entry DOI: 10.7270/Q2KS6S4D |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59215

(Pepstatin analog, 3)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(C)O)C(=O)NCCc1ccccc1 |r| Show InChI InChI=1S/C47H72N6O9/c1-33(2)30-37(44(58)49-29-28-35-22-16-14-17-23-35)51-46(60)43(34(3)54)53-42(57)27-21-13-11-9-7-5-4-6-8-10-12-20-26-41(56)50-38(32-40(48)55)45(59)52-39(47(61)62)31-36-24-18-15-19-25-36/h14-19,22-25,33-34,37-39,43,54H,4-13,20-21,26-32H2,1-3H3,(H2,48,55)(H,49,58)(H,50,56)(H,51,60)(H,52,59)(H,53,57)(H,61,62)/t34?,37-,38+,39-,43-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59218
(Pepstatin analog, 6)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(C)O)C(=O)NCCc1ccc(O)cc1 |r| Show InChI InChI=1S/C47H72N6O10/c1-32(2)29-37(44(59)49-28-27-34-23-25-36(55)26-24-34)51-46(61)43(33(3)54)53-42(58)22-18-13-11-9-7-5-4-6-8-10-12-17-21-41(57)50-38(31-40(48)56)45(60)52-39(47(62)63)30-35-19-15-14-16-20-35/h14-16,19-20,23-26,32-33,37-39,43,54-55H,4-13,17-18,21-22,27-31H2,1-3H3,(H2,48,56)(H,49,59)(H,50,57)(H,51,61)(H,52,60)(H,53,58)(H,62,63)/t33?,37-,38-,39+,43-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University
| Assay Description
A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. |
Chem Biol 12: 439-44 (2005)
Article DOI: 10.1016/j.chembiol.2005.02.004
BindingDB Entry DOI: 10.7270/Q2JD4V68 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366938

(CHEMBL1790133)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@@H](C(N)=O)C(=O)N[C@H](Cc1ccccc1)C(O)=O)[C@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C47H71N7O10/c1-31(2)28-36(44(60)50-35(42(48)58)29-33-22-16-14-17-23-33)51-45(61)40(32(3)55)53-38(56)26-20-12-10-8-6-4-5-7-9-11-13-21-27-39(57)54-41(43(49)59)46(62)52-37(47(63)64)30-34-24-18-15-19-25-34/h14-19,22-25,31-32,35-37,40-41,55H,4-13,20-21,26-30H2,1-3H3,(H2,48,58)(H2,49,59)(H,50,60)(H,51,61)(H,52,62)(H,53,56)(H,54,57)(H,63,64)/t32-,35-,36-,37+,40-,41-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protease |
Bioorg Med Chem Lett 14: 1395-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.099
BindingDB Entry DOI: 10.7270/Q2KS6S4D |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479605
(CHEMBL500554)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CSCCOCCOCCSC[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)CCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C54H70N6O10S2/c1-38(2)33-44(51(64)58-43(50(55)63)34-41-19-11-5-12-20-41)59-52(65)46(56-48(61)25-23-39-15-7-3-8-16-39)36-71-31-29-69-27-28-70-30-32-72-37-47(57-49(62)26-24-40-17-9-4-10-18-40)53(66)60-45(54(67)68)35-42-21-13-6-14-22-42/h3-22,38,43-47H,23-37H2,1-2H3,(H2,55,63)(H,56,61)(H,57,62)(H,58,64)(H,59,65)(H,60,66)(H,67,68)/t43-,44-,45-,46-,47-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by continuous fluorometric assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366936
(CHEMBL1790128)Show SMILES CC(C)C[C@H](N(C)C(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@@H](C(N)=O)C(=O)N[C@H](Cc1ccccc1)C(O)=O)[C@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H73N7O10/c1-32(2)29-38(45(61)51-36(43(49)59)30-34-23-17-15-18-24-34)55(4)47(63)41(33(3)56)53-39(57)27-21-13-11-9-7-5-6-8-10-12-14-22-28-40(58)54-42(44(50)60)46(62)52-37(48(64)65)31-35-25-19-16-20-26-35/h15-20,23-26,32-33,36-38,41-42,56H,5-14,21-22,27-31H2,1-4H3,(H2,49,59)(H2,50,60)(H,51,61)(H,52,62)(H,53,57)(H,54,58)(H,64,65)/t33-,36-,37+,38-,41-,42-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protease |
Bioorg Med Chem Lett 14: 1395-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.099
BindingDB Entry DOI: 10.7270/Q2KS6S4D |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366940
(CHEMBL1790135)Show SMILES CC(C)C[C@H](NC(=O)[C@H]([C@H](C)O)N(C)C(=O)CCCCCCCCCCCCCCC(=O)N[C@@H](C(N)=O)C(=O)N[C@H](Cc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H73N7O10/c1-32(2)29-37(45(61)51-36(43(49)59)30-34-23-17-15-18-24-34)52-47(63)42(33(3)56)55(4)40(58)28-22-14-12-10-8-6-5-7-9-11-13-21-27-39(57)54-41(44(50)60)46(62)53-38(48(64)65)31-35-25-19-16-20-26-35/h15-20,23-26,32-33,36-38,41-42,56H,5-14,21-22,27-31H2,1-4H3,(H2,49,59)(H2,50,60)(H,51,61)(H,52,63)(H,53,62)(H,54,57)(H,64,65)/t33-,36-,37-,38+,41-,42-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protease |
Bioorg Med Chem Lett 14: 1395-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.099
BindingDB Entry DOI: 10.7270/Q2KS6S4D |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479595
(CHEMBL507080)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CSCCOCCOCCSC[C@H](NC(=O)CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C52H78N6O10S2/c1-36(2)29-42(49(62)56-41(48(53)61)30-37-15-7-3-8-16-37)57-50(63)44(54-46(59)32-39-19-11-5-12-20-39)34-69-27-25-67-23-24-68-26-28-70-35-45(55-47(60)33-40-21-13-6-14-22-40)51(64)58-43(52(65)66)31-38-17-9-4-10-18-38/h3-4,7-10,15-18,36,39-45H,5-6,11-14,19-35H2,1-2H3,(H2,53,61)(H,54,59)(H,55,60)(H,56,62)(H,57,63)(H,58,64)(H,65,66)/t41-,42-,43-,44-,45-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by continuous fluorometric assay |
Bioorg Med Chem 17: 967-76 (2009)
Article DOI: 10.1016/j.bmc.2008.02.060
BindingDB Entry DOI: 10.7270/Q2QN69K9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366937
(CHEMBL1790127)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)CCCCCCCCCCCCCCC(=O)N[C@@H](C(N)=O)C(=O)N(C)[C@H](Cc1ccccc1)C(O)=O)[C@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H73N7O10/c1-32(2)29-37(45(61)51-36(43(49)59)30-34-23-17-15-18-24-34)52-46(62)41(33(3)56)53-39(57)27-21-13-11-9-7-5-6-8-10-12-14-22-28-40(58)54-42(44(50)60)47(63)55(4)38(48(64)65)31-35-25-19-16-20-26-35/h15-20,23-26,32-33,36-38,41-42,56H,5-14,21-22,27-31H2,1-4H3,(H2,49,59)(H2,50,60)(H,51,61)(H,52,62)(H,53,57)(H,54,58)(H,64,65)/t33-,36-,37-,38+,41-,42-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protease |
Bioorg Med Chem Lett 14: 1395-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.099
BindingDB Entry DOI: 10.7270/Q2KS6S4D |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50150695
(CHEMBL414031 | methyl 2-[(2S,3S)-2-[(2S)-2-{2-[(2S...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H]([C@@H](C)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NC(C)(C)C(=O)OC Show InChI InChI=1S/C61H104N10O14/c1-24-36(11)44(64-47(72)40(32(3)4)62-49(74)42(34(7)8)68-57(82)85-58(14,15)16)52(77)70-60(19,20)55(80)67-46(38(13)84-31-39-29-27-26-28-30-39)50(75)63-43(35(9)10)51(76)69-59(17,18)54(79)66-41(33(5)6)48(73)65-45(37(12)25-2)53(78)71-61(21,22)56(81)83-23/h26-30,32-38,40-46H,24-25,31H2,1-23H3,(H,62,74)(H,63,75)(H,64,72)(H,65,73)(H,66,79)(H,67,80)(H,68,82)(H,69,76)(H,70,77)(H,71,78)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Gamma-secretase in HeLa cells expressing APP-reporter |
J Med Chem 47: 3931-3 (2004)
Article DOI: 10.1021/jm049788c
BindingDB Entry DOI: 10.7270/Q29Z94C2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data