

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205093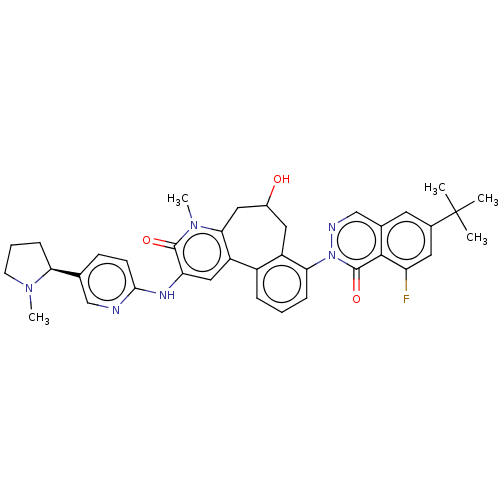 (US9556147, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205094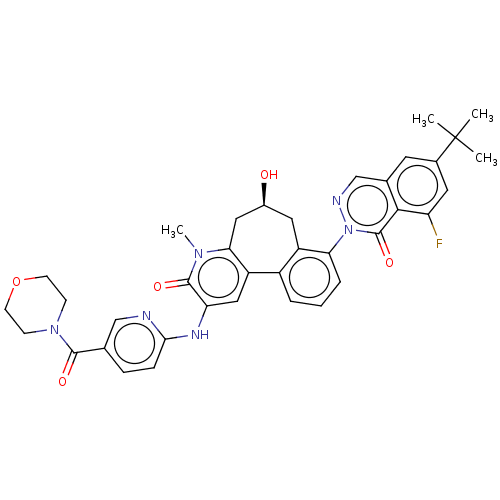 (US9556147, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205082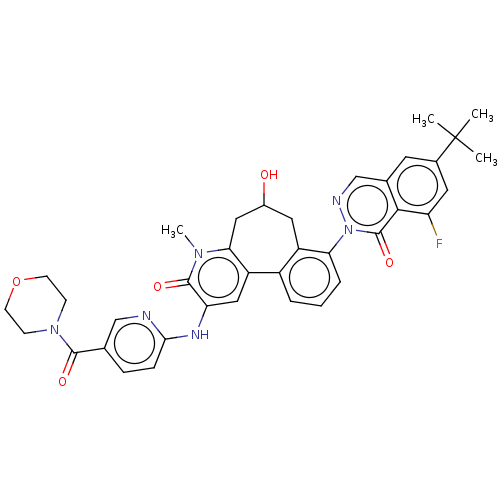 (US9556147, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50505177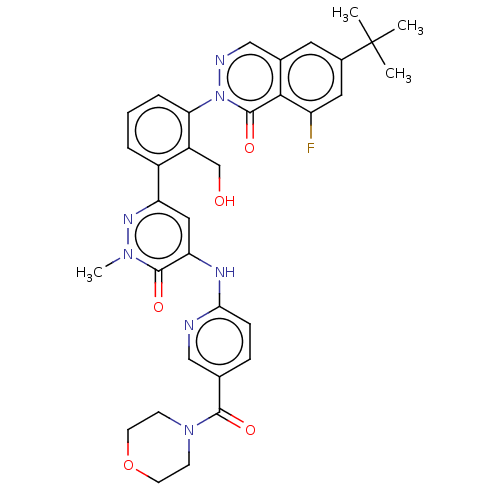 (CHEMBL4117175) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Kinase Tracer 178 binding to biotinylated-BTK (unknown origin) measured after 18 to 24 hrs by Europium-labeled Streptavidin conjugate b... | Bioorg Med Chem Lett 29: 1074-1078 (2019) Article DOI: 10.1016/j.bmcl.2019.03.001 BindingDB Entry DOI: 10.7270/Q21G0QJM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205094 (US9556147, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Kinase Tracer 178 binding to biotinylated-BTK (unknown origin) measured after 18 to 24 hrs by Europium-labeled Streptavidin conjugate b... | Bioorg Med Chem Lett 29: 1074-1078 (2019) Article DOI: 10.1016/j.bmcl.2019.03.001 BindingDB Entry DOI: 10.7270/Q21G0QJM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205082 (US9556147, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Kinase Tracer 178 binding to biotinylated-BTK (unknown origin) measured after 18 to 24 hrs by Europium-labeled Streptavidin conjugate b... | Bioorg Med Chem Lett 29: 1074-1078 (2019) Article DOI: 10.1016/j.bmcl.2019.03.001 BindingDB Entry DOI: 10.7270/Q21G0QJM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205095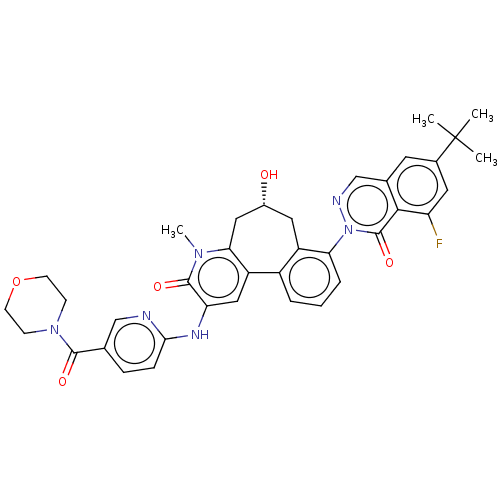 (US9556147, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Kinase Tracer 178 binding to biotinylated-BTK (unknown origin) measured after 18 to 24 hrs by Europium-labeled Streptavidin conjugate b... | Bioorg Med Chem Lett 29: 1074-1078 (2019) Article DOI: 10.1016/j.bmcl.2019.03.001 BindingDB Entry DOI: 10.7270/Q21G0QJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205095 (US9556147, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263528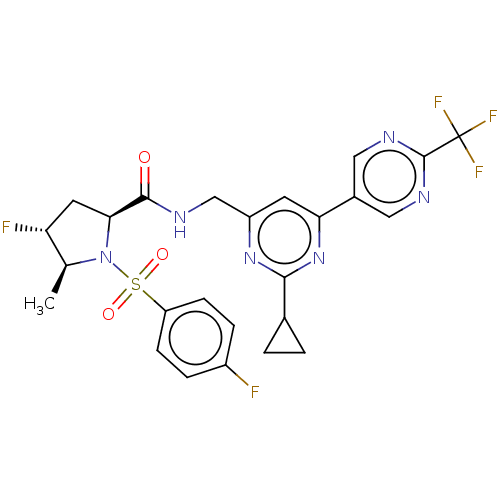 (CHEMBL4079886) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA , Beijing 100176 , P. R. China. Curated by ChEMBL | Assay Description Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by... | J Med Chem 61: 3641-3659 (2018) Article DOI: 10.1021/acs.jmedchem.8b00117 BindingDB Entry DOI: 10.7270/Q2VX0JZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263509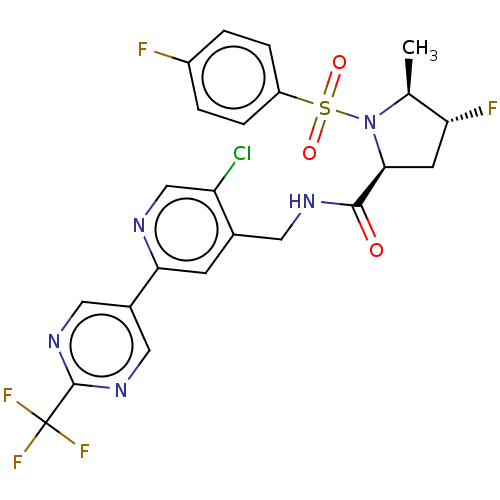 (CHEMBL4103545) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA , Beijing 100176 , P. R. China. Curated by ChEMBL | Assay Description Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by... | J Med Chem 61: 3641-3659 (2018) Article DOI: 10.1021/acs.jmedchem.8b00117 BindingDB Entry DOI: 10.7270/Q2VX0JZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499493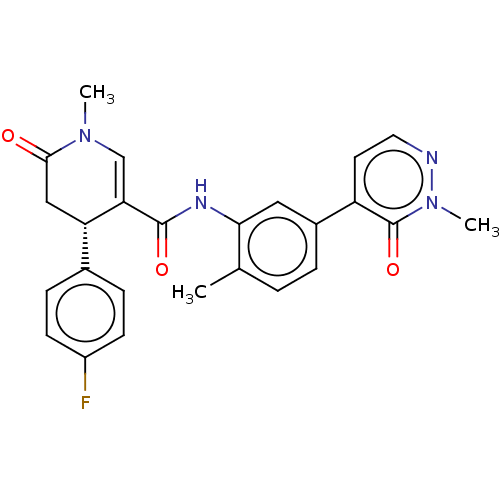 (CHEMBL3739648) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310731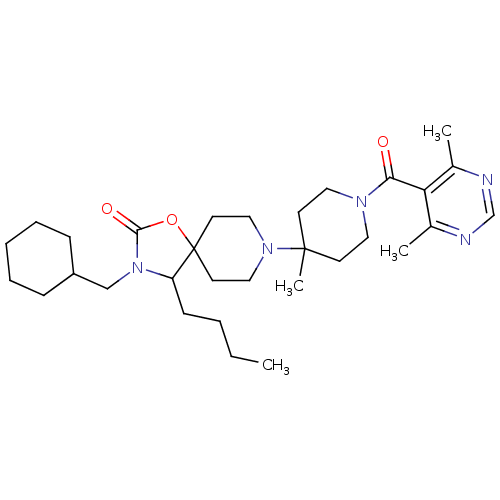 (4-butyl-3-(cyclohexylmethyl)-8-(1-(4,6-dimethylpyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263519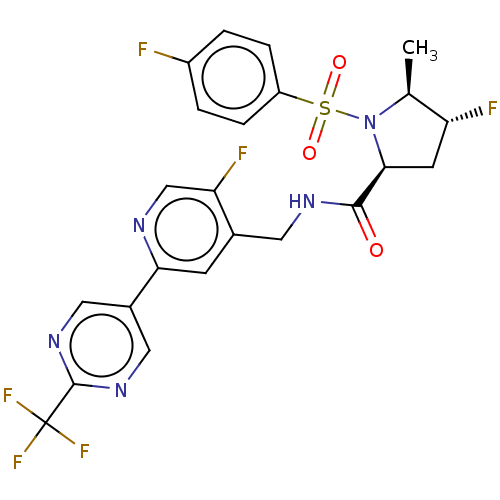 (CHEMBL4092287) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA , Beijing 100176 , P. R. China. Curated by ChEMBL | Assay Description Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by... | J Med Chem 61: 3641-3659 (2018) Article DOI: 10.1021/acs.jmedchem.8b00117 BindingDB Entry DOI: 10.7270/Q2VX0JZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499492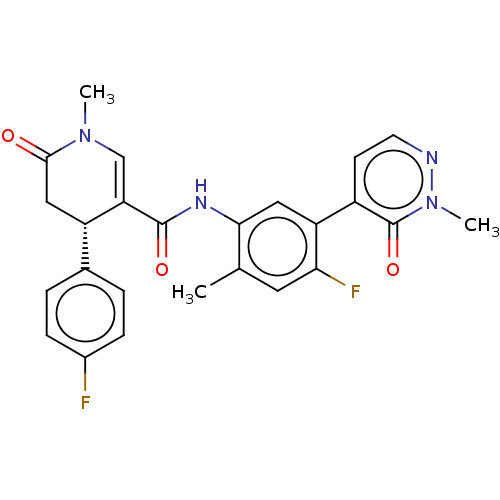 (CHEMBL3740684) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499491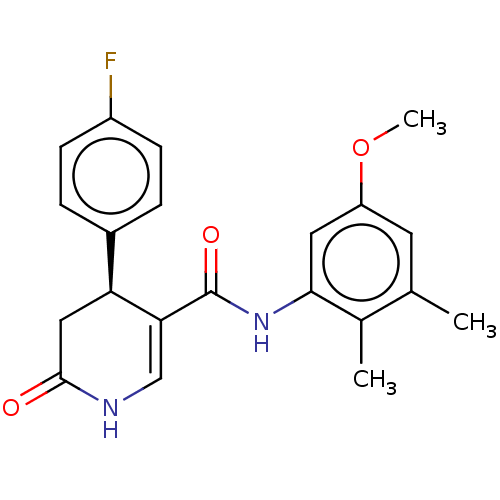 (CHEMBL3741668) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499501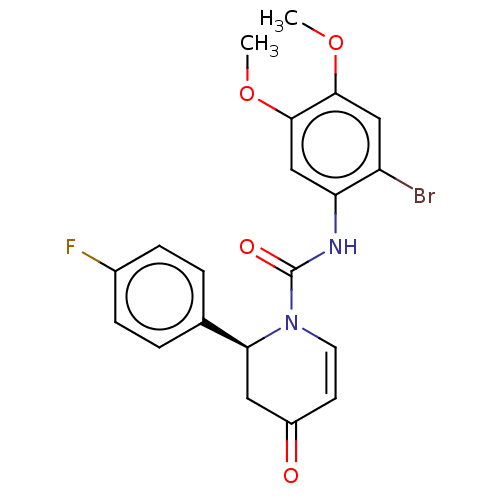 (CHEMBL3741934) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291171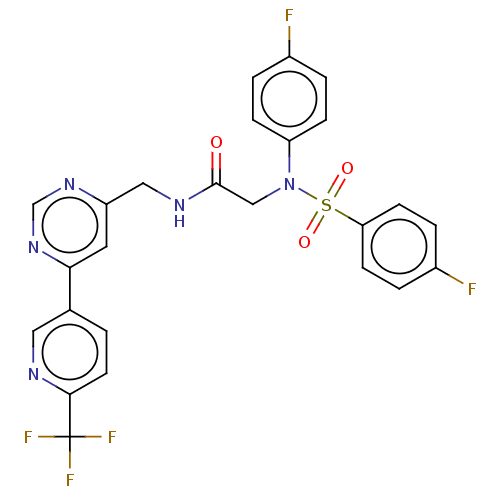 (US9580411, Example 86) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499504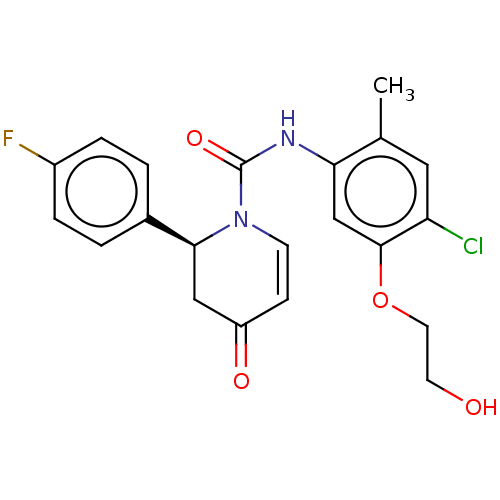 (CHEMBL3741412) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499502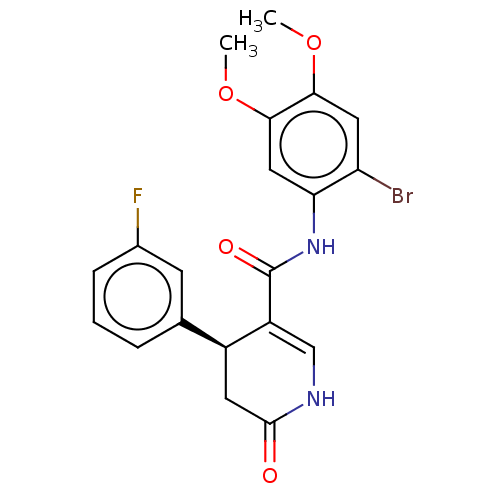 (CHEMBL3740237) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310730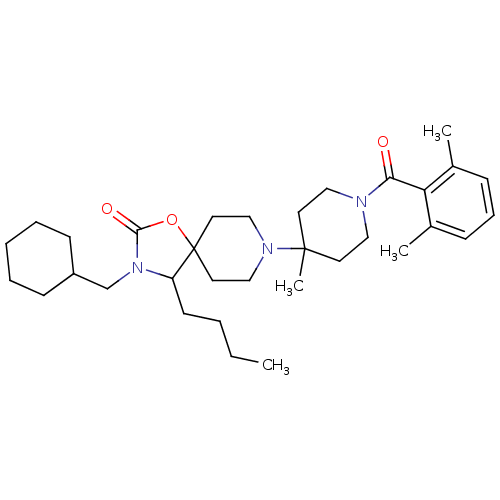 (4-butyl-3-(cyclohexylmethyl)-8-(1-(2,6-dimethylben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263567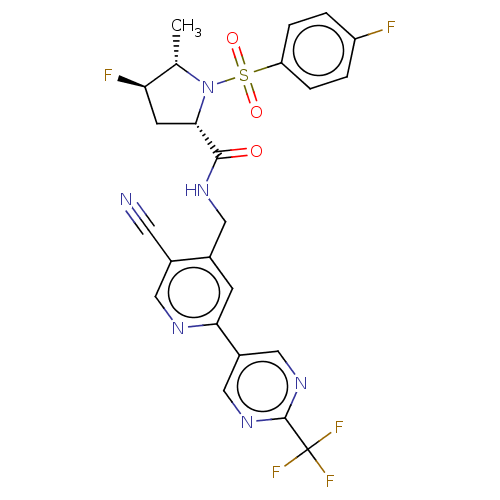 (CHEMBL4098855) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA , Beijing 100176 , P. R. China. Curated by ChEMBL | Assay Description Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by... | J Med Chem 61: 3641-3659 (2018) Article DOI: 10.1021/acs.jmedchem.8b00117 BindingDB Entry DOI: 10.7270/Q2VX0JZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310747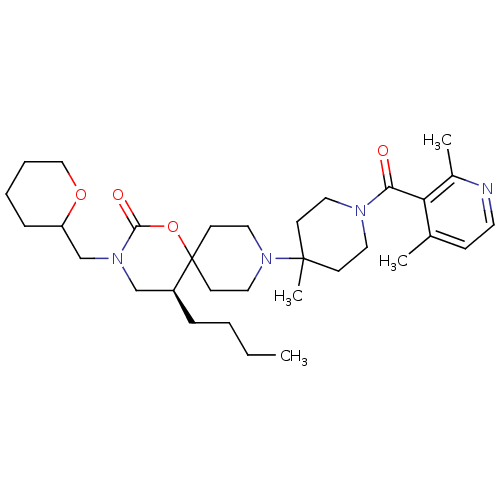 ((5S)-5-butyl-9-(1-(2,4-dimethylnicotinoyl)-4-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310744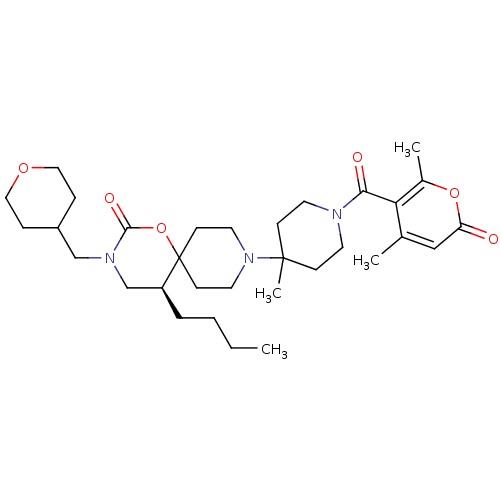 ((S)-5-butyl-9-(1-(4,6-dimethyl-2-oxo-2H-pyran-5-ca...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499508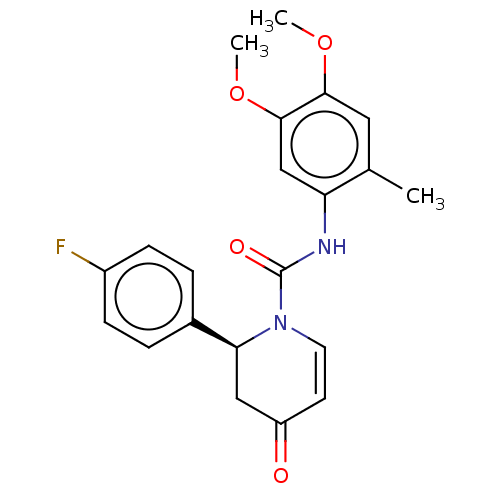 (CHEMBL3740363) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499492 (CHEMBL3740684) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263547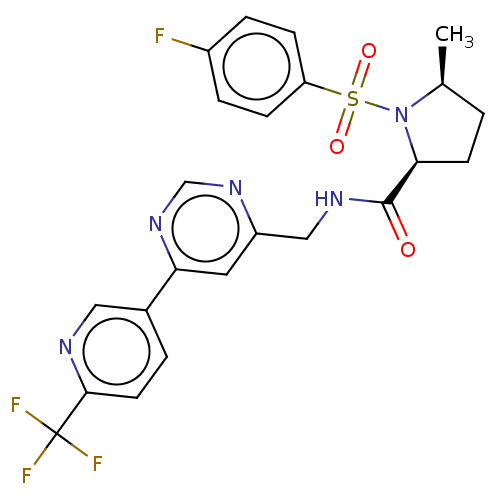 (CHEMBL4077957 | US11236046, Example 41) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA , Beijing 100176 , P. R. China. Curated by ChEMBL | Assay Description Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by... | J Med Chem 61: 3641-3659 (2018) Article DOI: 10.1021/acs.jmedchem.8b00117 BindingDB Entry DOI: 10.7270/Q2VX0JZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263536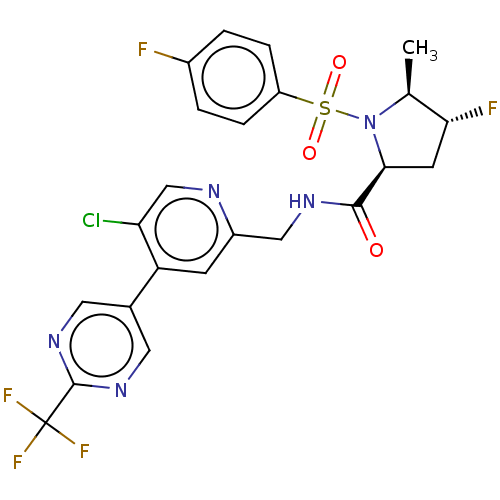 (CHEMBL4086464) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA , Beijing 100176 , P. R. China. Curated by ChEMBL | Assay Description Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by... | J Med Chem 61: 3641-3659 (2018) Article DOI: 10.1021/acs.jmedchem.8b00117 BindingDB Entry DOI: 10.7270/Q2VX0JZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM291170 (2-[N-(Propan-2-yl)(4-fluorobenzene)sulfonamido]-N-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 25 |
Hoffmann-La Roche Inc. US Patent | Assay Description IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e... | US Patent US9580411 (2017) BindingDB Entry DOI: 10.7270/Q25T3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482300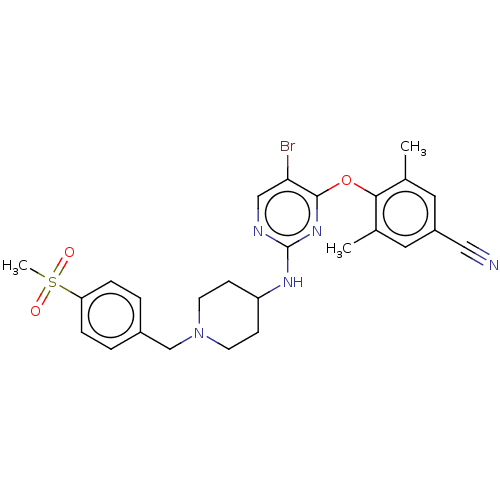 (CHEMBL1170386) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482304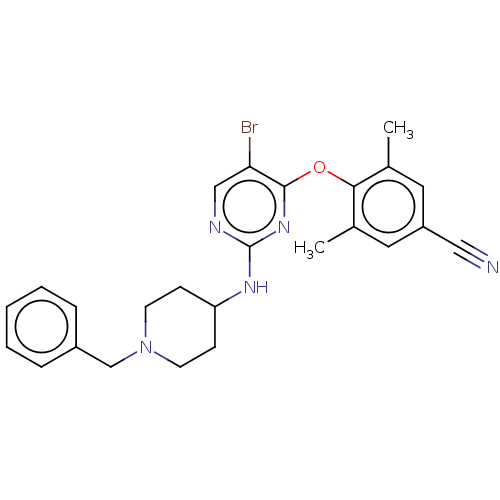 (CHEMBL1170190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499495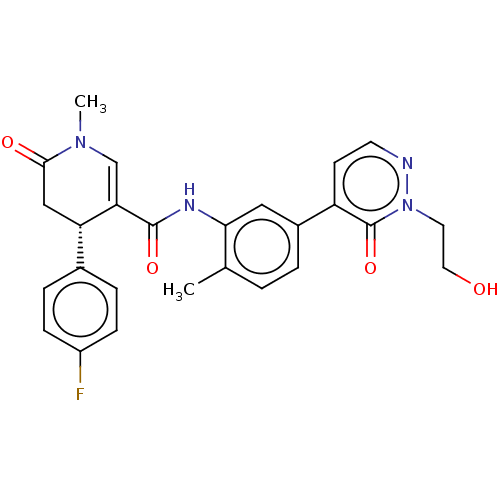 (CHEMBL3740645) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499488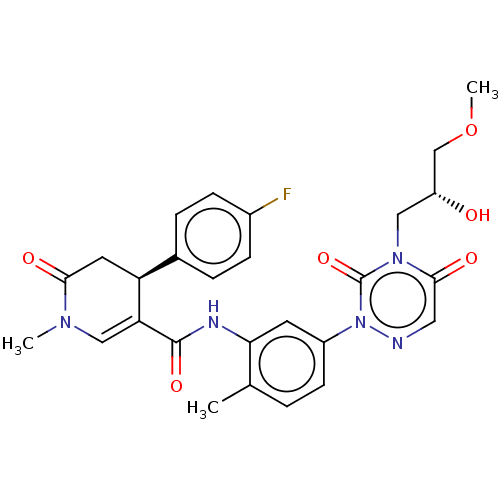 (CHEMBL3739741) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482308 (CHEMBL1169643) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310726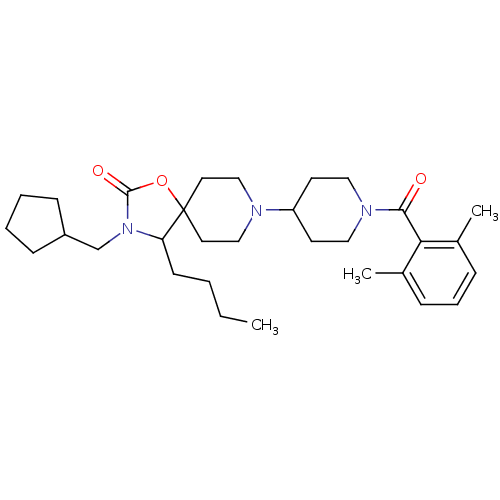 (4-butyl-3-(cyclopentylmethyl)-8-(1-(2,6-dimethylbe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482304 (CHEMBL1170190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499494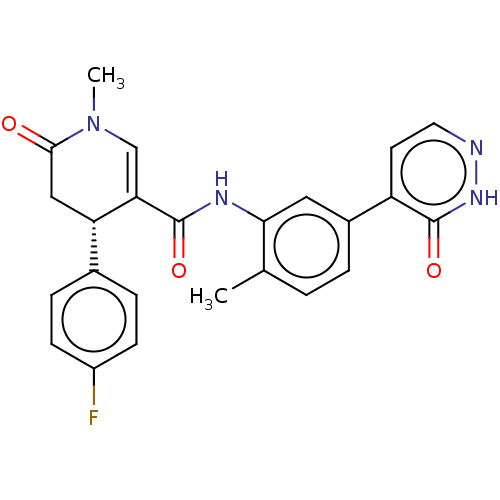 (CHEMBL3740130) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499489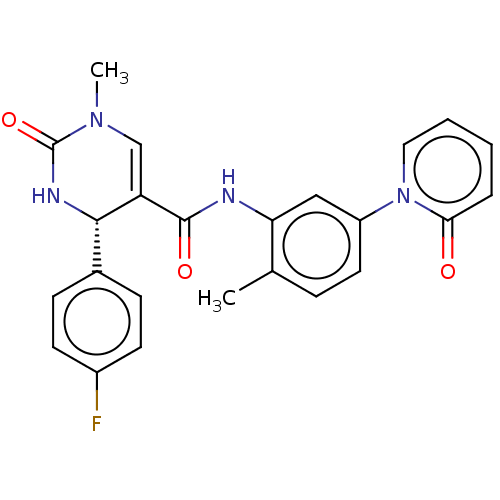 (CHEMBL3741681) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482308 (CHEMBL1169643) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482298 (CHEMBL1171403) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499489 (CHEMBL3741681) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482299 (CHEMBL1170387) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310743 ((S)-5-(4-(5-butyl-3-methyl-2-oxo-1-oxa-3,9-diazasp...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482301 (CHEMBL1170591) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50505177 (CHEMBL4117175) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of BTK in goat F(ab')2 anti-human IgM-stimulated human whole blood assessed as suppression of CD69 expression on B cells pretreated for 30... | Bioorg Med Chem Lett 29: 1074-1078 (2019) Article DOI: 10.1016/j.bmcl.2019.03.001 BindingDB Entry DOI: 10.7270/Q21G0QJM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499506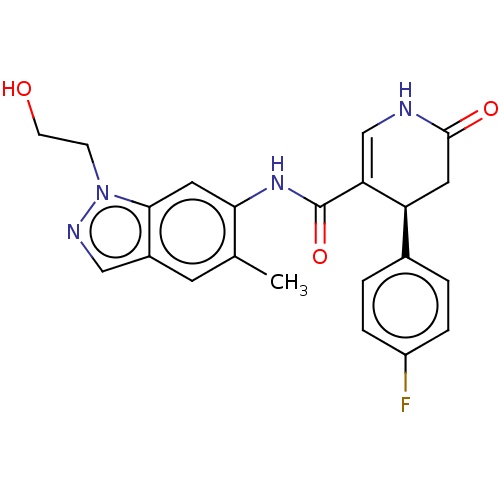 (CHEMBL3741276) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499490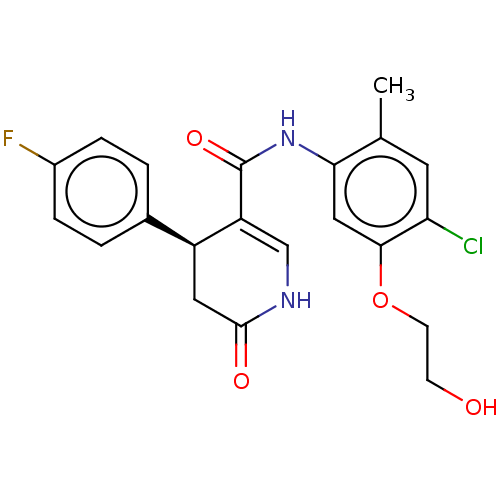 (CHEMBL3741992) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205082 (US9556147, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of BTK in goat F(ab')2 anti-human IgM-stimulated human whole blood assessed as suppression of CD69 expression on B cells pretreated for 30... | Bioorg Med Chem Lett 29: 1074-1078 (2019) Article DOI: 10.1016/j.bmcl.2019.03.001 BindingDB Entry DOI: 10.7270/Q21G0QJM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310742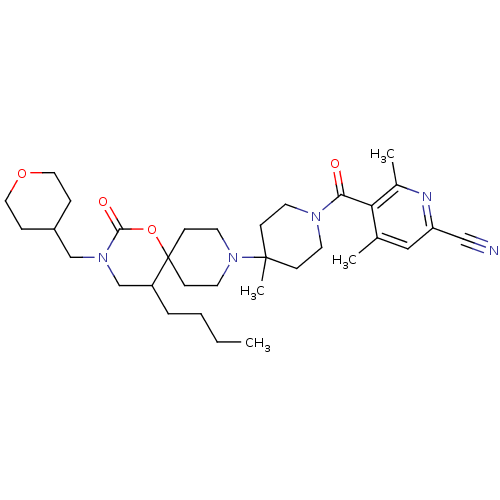 ((+/-)-5-(4-(5-butyl-2-oxo-3-((tetrahydro-2H-pyran-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310727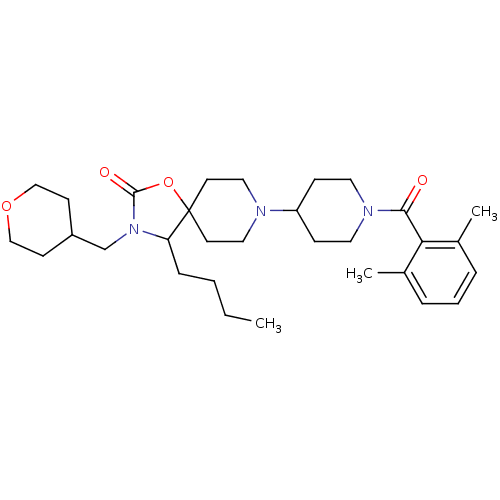 (4-butyl-8-(1-(2,6-dimethylbenzoyl)piperidin-4-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482310 (CHEMBL1170006) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 559 total ) | Next | Last >> |