 Found 1972 hits with Last Name = 'brown' and Initial = 'c'
Found 1972 hits with Last Name = 'brown' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B2 bradykinin receptor
(RAT) | BDBM50370083
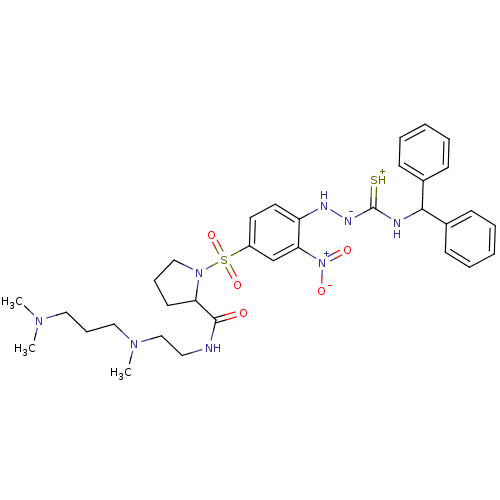
(CHEMBL1907651)Show SMILES CN(C)CCCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C33H44N8O5S2/c1-38(2)20-11-21-39(3)23-19-34-32(42)29-16-10-22-40(29)48(45,46)27-17-18-28(30(24-27)41(43)44)36-37-33(47)35-31(25-12-6-4-7-13-25)26-14-8-5-9-15-26/h4-9,12-15,17-18,24,29,31,36H,10-11,16,19-23H2,1-3H3,(H3,34,35,37,42,47) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50035179
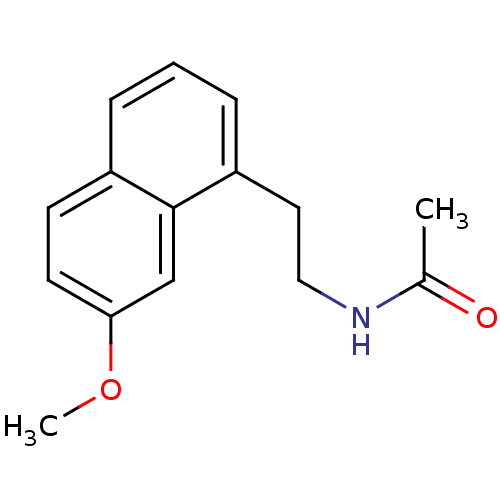
(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418268
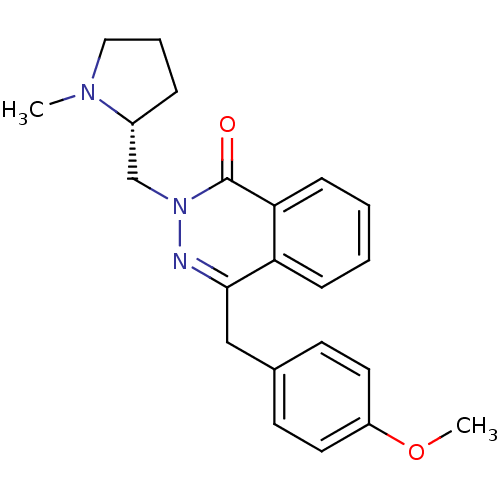
(CHEMBL1767137)Show SMILES COc1ccc(Cc2nn(C[C@H]3CCCN3C)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C22H25N3O2/c1-24-13-5-6-17(24)15-25-22(26)20-8-4-3-7-19(20)21(23-25)14-16-9-11-18(27-2)12-10-16/h3-4,7-12,17H,5-6,13-15H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50043289
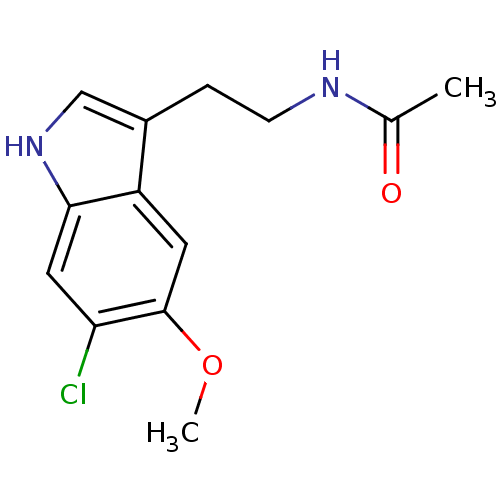
(CHEMBL34730 | N-[2-(6-Chloro-5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H15ClN2O2/c1-8(17)15-4-3-9-7-16-12-6-11(14)13(18-2)5-10(9)12/h5-7,16H,3-4H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM85064
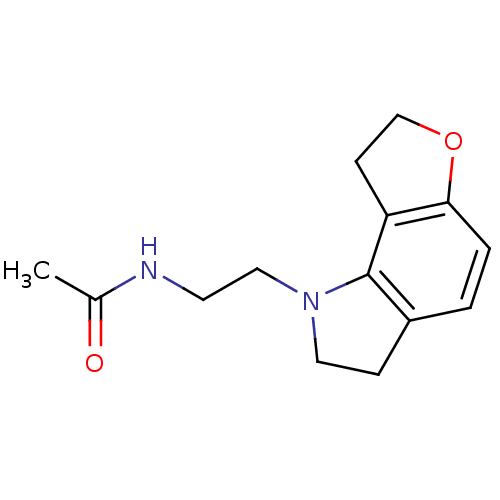
(CAS_5311134 | GR 196429 | NSC_5311134)Show InChI InChI=1S/C14H18N2O2/c1-10(17)15-6-8-16-7-4-11-2-3-13-12(14(11)16)5-9-18-13/h2-3H,4-9H2,1H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391708

(CHEMBL1767136)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C21H22ClN3O/c1-24-12-4-5-17(24)14-25-21(26)19-7-3-2-6-18(19)20(23-25)13-15-8-10-16(22)11-9-15/h2-3,6-11,17H,4-5,12-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418267

(CHEMBL1767138)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(O)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C21H23N3O2/c1-23-12-4-5-16(23)14-24-21(26)19-7-3-2-6-18(19)20(22-24)13-15-8-10-17(25)11-9-15/h2-3,6-11,16,25H,4-5,12-14H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM85064

(CAS_5311134 | GR 196429 | NSC_5311134)Show InChI InChI=1S/C14H18N2O2/c1-10(17)15-6-8-16-7-4-11-2-3-13-12(14(11)16)5-9-18-13/h2-3H,4-9H2,1H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391708

(CHEMBL1767136)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C21H22ClN3O/c1-24-12-4-5-17(24)14-25-21(26)19-7-3-2-6-18(19)20(23-25)13-15-8-10-16(22)11-9-15/h2-3,6-11,17H,4-5,12-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418266

(CHEMBL1767141)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(C)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C22H25N3O/c1-16-9-11-17(12-10-16)14-21-19-7-3-4-8-20(19)22(26)25(23-21)15-18-6-5-13-24(18)2/h3-4,7-12,18H,5-6,13-15H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418298
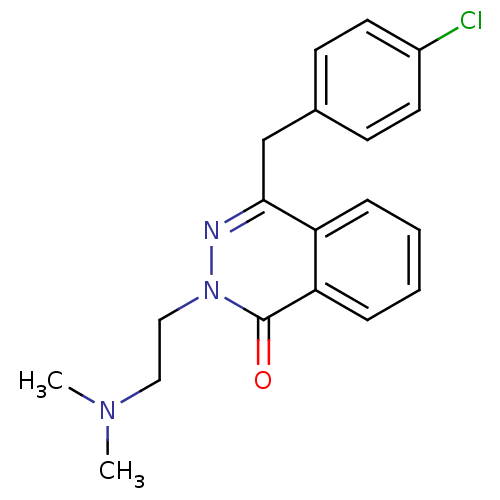
(CHEMBL1767134)Show InChI InChI=1S/C19H20ClN3O/c1-22(2)11-12-23-19(24)17-6-4-3-5-16(17)18(21-23)13-14-7-9-15(20)10-8-14/h3-10H,11-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50341447
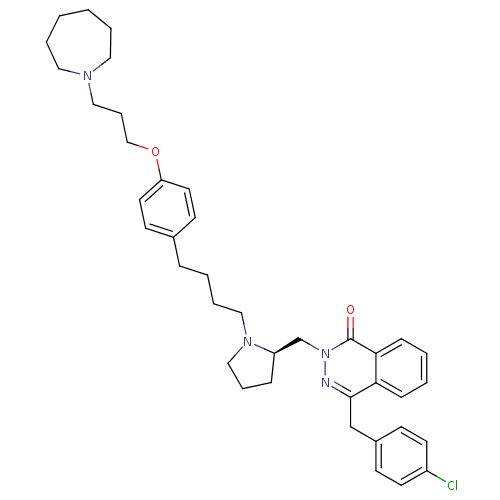
(4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...)Show SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C39H49ClN4O2/c40-33-19-15-32(16-20-33)29-38-36-13-3-4-14-37(36)39(45)44(41-38)30-34-12-9-27-43(34)26-8-5-11-31-17-21-35(22-18-31)46-28-10-25-42-23-6-1-2-7-24-42/h3-4,13-22,34H,1-2,5-12,23-30H2/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H3 receptor expressed in CHO cells assessed as inhibition of histamine-induced GTPgamma[S] binding by scintillation prox... |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391698
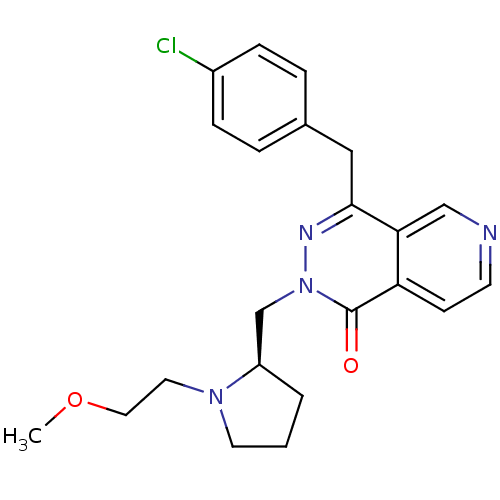
(CHEMBL2146801)Show SMILES COCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cnccc2c1=O |r| Show InChI InChI=1S/C22H25ClN4O2/c1-29-12-11-26-10-2-3-18(26)15-27-22(28)19-8-9-24-14-20(19)21(25-27)13-16-4-6-17(23)7-5-16/h4-9,14,18H,2-3,10-13,15H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418265
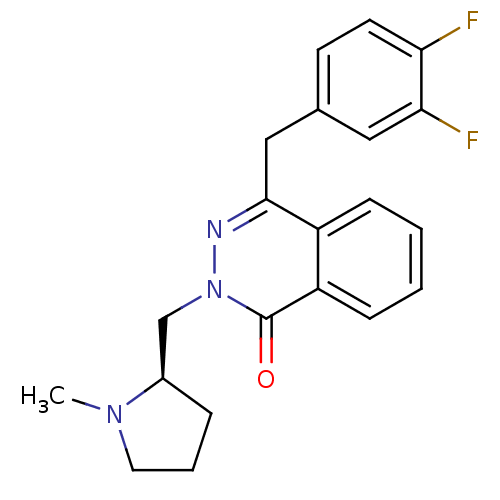
(CHEMBL1767149)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(F)c(F)c2)c2ccccc2c1=O |r| Show InChI InChI=1S/C21H21F2N3O/c1-25-10-4-5-15(25)13-26-21(27)17-7-3-2-6-16(17)20(24-26)12-14-8-9-18(22)19(23)11-14/h2-3,6-9,11,15H,4-5,10,12-13H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370077
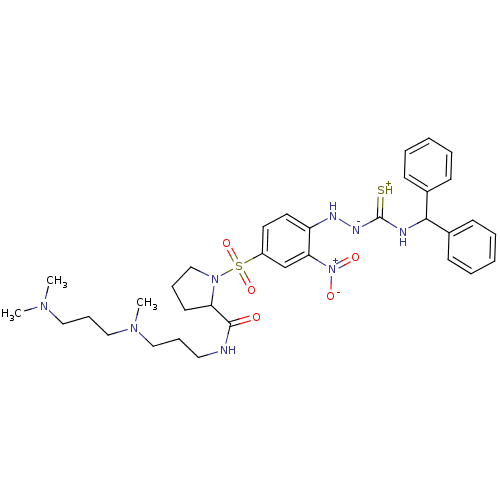
(CHEMBL1907652)Show SMILES CN(C)CCCN(C)CCCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H46N8O5S2/c1-39(2)21-12-23-40(3)22-11-20-35-33(43)30-17-10-24-41(30)49(46,47)28-18-19-29(31(25-28)42(44)45)37-38-34(48)36-32(26-13-6-4-7-14-26)27-15-8-5-9-16-27/h4-9,13-16,18-19,25,30,32,37H,10-12,17,20-24H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418297
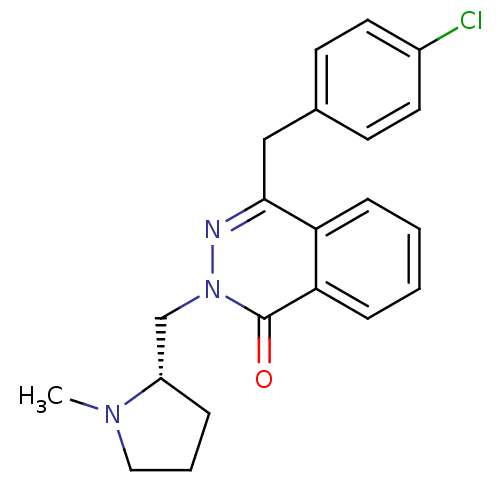
(CHEMBL1767154)Show SMILES CN1CCC[C@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C21H22ClN3O/c1-24-12-4-5-17(24)14-25-21(26)19-7-3-2-6-18(19)20(23-25)13-15-8-10-16(22)11-9-15/h2-3,6-11,17H,4-5,12-14H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391702
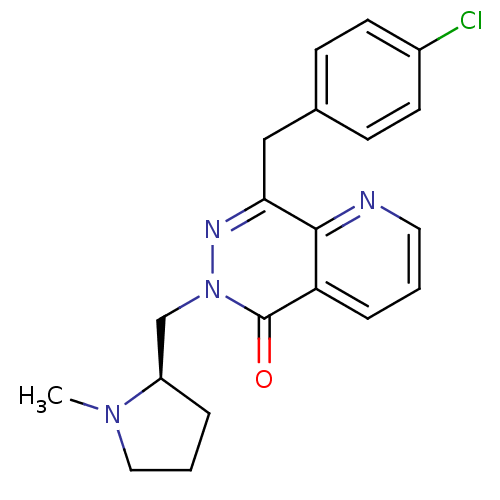
(CHEMBL2146809)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ncccc2c1=O |r| Show InChI InChI=1S/C20H21ClN4O/c1-24-11-3-4-16(24)13-25-20(26)17-5-2-10-22-19(17)18(23-25)12-14-6-8-15(21)9-7-14/h2,5-10,16H,3-4,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418296

(CHEMBL1767140)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(F)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C21H22FN3O/c1-24-12-4-5-17(24)14-25-21(26)19-7-3-2-6-18(19)20(23-25)13-15-8-10-16(22)11-9-15/h2-3,6-11,17H,4-5,12-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM85064

(CAS_5311134 | GR 196429 | NSC_5311134)Show InChI InChI=1S/C14H18N2O2/c1-10(17)15-6-8-16-7-4-11-2-3-13-12(14(11)16)5-9-18-13/h2-3H,4-9H2,1H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391707
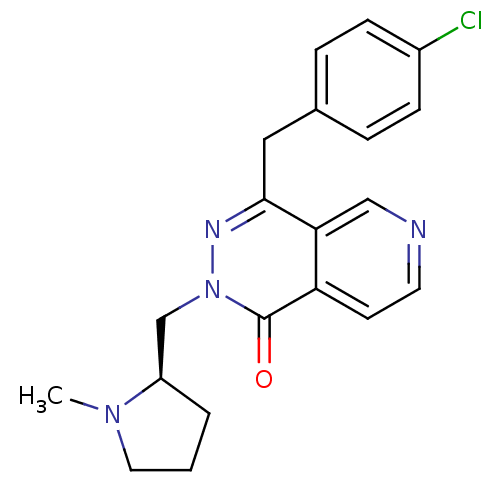
(CHEMBL2146805)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cnccc2c1=O |r| Show InChI InChI=1S/C20H21ClN4O/c1-24-10-2-3-16(24)13-25-20(26)17-8-9-22-12-18(17)19(23-25)11-14-4-6-15(21)7-5-14/h4-9,12,16H,2-3,10-11,13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228318
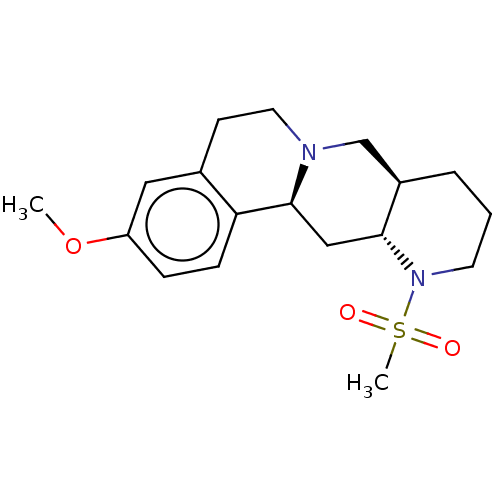
(CHEMBL279807)Show SMILES [H][C@]12CCCN([C@]1([H])C[C@]1([H])N(CCc3cc(OC)ccc13)C2)S(C)(=O)=O Show InChI InChI=1S/C18H26N2O3S/c1-23-15-5-6-16-13(10-15)7-9-19-12-14-4-3-8-20(24(2,21)22)17(14)11-18(16)19/h5-6,10,14,17-18H,3-4,7-9,11-12H2,1-2H3/t14-,17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228318

(CHEMBL279807)Show SMILES [H][C@]12CCCN([C@]1([H])C[C@]1([H])N(CCc3cc(OC)ccc13)C2)S(C)(=O)=O Show InChI InChI=1S/C18H26N2O3S/c1-23-15-5-6-16-13(10-15)7-9-19-12-14-4-3-8-20(24(2,21)22)17(14)11-18(16)19/h5-6,10,14,17-18H,3-4,7-9,11-12H2,1-2H3/t14-,17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2034-6 (1989)
BindingDB Entry DOI: 10.7270/Q2WH2S78 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391699
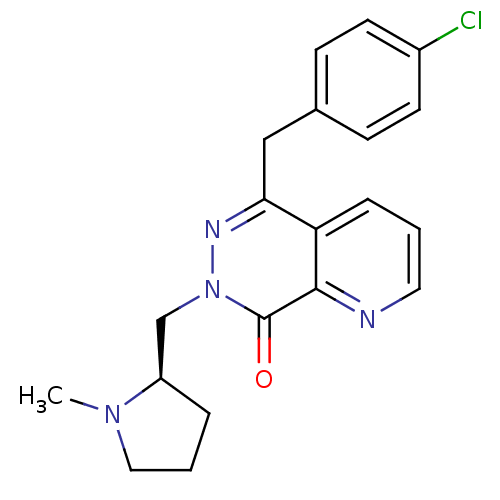
(CHEMBL2146484)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cccnc2c1=O |r| Show InChI InChI=1S/C20H21ClN4O/c1-24-11-3-4-16(24)13-25-20(26)19-17(5-2-10-22-19)18(23-25)12-14-6-8-15(21)9-7-14/h2,5-10,16H,3-4,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391697
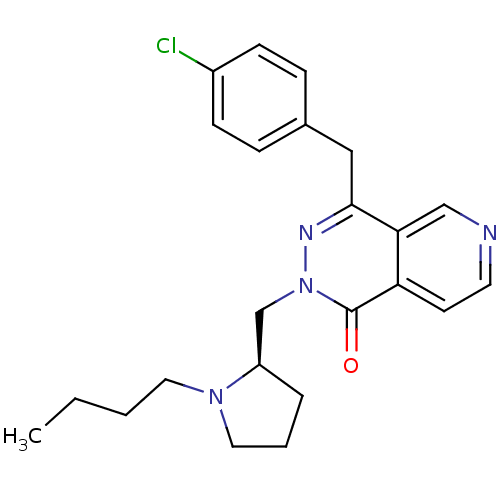
(CHEMBL2146806)Show SMILES CCCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cnccc2c1=O |r| Show InChI InChI=1S/C23H27ClN4O/c1-2-3-12-27-13-4-5-19(27)16-28-23(29)20-10-11-25-15-21(20)22(26-28)14-17-6-8-18(24)9-7-17/h6-11,15,19H,2-5,12-14,16H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50035179

(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50086706
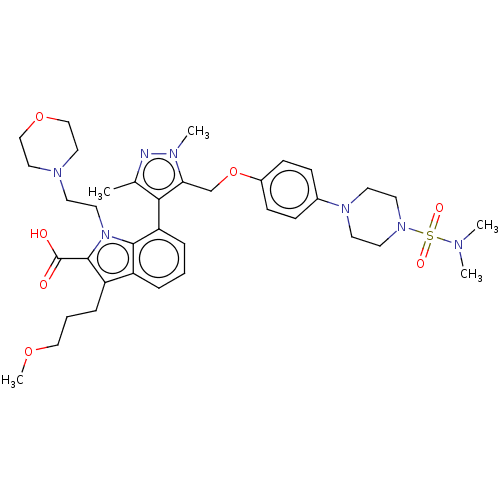
(CHEMBL3426335)Show SMILES COCCCc1c(C(O)=O)n(CCN2CCOCC2)c2c(cccc12)-c1c(C)nn(C)c1COc1ccc(cc1)N1CCN(CC1)S(=O)(=O)N(C)C |(6.12,5.96,;5.74,4.79,;4.23,4.48,;3.75,3.01,;2.24,2.7,;1.76,1.24,;2.66,.02,;4.2,.04,;4.83,-1.02,;4.8,1.11,;1.76,-1.24,;2.24,-2.7,;3.75,-3.01,;4.24,-4.47,;5.75,-4.78,;6.23,-6.25,;5.2,-7.4,;3.69,-7.08,;3.21,-5.62,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;.3,.77,;-1.02,-3.09,;.23,-3.96,;1.4,-3.56,;-.23,-5.43,;-1.77,-5.45,;-2.48,-6.45,;-2.26,-3.99,;-3.73,-3.52,;-4.87,-4.56,;-6.33,-4.09,;-7.48,-5.12,;-8.94,-4.65,;-9.27,-3.14,;-8.12,-2.11,;-6.66,-2.58,;-10.73,-2.67,;-11.88,-3.7,;-13.34,-3.23,;-13.66,-1.72,;-12.52,-.69,;-11.05,-1.16,;-15.13,-1.25,;-15.39,-.04,;-16.3,-.87,;-16.27,-2.28,;-17.45,-1.9,;-16.02,-3.48,)| Show InChI InChI=1S/C37H51N7O7S/c1-27-34(33(40(4)38-27)26-51-29-13-11-28(12-14-29)42-16-18-43(19-17-42)52(47,48)39(2)3)32-9-6-8-30-31(10-7-23-49-5)36(37(45)46)44(35(30)32)20-15-41-21-24-50-25-22-41/h6,8-9,11-14H,7,10,15-26H2,1-5H3,(H,45,46) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.454 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged Mcl-1 (unknown origin) incubated for 60 mins by TR-FRET-binding affinity assay |
J Med Chem 58: 3794-805 (2015)
Article DOI: 10.1021/jm501984f
BindingDB Entry DOI: 10.7270/Q24X59HH |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229080
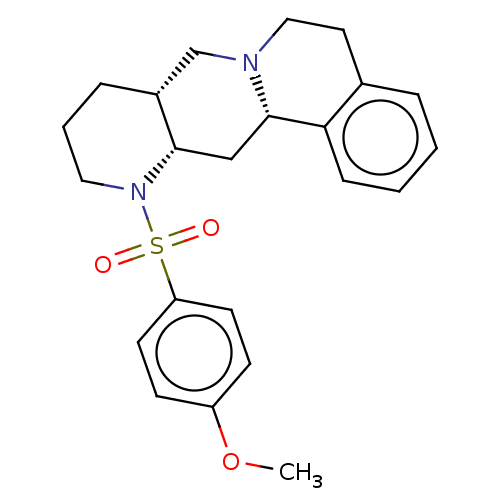
(CHEMBL349665)Show SMILES [H][C@]12CCCN([C@@]1([H])C[C@]1([H])N(CCc3ccccc13)C2)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C23H28N2O3S/c1-28-19-8-10-20(11-9-19)29(26,27)25-13-4-6-18-16-24-14-12-17-5-2-3-7-21(17)23(24)15-22(18)25/h2-3,5,7-11,18,22-23H,4,6,12-16H2,1H3/t18-,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane |
J Med Chem 34: 705-17 (1991)
BindingDB Entry DOI: 10.7270/Q2MW2KBF |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(GUINEA PIG) | BDBM50043289

(CHEMBL34730 | N-[2-(6-Chloro-5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H15ClN2O2/c1-8(17)15-4-3-9-7-16-12-6-11(14)13(18-2)5-10(9)12/h5-7,16H,3-4H2,1-2H3,(H,15,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50113263
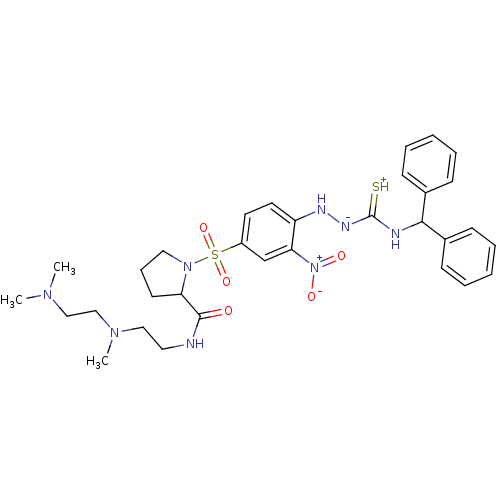
((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H3,33,34,36,41,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50409120
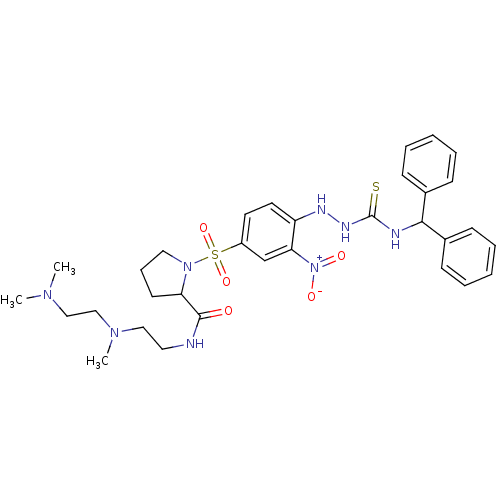
(CHEMBL2112044 | CHEMBL2112937)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(NN\C([S-])=[NH+]\C(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H,33,41)(H2,34,36,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes |
J Med Chem 43: 769-71 (2000)
BindingDB Entry DOI: 10.7270/Q2XD10W5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418295
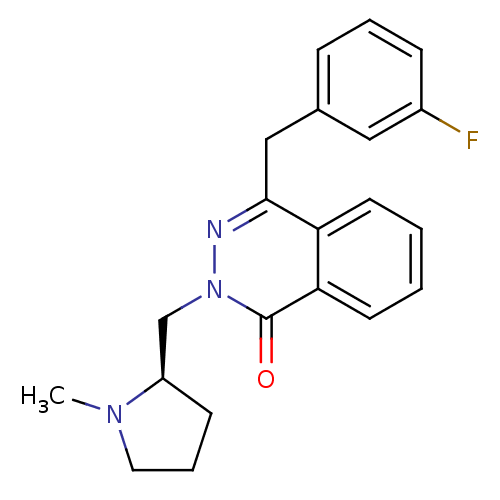
(CHEMBL1767145)Show SMILES CN1CCC[C@@H]1Cn1nc(Cc2cccc(F)c2)c2ccccc2c1=O |r| Show InChI InChI=1S/C21H22FN3O/c1-24-11-5-8-17(24)14-25-21(26)19-10-3-2-9-18(19)20(23-25)13-15-6-4-7-16(22)12-15/h2-4,6-7,9-10,12,17H,5,8,11,13-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391710
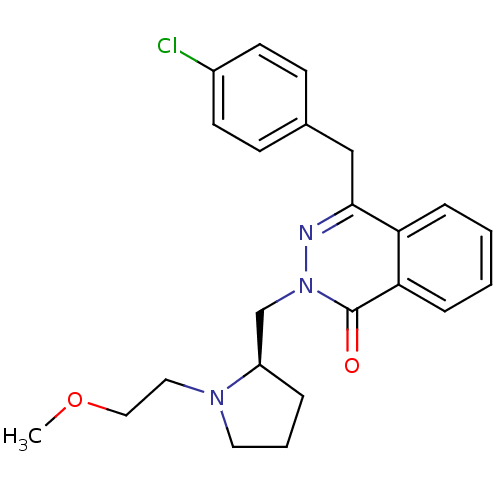
(CHEMBL2146803)Show SMILES COCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O |r| Show InChI InChI=1S/C23H26ClN3O2/c1-29-14-13-26-12-4-5-19(26)16-27-23(28)21-7-3-2-6-20(21)22(25-27)15-17-8-10-18(24)11-9-17/h2-3,6-11,19H,4-5,12-16H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50391704
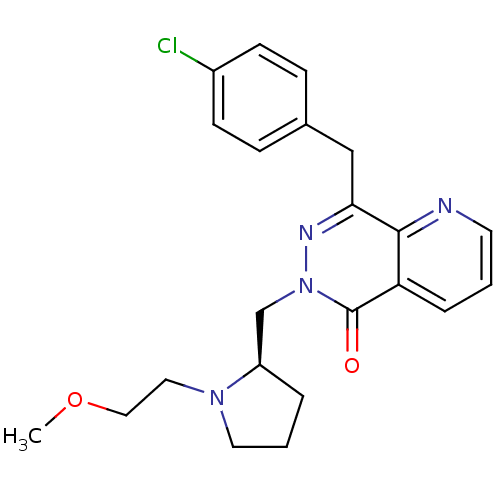
(CHEMBL2146811)Show SMILES COCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2ncccc2c1=O |r| Show InChI InChI=1S/C22H25ClN4O2/c1-29-13-12-26-11-3-4-18(26)15-27-22(28)19-5-2-10-24-21(19)20(25-27)14-16-6-8-17(23)9-7-16/h2,5-10,18H,3-4,11-15H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM82509
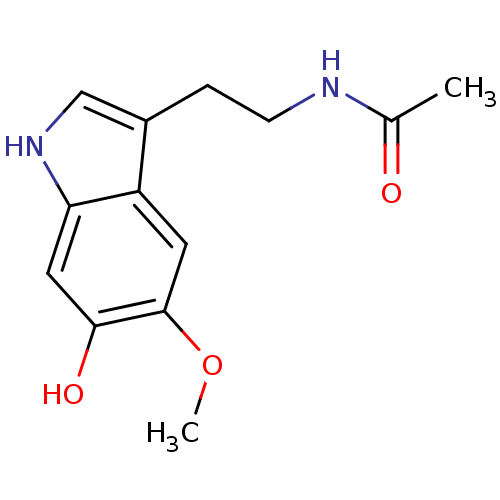
(Melatonin,6-Hydroxy | melatonin, 6-Hydroxy)Show InChI InChI=1S/C13H16N2O3/c1-8(16)14-4-3-9-7-15-11-6-12(17)13(18-2)5-10(9)11/h5-7,15,17H,3-4H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50391696
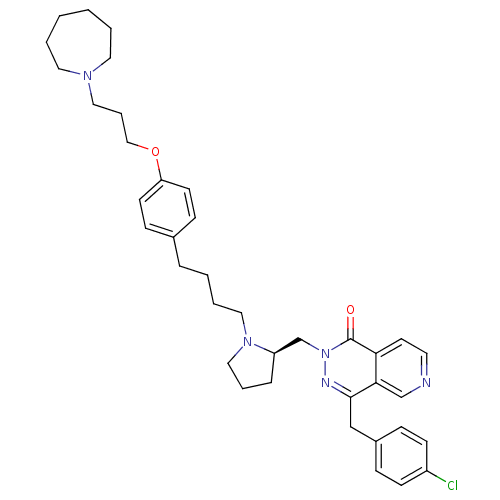
(CHEMBL2146813)Show SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccncc23)cc1 |r| Show InChI InChI=1S/C38H48ClN5O2/c39-32-15-11-31(12-16-32)27-37-36-28-40-20-19-35(36)38(45)44(41-37)29-33-10-7-25-43(33)24-6-3-9-30-13-17-34(18-14-30)46-26-8-23-42-21-4-1-2-5-22-42/h11-20,28,33H,1-10,21-27,29H2/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human H3 receptor expressed in CHO cells assessed as inhibition of histamine-induced GTPgamma[S] binding by scintillation prox... |
Bioorg Med Chem 20: 6097-108 (2012)
Article DOI: 10.1016/j.bmc.2012.08.032
BindingDB Entry DOI: 10.7270/Q2NG4RQC |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50113263

((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H3,33,34,36,41,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50409120

(CHEMBL2112044 | CHEMBL2112937)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(NN\C([S-])=[NH+]\C(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H,33,41)(H2,34,36,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes |
J Med Chem 43: 769-71 (2000)
BindingDB Entry DOI: 10.7270/Q2XD10W5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data