

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM254888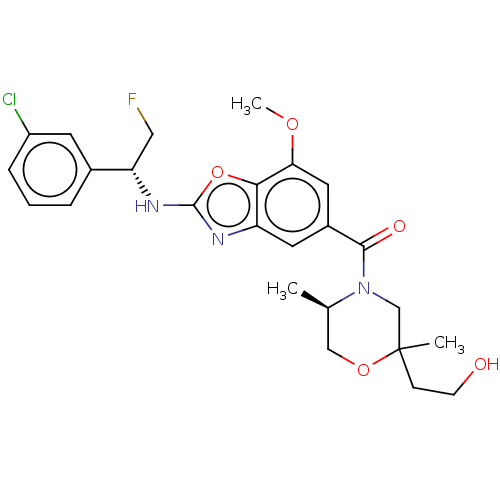 (US9493472, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254889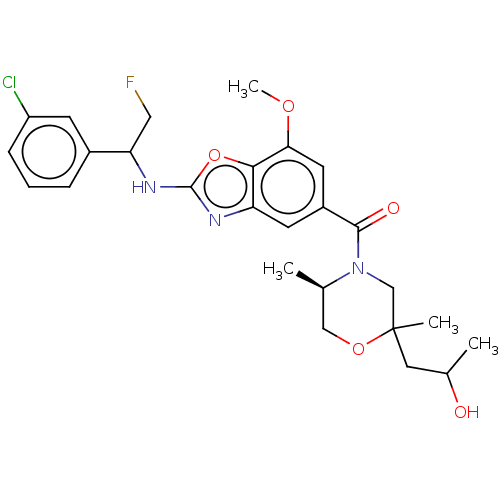 (US9493472, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254905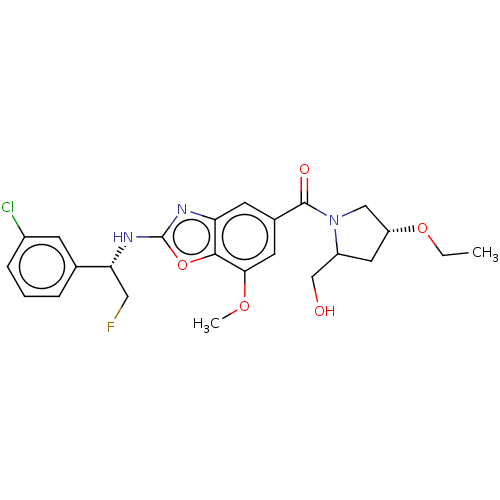 (US9493472, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254904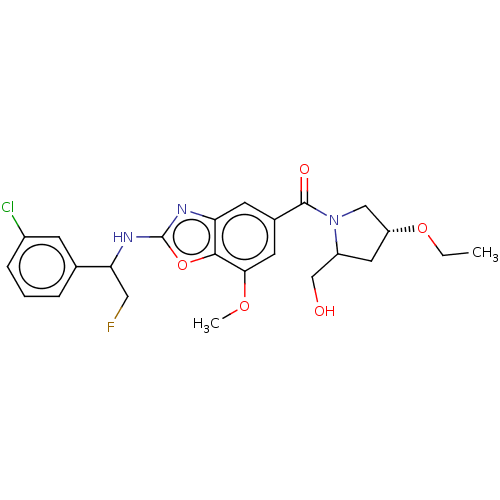 (US9493472, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50443853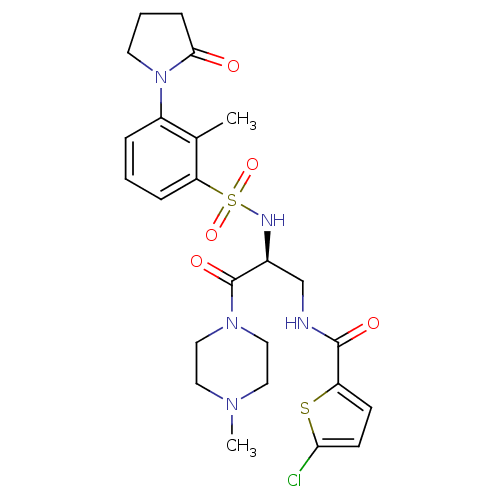 (CHEMBL3091519 | US20230391761, Reference 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254886 (US9493472, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227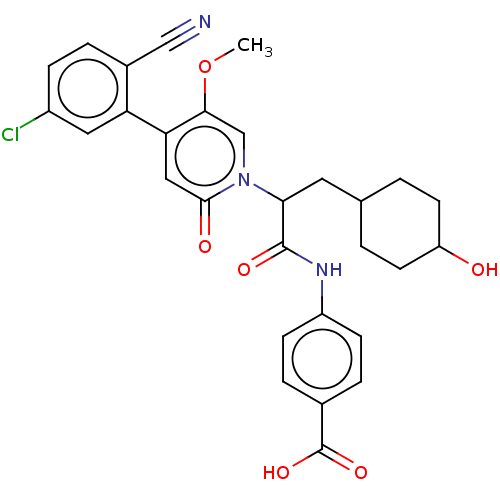 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254896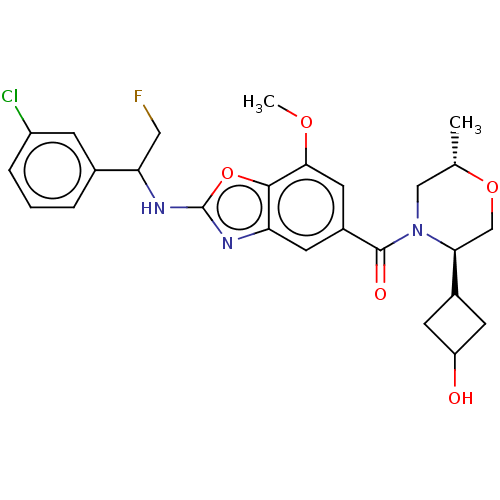 (US9493472, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254895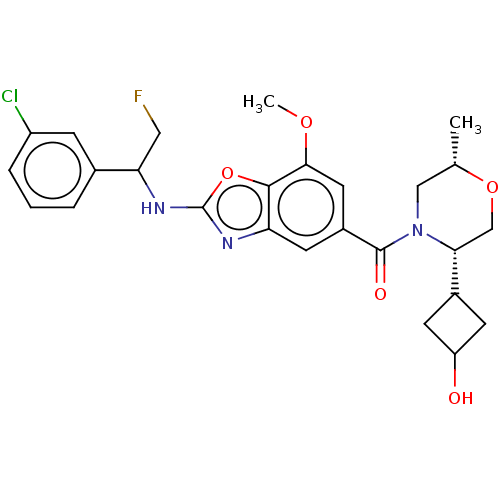 (US9493472, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254911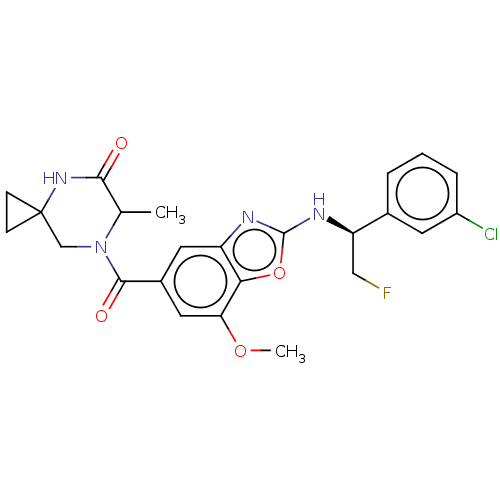 (US9493472, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443853 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639340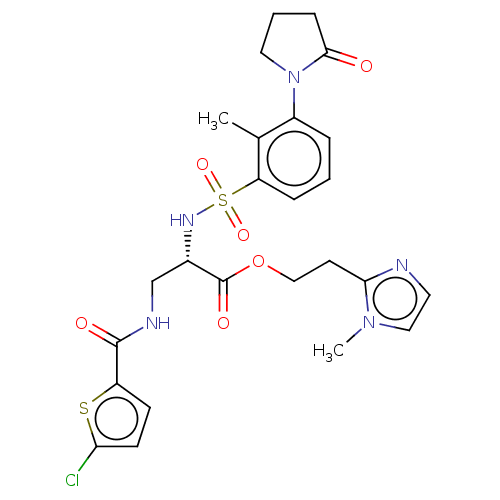 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254894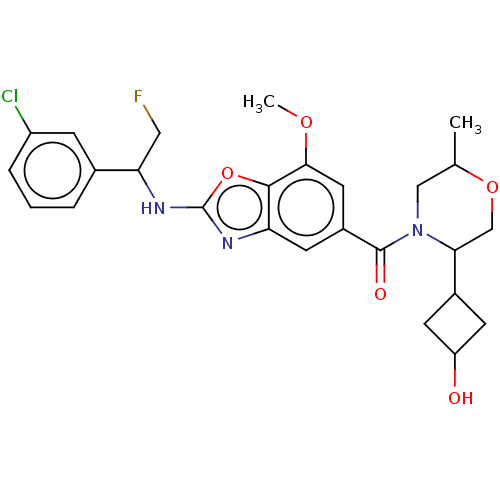 (US9493472, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639343 (3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639349 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639321 (Methyl 3-{[(5-chloro-2-thienyl)carbonyl]amino}-N-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM341292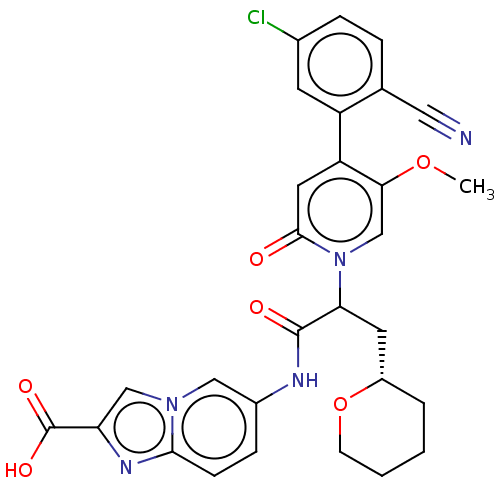 (6-({2-[4-(5-Chloro-2-cyanophenyl)-5-methoxy-2-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9765070 (2017) BindingDB Entry DOI: 10.7270/Q25D8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639349 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639346 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639322 (Methyl 3-[(5-chlorothiophene-2-carbonyl)amino]-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907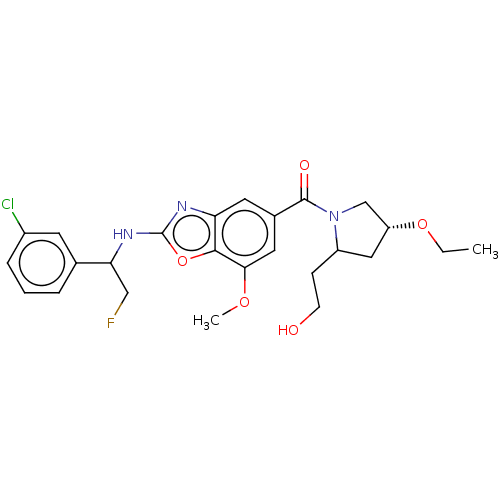 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639349 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246220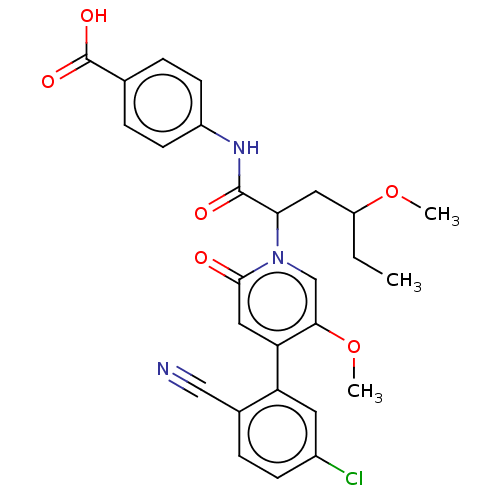 (US10183932, Example 144 | US9434690, 125 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246220 (US10183932, Example 144 | US9434690, 125 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246220 (US10183932, Example 144 | US9434690, 125 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639344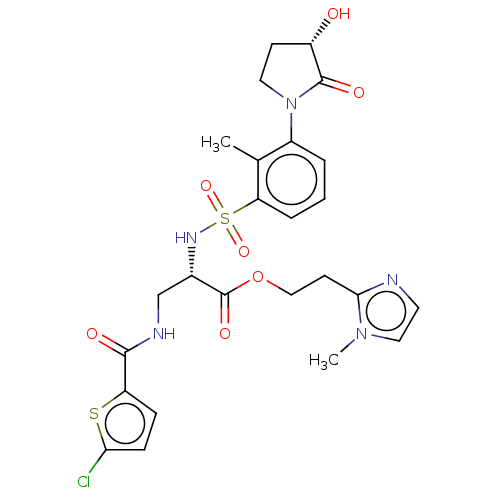 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639342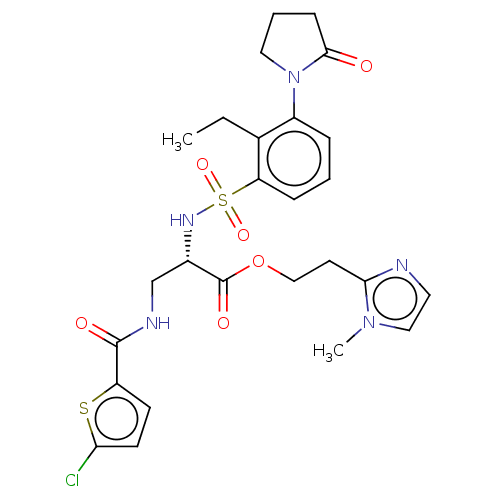 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639349 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639346 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639338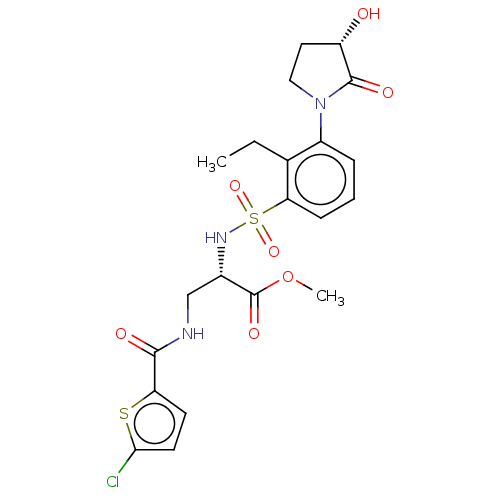 (Methyl 3-[(5-chlorothiophene-2-carbonyl)amino]-N-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254910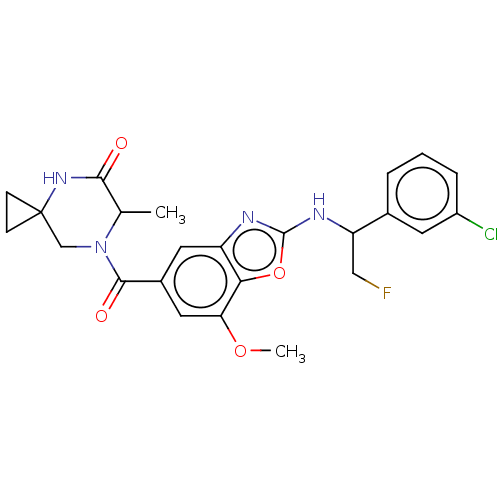 (US9493472, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246174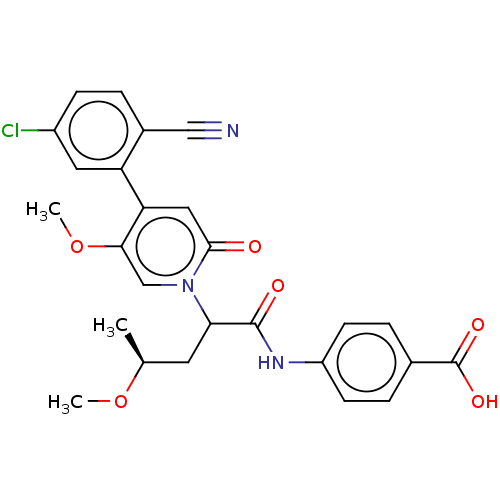 (US10183932, Example 81 | US9434690, 79 | US9434690...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246174 (US10183932, Example 81 | US9434690, 79 | US9434690...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246174 (US10183932, Example 81 | US9434690, 79 | US9434690...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639349 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254892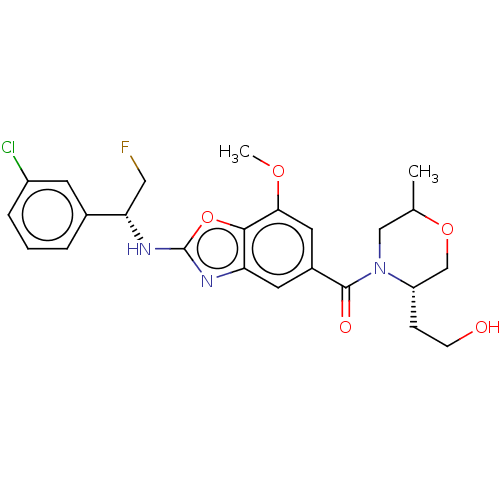 (US9493472, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM639342 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639339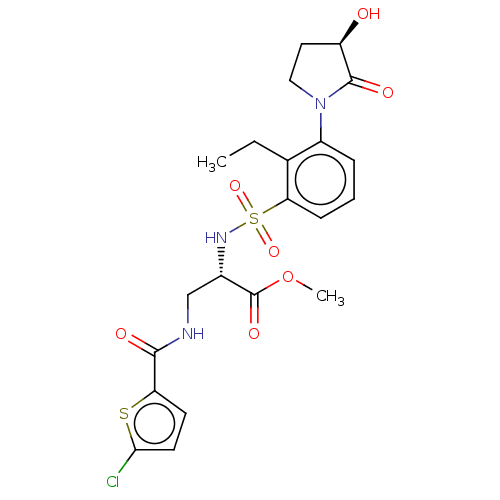 (Methyl 3-[(5-chlorothiophene-2-carbonyl)amino]-N-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639349 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639349 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM639348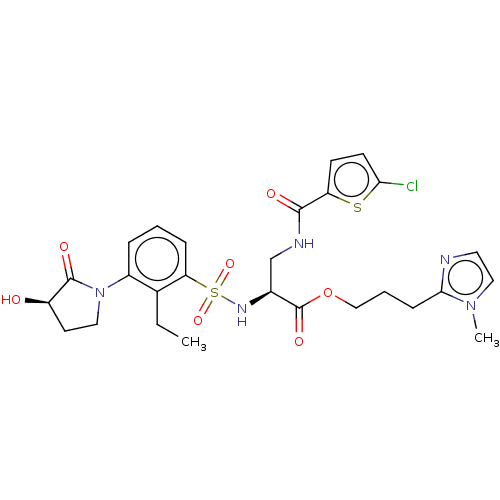 (3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246249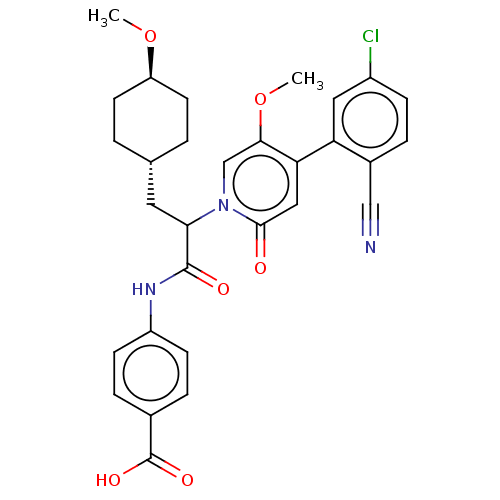 (US10183932, Example 157 | US9434690, 155 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246249 (US10183932, Example 157 | US9434690, 155 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM285517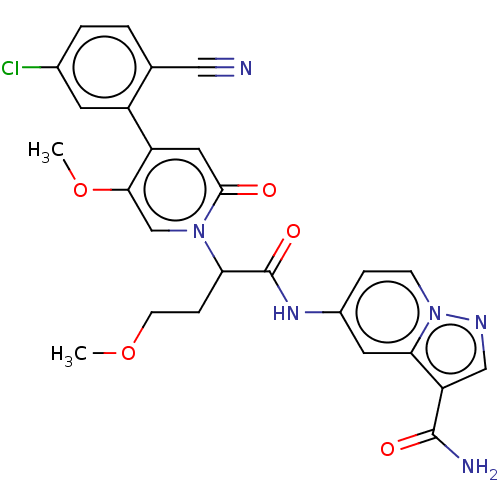 (5-({2-[4-(5-Chloro-2-cyanophenyl)-5-methoxy-2-oxop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description To determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reac... | US Patent US10077265 (2018) BindingDB Entry DOI: 10.7270/Q2XS5XFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246249 (US10183932, Example 157 | US9434690, 155 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1498 total ) | Next | Last >> |