 Found 227 hits with Last Name = 'calo' and Initial = 'g'
Found 227 hits with Last Name = 'calo' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM85822
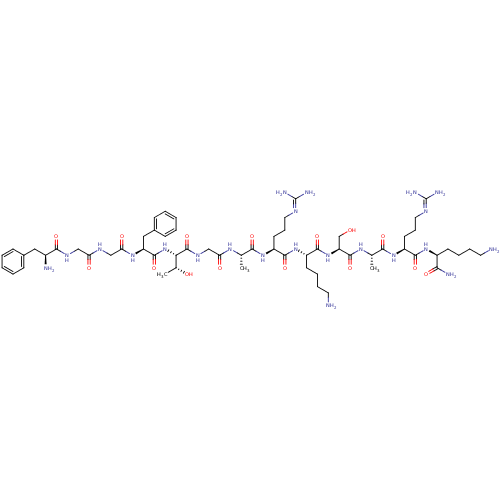
(Demethyl-AMPA | OFQ/N | OFQ/N (1-11) | OFQ/N (1-11...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50181392
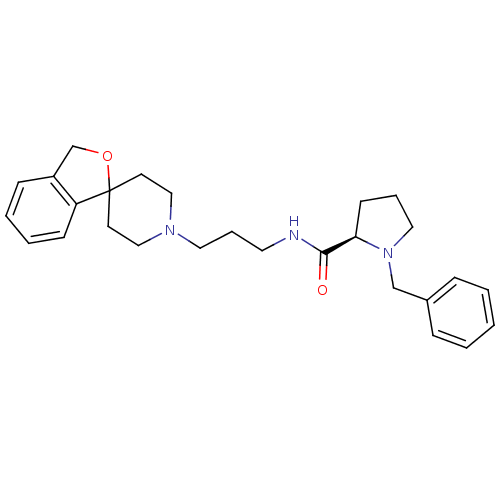
((R)-N-(3-(3H-spiro[isobenzofuran-1,4'-piperidine]-...)Show SMILES O=C(NCCCN1CCC2(CC1)OCc1ccccc21)[C@H]1CCCN1Cc1ccccc1 Show InChI InChI=1S/C27H35N3O2/c31-26(25-12-6-17-30(25)20-22-8-2-1-3-9-22)28-15-7-16-29-18-13-27(14-19-29)24-11-5-4-10-23(24)21-32-27/h1-5,8-11,25H,6-7,12-21H2,(H,28,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143182
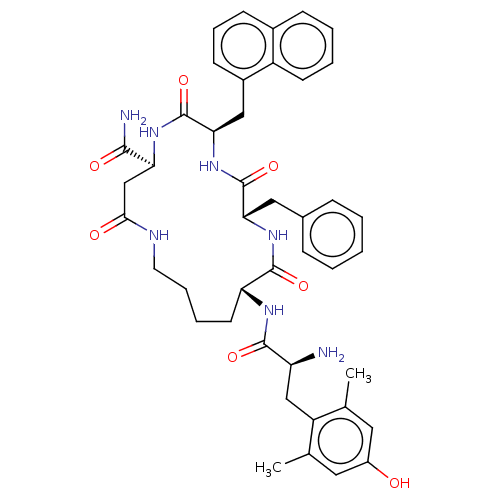
(CHEMBL3759167)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2cccc3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-19-30(51)20-26(2)32(25)23-33(44)40(54)47-34-17-8-9-18-46-38(52)24-35(39(45)53)48-43(57)37(22-29-15-10-14-28-13-6-7-16-31(28)29)50-42(56)36(49-41(34)55)21-27-11-4-3-5-12-27/h3-7,10-16,19-20,33-37,51H,8-9,17-18,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,57)(H,49,55)(H,50,56)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50359546

(CHEMBL1927270)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C37H45N7O7/c38-27(19-25-14-16-26(45)17-15-25)34(48)41-28-13-7-8-18-40-32(46)22-29(33(39)47)42-36(50)30(20-23-9-3-1-4-10-23)44-37(51)31(43-35(28)49)21-24-11-5-2-6-12-24/h1-6,9-12,14-17,27-31,45H,7-8,13,18-22,38H2,(H2,39,47)(H,40,46)(H,41,48)(H,42,50)(H,43,49)(H,44,51)/t27-,28+,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50148259
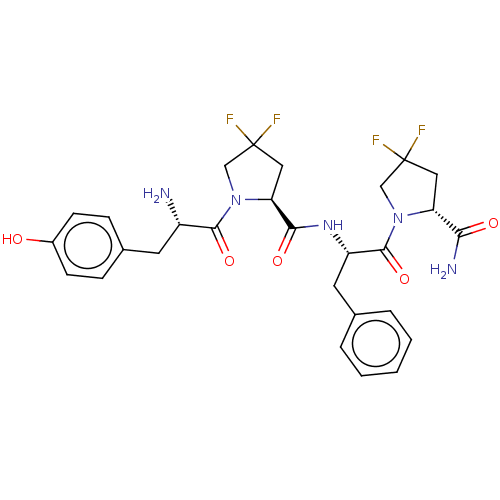
(CHEMBL3765406)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CC(F)(F)C[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CC(F)(F)C[C@@H]1C(N)=O |r| Show InChI InChI=1S/C28H31F4N5O5/c29-27(30)12-21(23(34)39)36(14-27)26(42)20(11-16-4-2-1-3-5-16)35-24(40)22-13-28(31,32)15-37(22)25(41)19(33)10-17-6-8-18(38)9-7-17/h1-9,19-22,38H,10-15,33H2,(H2,34,39)(H,35,40)/t19-,20-,21+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenate by scintillation counting analysis |
Bioorg Med Chem 24: 1582-8 (2016)
Article DOI: 10.1016/j.bmc.2016.02.034
BindingDB Entry DOI: 10.7270/Q2Z321H7 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143183

(CHEMBL3759179)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-18-31(51)19-26(2)32(25)23-33(44)40(54)47-34-14-8-9-17-46-38(52)24-35(39(45)53)48-42(56)37(22-28-15-16-29-12-6-7-13-30(29)20-28)50-43(57)36(49-41(34)55)21-27-10-4-3-5-11-27/h3-7,10-13,15-16,18-20,33-37,51H,8-9,14,17,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,55)(H,50,57)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from human recombinant mu-type opioid receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(GUINEA PIG) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50418594
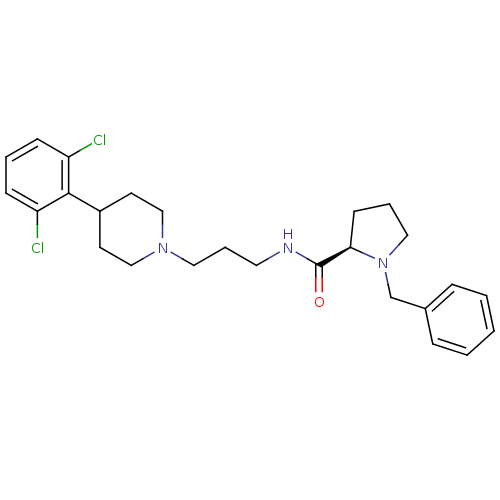
(CHEMBL1783826)Show SMILES Clc1cccc(Cl)c1C1CCN(CCCNC(=O)[C@H]2CCCN2Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C26H33Cl2N3O/c27-22-9-4-10-23(28)25(22)21-12-17-30(18-13-21)15-6-14-29-26(32)24-11-5-16-31(24)19-20-7-2-1-3-8-20/h1-4,7-10,21,24H,5-6,11-19H2,(H,29,32)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50237596

(CHEMBL4099423)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C39H49N7O7/c1-41-31(21-27-16-18-28(47)19-17-27)37(51)43-29-15-9-10-20-42-34(48)24-30(35(40)49)44-38(52)33(23-26-13-7-4-8-14-26)46(2)39(53)32(45-36(29)50)22-25-11-5-3-6-12-25/h3-8,11-14,16-19,29-33,41,47H,9-10,15,20-24H2,1-2H3,(H2,40,49)(H,42,48)(H,43,51)(H,44,52)(H,45,50)/t29-,30+,31+,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates after 180 mins by liquid scintillation counting analysis |
Bioorg Med Chem Lett 27: 1644-1648 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.016
BindingDB Entry DOI: 10.7270/Q2BG2R9F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50143183

(CHEMBL3759179)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-18-31(51)19-26(2)32(25)23-33(44)40(54)47-34-14-8-9-17-46-38(52)24-35(39(45)53)48-42(56)37(22-28-15-16-29-12-6-7-13-30(29)20-28)50-43(57)36(49-41(34)55)21-27-10-4-3-5-11-27/h3-7,10-13,15-16,18-20,33-37,51H,8-9,14,17,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,55)(H,50,57)/t33-,34+,35-,36-,37+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]nor-BNI from KOR in Dunkin Hartley guinea pig brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50359546

(CHEMBL1927270)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C37H45N7O7/c38-27(19-25-14-16-26(45)17-15-25)34(48)41-28-13-7-8-18-40-32(46)22-29(33(39)47)42-36(50)30(20-23-9-3-1-4-10-23)44-37(51)31(43-35(28)49)21-24-11-5-2-6-12-24/h1-6,9-12,14-17,27-31,45H,7-8,13,18-22,38H2,(H2,39,47)(H,40,46)(H,41,48)(H,42,50)(H,43,49)(H,44,51)/t27-,28+,29-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]nor-BNI from KOR in Dunkin Hartley guinea pig brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM85822

(Demethyl-AMPA | OFQ/N | OFQ/N (1-11) | OFQ/N (1-11...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50143182

(CHEMBL3759167)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2cccc3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-19-30(51)20-26(2)32(25)23-33(44)40(54)47-34-17-8-9-18-46-38(52)24-35(39(45)53)48-43(57)37(22-29-15-10-14-28-13-6-7-16-31(28)29)50-42(56)36(49-41(34)55)21-27-11-4-3-5-12-27/h3-7,10-16,19-20,33-37,51H,8-9,17-18,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,57)(H,49,55)(H,50,56)/t33-,34+,35-,36-,37+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]nor-BNI from KOR in Dunkin Hartley guinea pig brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM85822

(Demethyl-AMPA | OFQ/N | OFQ/N (1-11) | OFQ/N (1-11...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from human recombinant kappa-type opioid receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(GUINEA PIG) | BDBM85822

(Demethyl-AMPA | OFQ/N | OFQ/N (1-11) | OFQ/N (1-11...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50148257
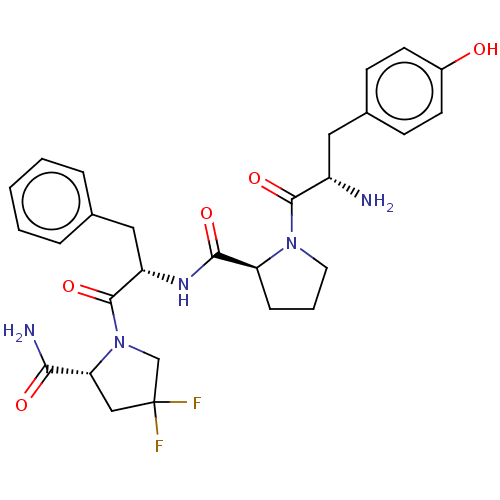
(CHEMBL3764219)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CC(F)(F)C[C@@H]1C(N)=O |r| Show InChI InChI=1S/C28H33F2N5O5/c29-28(30)15-23(24(32)37)35(16-28)27(40)21(14-17-5-2-1-3-6-17)33-25(38)22-7-4-12-34(22)26(39)20(31)13-18-8-10-19(36)11-9-18/h1-3,5-6,8-11,20-23,36H,4,7,12-16,31H2,(H2,32,37)(H,33,38)/t20-,21-,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenate by scintillation counting analysis |
Bioorg Med Chem 24: 1582-8 (2016)
Article DOI: 10.1016/j.bmc.2016.02.034
BindingDB Entry DOI: 10.7270/Q2Z321H7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143181
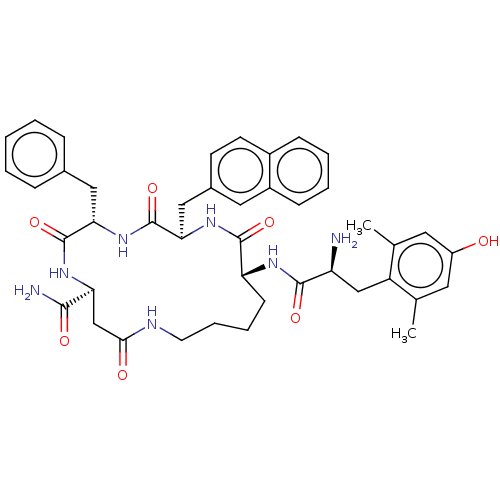
(CHEMBL3759981)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-18-31(51)19-26(2)32(25)23-33(44)40(54)47-34-14-8-9-17-46-38(52)24-35(39(45)53)48-42(56)36(21-27-10-4-3-5-11-27)50-43(57)37(49-41(34)55)22-28-15-16-29-12-6-7-13-30(29)20-28/h3-7,10-13,15-16,18-20,33-37,51H,8-9,14,17,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,55)(H,50,57)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM85822

(Demethyl-AMPA | OFQ/N | OFQ/N (1-11) | OFQ/N (1-11...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from human recombinant delta-type opioid receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50166066
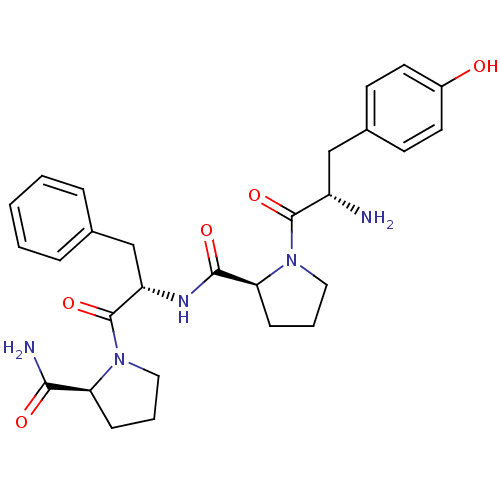
(CHEMBL362991 | H-Tyr-Pro-Phe-Pro-NH2 | MORPHICEPTI...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C28H35N5O5/c29-21(16-19-10-12-20(34)13-11-19)27(37)33-15-5-9-24(33)26(36)31-22(17-18-6-2-1-3-7-18)28(38)32-14-4-8-23(32)25(30)35/h1-3,6-7,10-13,21-24,34H,4-5,8-9,14-17,29H2,(H2,30,35)(H,31,36)/t21-,22-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenate by scintillation counting analysis |
Bioorg Med Chem 24: 1582-8 (2016)
Article DOI: 10.1016/j.bmc.2016.02.034
BindingDB Entry DOI: 10.7270/Q2Z321H7 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50418589
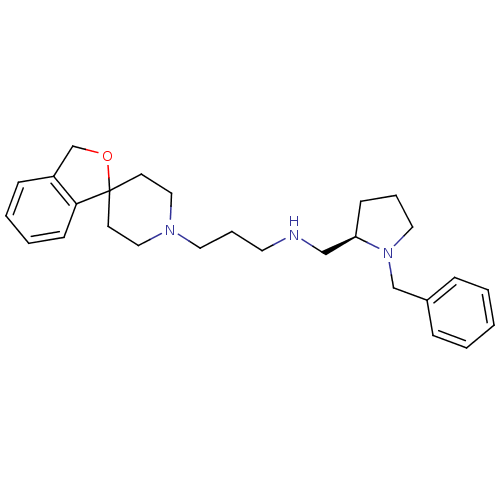
(CHEMBL1783821)Show SMILES C(CNC[C@H]1CCCN1Cc1ccccc1)CN1CCC2(CC1)OCc1ccccc21 |r| Show InChI InChI=1S/C27H37N3O/c1-2-8-23(9-3-1)21-30-17-6-11-25(30)20-28-15-7-16-29-18-13-27(14-19-29)26-12-5-4-10-24(26)22-31-27/h1-5,8-10,12,25,28H,6-7,11,13-22H2/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143182

(CHEMBL3759167)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2cccc3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-19-30(51)20-26(2)32(25)23-33(44)40(54)47-34-17-8-9-18-46-38(52)24-35(39(45)53)48-43(57)37(22-29-15-10-14-28-13-6-7-16-31(28)29)50-42(56)36(49-41(34)55)21-27-11-4-3-5-12-27/h3-7,10-16,19-20,33-37,51H,8-9,17-18,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,57)(H,49,55)(H,50,56)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H][Ile5,6]deltorphin-2 from DOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50143180

(CHEMBL3758969)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cccc3ccccc23)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-19-30(51)20-26(2)32(25)23-33(44)40(54)47-34-17-8-9-18-46-38(52)24-35(39(45)53)48-42(56)36(21-27-11-4-3-5-12-27)49-43(57)37(50-41(34)55)22-29-15-10-14-28-13-6-7-16-31(28)29/h3-7,10-16,19-20,33-37,51H,8-9,17-18,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,57)(H,50,55)/t33-,34+,35-,36-,37+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]nor-BNI from KOR in Dunkin Hartley guinea pig brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM85822

(Demethyl-AMPA | OFQ/N | OFQ/N (1-11) | OFQ/N (1-11...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50418587

(CHEMBL1783817)Show SMILES N[C@H](Cc1ccccc1)C(=O)NCCCN1CCC2(CC1)OCc1ccccc21 |r| Show InChI InChI=1S/C24H31N3O2/c25-22(17-19-7-2-1-3-8-19)23(28)26-13-6-14-27-15-11-24(12-16-27)21-10-5-4-9-20(21)18-29-24/h1-5,7-10,22H,6,11-18,25H2,(H,26,28)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50418598

(CHEMBL1783818)Show SMILES N[C@@H](Cc1ccccc1)C(=O)NCCCN1CCC2(CC1)OCc1ccccc21 |r| Show InChI InChI=1S/C24H31N3O2/c25-22(17-19-7-2-1-3-8-19)23(28)26-13-6-14-27-15-11-24(12-16-27)21-10-5-4-9-20(21)18-29-24/h1-5,7-10,22H,6,11-18,25H2,(H,26,28)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50418588

(CHEMBL1783819)Show InChI InChI=1S/C24H31N3O2/c28-23(18-25-17-20-7-2-1-3-8-20)26-13-6-14-27-15-11-24(12-16-27)22-10-5-4-9-21(22)19-29-24/h1-5,7-10,25H,6,11-19H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143180

(CHEMBL3758969)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cccc3ccccc23)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-19-30(51)20-26(2)32(25)23-33(44)40(54)47-34-17-8-9-18-46-38(52)24-35(39(45)53)48-42(56)36(21-27-11-4-3-5-12-27)49-43(57)37(50-41(34)55)22-29-15-10-14-28-13-6-7-16-31(28)29/h3-7,10-16,19-20,33-37,51H,8-9,17-18,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,57)(H,50,55)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50237595

(CHEMBL4065881)Show SMILES CN[C@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)CNC(=O)[C@H](Cc2ccccc2)N(C)C1=O)C(N)=O |r| Show InChI InChI=1S/C42H54N8O8/c1-44-33(22-29-17-19-30(51)20-18-29)39(55)47-31-16-10-11-21-45-36(52)25-32(38(43)54)48-41(57)35(24-28-14-8-5-9-15-28)49(2)37(53)26-46-40(56)34(50(3)42(31)58)23-27-12-6-4-7-13-27/h4-9,12-15,17-20,31-35,44,51H,10-11,16,21-26H2,1-3H3,(H2,43,54)(H,45,52)(H,46,56)(H,47,55)(H,48,57)/t31-,32-,33+,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates after 180 mins by liquid scintillation counting analysis |
Bioorg Med Chem Lett 27: 1644-1648 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.016
BindingDB Entry DOI: 10.7270/Q2BG2R9F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50418595
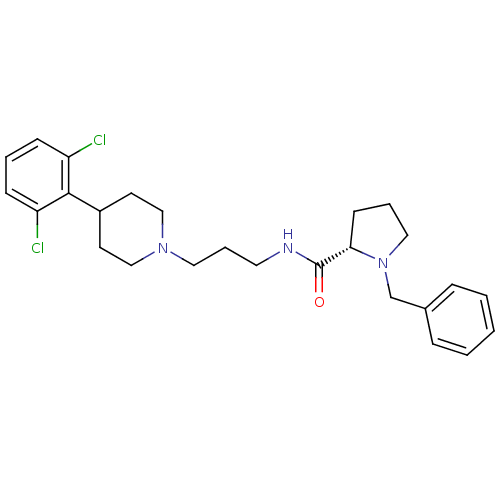
(CHEMBL1783827)Show SMILES Clc1cccc(Cl)c1C1CCN(CCCNC(=O)[C@@H]2CCCN2Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C26H33Cl2N3O/c27-22-9-4-10-23(28)25(22)21-12-17-30(18-13-21)15-6-14-29-26(32)24-11-5-16-31(24)19-20-7-2-1-3-8-20/h1-4,7-10,21,24H,5-6,11-19H2,(H,29,32)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50148258
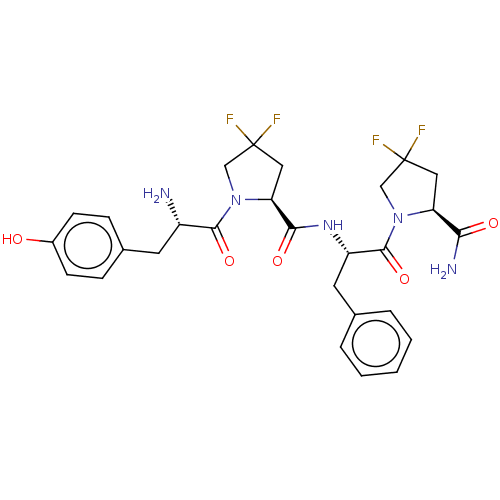
(CHEMBL3764033)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CC(F)(F)C[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CC(F)(F)C[C@H]1C(N)=O |r| Show InChI InChI=1S/C28H31F4N5O5/c29-27(30)12-21(23(34)39)36(14-27)26(42)20(11-16-4-2-1-3-5-16)35-24(40)22-13-28(31,32)15-37(22)25(41)19(33)10-17-6-8-18(38)9-7-17/h1-9,19-22,38H,10-15,33H2,(H2,34,39)(H,35,40)/t19-,20-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenate by scintillation counting analysis |
Bioorg Med Chem 24: 1582-8 (2016)
Article DOI: 10.1016/j.bmc.2016.02.034
BindingDB Entry DOI: 10.7270/Q2Z321H7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50237594

(CHEMBL4087301)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)CNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C(=O)CNC1=O)C(N)=O |r| Show InChI InChI=1S/C44H57N9O9/c1-46-34(22-30-17-19-31(54)20-18-30)42(60)51-32-16-10-11-21-47-37(55)25-33(40(45)58)50-38(56)26-48-43(61)35(23-28-12-6-4-7-13-28)53(3)44(62)36(24-29-14-8-5-9-15-29)52(2)39(57)27-49-41(32)59/h4-9,12-15,17-20,32-36,46,54H,10-11,16,21-27H2,1-3H3,(H2,45,58)(H,47,55)(H,48,61)(H,49,59)(H,50,56)(H,51,60)/t32-,33+,34+,35+,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 27: 1644-1648 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.016
BindingDB Entry DOI: 10.7270/Q2BG2R9F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50418590

(CHEMBL1783822)Show SMILES O=C(OCCCN1CCC2(CC1)OCc1ccccc21)[C@H]1CCCN1Cc1ccccc1 |r| Show InChI InChI=1S/C27H34N2O3/c30-26(25-12-6-16-29(25)20-22-8-2-1-3-9-22)31-19-7-15-28-17-13-27(14-18-28)24-11-5-4-10-23(24)21-32-27/h1-5,8-11,25H,6-7,12-21H2/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50143183

(CHEMBL3759179)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C43H51N7O7/c1-25-18-31(51)19-26(2)32(25)23-33(44)40(54)47-34-14-8-9-17-46-38(52)24-35(39(45)53)48-42(56)37(22-28-15-16-29-12-6-7-13-30(29)20-28)50-43(57)36(49-41(34)55)21-27-10-4-3-5-11-27/h3-7,10-13,15-16,18-20,33-37,51H,8-9,14,17,21-24,44H2,1-2H3,(H2,45,53)(H,46,52)(H,47,54)(H,48,56)(H,49,55)(H,50,57)/t33-,34+,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H][Ile5,6]deltorphin-2 from DOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50418591
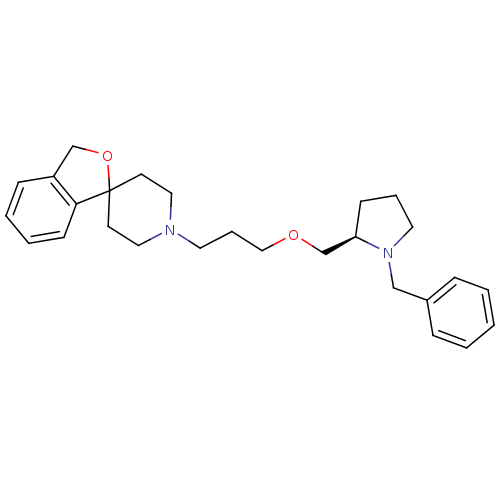
(CHEMBL1783823)Show SMILES C(COC[C@H]1CCCN1Cc1ccccc1)CN1CCC2(CC1)OCc1ccccc21 |r| Show InChI InChI=1S/C27H36N2O2/c1-2-8-23(9-3-1)20-29-16-6-11-25(29)22-30-19-7-15-28-17-13-27(14-18-28)26-12-5-4-10-24(26)21-31-27/h1-5,8-10,12,25H,6-7,11,13-22H2/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human recombinant NOP receptor expressed in CHO cells |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50359546

(CHEMBL1927270)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C37H45N7O7/c38-27(19-25-14-16-26(45)17-15-25)34(48)41-28-13-7-8-18-40-32(46)22-29(33(39)47)42-36(50)30(20-23-9-3-1-4-10-23)44-37(51)31(43-35(28)49)21-24-11-5-2-6-12-24/h1-6,9-12,14-17,27-31,45H,7-8,13,18-22,38H2,(H2,39,47)(H,40,46)(H,41,48)(H,42,50)(H,43,49)(H,44,51)/t27-,28+,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Displacement of [3H][Ile5,6]deltorphin-2 from DOR in Wistar rat brain homogenates by liquid scintillation counting analysis |
Eur J Med Chem 109: 276-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.012
BindingDB Entry DOI: 10.7270/Q22809FJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50418594

(CHEMBL1783826)Show SMILES Clc1cccc(Cl)c1C1CCN(CCCNC(=O)[C@H]2CCCN2Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C26H33Cl2N3O/c27-22-9-4-10-23(28)25(22)21-12-17-30(18-13-21)15-6-14-29-26(32)24-11-5-16-31(24)19-20-7-2-1-3-8-20/h1-4,7-10,21,24H,5-6,11-19H2,(H,29,32)/t24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from human recombinant mu-type opioid receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50418594

(CHEMBL1783826)Show SMILES Clc1cccc(Cl)c1C1CCN(CCCNC(=O)[C@H]2CCCN2Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C26H33Cl2N3O/c27-22-9-4-10-23(28)25(22)21-12-17-30(18-13-21)15-6-14-29-26(32)24-11-5-16-31(24)19-20-7-2-1-3-8-20/h1-4,7-10,21,24H,5-6,11-19H2,(H,29,32)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from human recombinant kappa-type opioid receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 5080-95 (2009)
Article DOI: 10.1016/j.bmc.2009.05.068
BindingDB Entry DOI: 10.7270/Q2P270C3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data