 Found 55 hits with Last Name = 'cancilla' and Initial = 'b'
Found 55 hits with Last Name = 'cancilla' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807
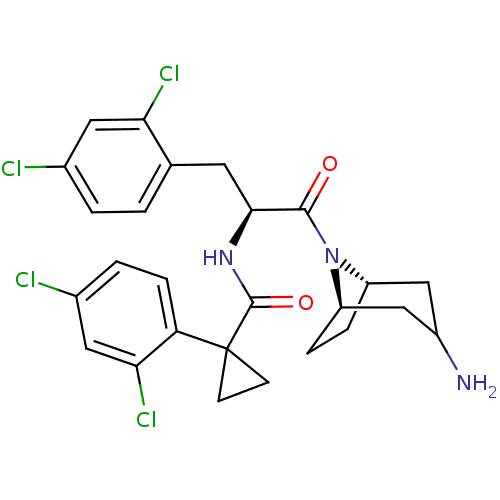
(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394820
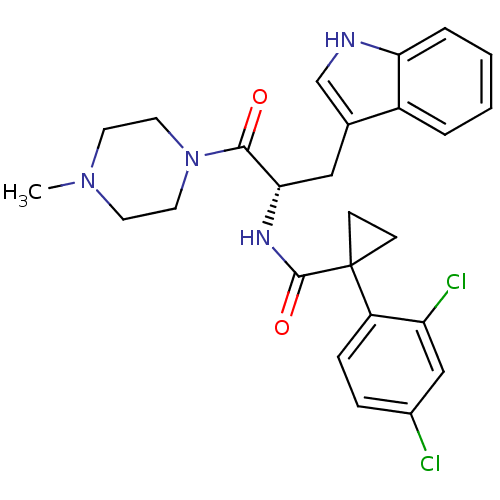
(CHEMBL2163829)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O2/c1-31-10-12-32(13-11-31)24(33)23(14-17-16-29-22-5-3-2-4-19(17)22)30-25(34)26(8-9-26)20-7-6-18(27)15-21(20)28/h2-7,15-16,23,29H,8-14H2,1H3,(H,30,34)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090
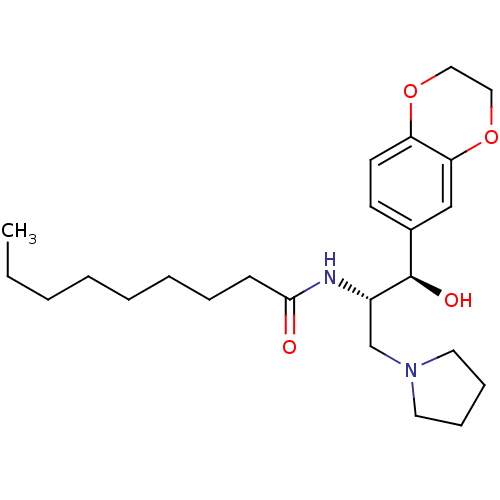
(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394812
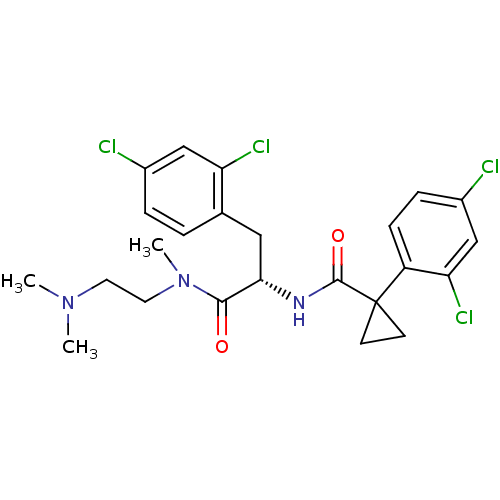
(CHEMBL2163838)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl4N3O2/c1-30(2)10-11-31(3)22(32)21(12-15-4-5-16(25)13-19(15)27)29-23(33)24(8-9-24)18-7-6-17(26)14-20(18)28/h4-7,13-14,21H,8-12H2,1-3H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394804
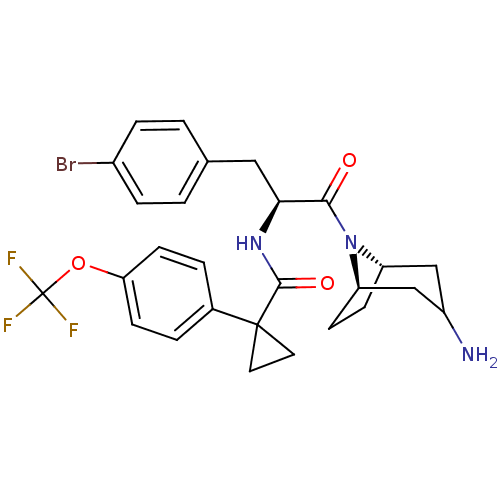
(CHEMBL2163828)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H29BrF3N3O3/c28-18-5-1-16(2-6-18)13-23(24(35)34-20-7-8-21(34)15-19(32)14-20)33-25(36)26(11-12-26)17-3-9-22(10-4-17)37-27(29,30)31/h1-6,9-10,19-21,23H,7-8,11-15,32H2,(H,33,36)/t19?,20-,21+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091
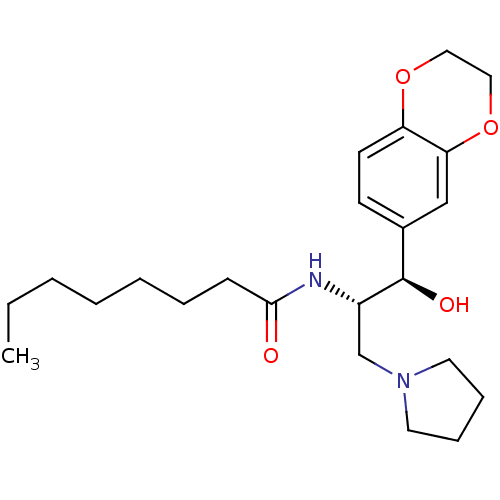
(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394816
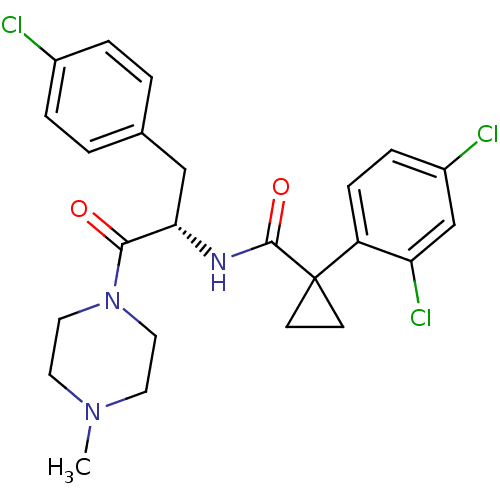
(CHEMBL2163833)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26Cl3N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394805
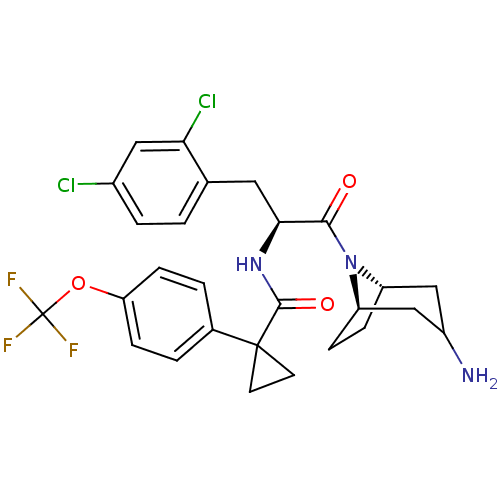
(CHEMBL2163827)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H28Cl2F3N3O3/c28-17-4-1-15(22(29)12-17)11-23(24(36)35-19-5-6-20(35)14-18(33)13-19)34-25(37)26(9-10-26)16-2-7-21(8-3-16)38-27(30,31)32/h1-4,7-8,12,18-20,23H,5-6,9-11,13-14,33H2,(H,34,37)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394806

(CHEMBL2163826)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H28BrCl2N3O2/c27-16-3-1-15(2-4-16)11-23(24(33)32-19-6-7-20(32)14-18(30)13-19)31-25(34)26(9-10-26)21-8-5-17(28)12-22(21)29/h1-5,8,12,18-20,23H,6-7,9-11,13-14,30H2,(H,31,34)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394809
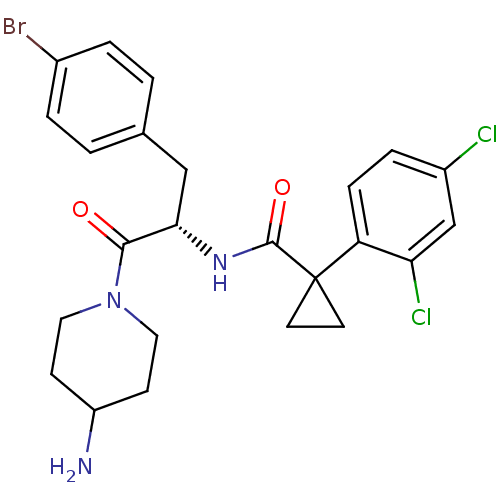
(CHEMBL2163823)Show SMILES NC1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26BrCl2N3O2/c25-16-3-1-15(2-4-16)13-21(22(31)30-11-7-18(28)8-12-30)29-23(32)24(9-10-24)19-6-5-17(26)14-20(19)27/h1-6,14,18,21H,7-13,28H2,(H,29,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394817
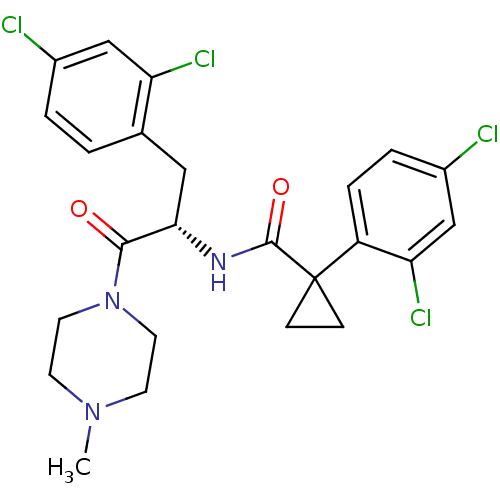
(CHEMBL2163832)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-30-8-10-31(11-9-30)22(32)21(12-15-2-3-16(25)13-19(15)27)29-23(33)24(6-7-24)18-5-4-17(26)14-20(18)28/h2-5,13-14,21H,6-12H2,1H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394811
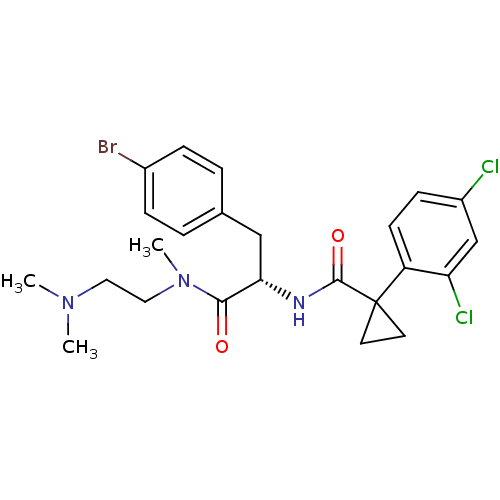
(CHEMBL2163839)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H28BrCl2N3O2/c1-29(2)12-13-30(3)22(31)21(14-16-4-6-17(25)7-5-16)28-23(32)24(10-11-24)19-9-8-18(26)15-20(19)27/h4-9,15,21H,10-14H2,1-3H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807

(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394820

(CHEMBL2163829)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O2/c1-31-10-12-32(13-11-31)24(33)23(14-17-16-29-22-5-3-2-4-19(17)22)30-25(34)26(8-9-26)20-7-6-18(27)15-21(20)28/h2-7,15-16,23,29H,8-14H2,1H3,(H,30,34)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394815
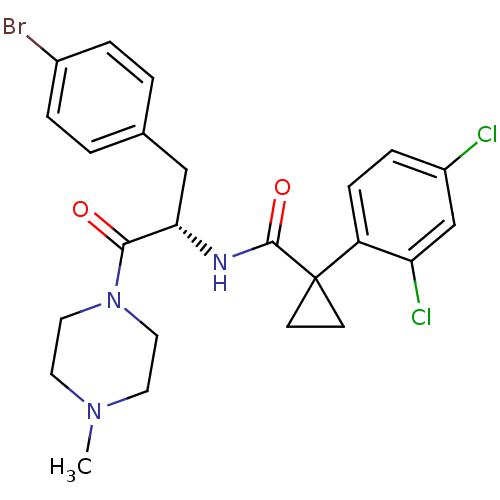
(CHEMBL2163834)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26BrCl2N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394805

(CHEMBL2163827)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H28Cl2F3N3O3/c28-17-4-1-15(22(29)12-17)11-23(24(36)35-19-5-6-20(35)14-18(33)13-19)34-25(37)26(9-10-26)16-2-7-21(8-3-16)38-27(30,31)32/h1-4,7-8,12,18-20,23H,5-6,9-11,13-14,33H2,(H,34,37)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090

(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394820

(CHEMBL2163829)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O2/c1-31-10-12-32(13-11-31)24(33)23(14-17-16-29-22-5-3-2-4-19(17)22)30-25(34)26(8-9-26)20-7-6-18(27)15-21(20)28/h2-7,15-16,23,29H,8-14H2,1H3,(H,30,34)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394816

(CHEMBL2163833)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26Cl3N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394811

(CHEMBL2163839)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H28BrCl2N3O2/c1-29(2)12-13-30(3)22(31)21(14-16-4-6-17(25)7-5-16)28-23(32)24(10-11-24)19-9-8-18(26)15-20(19)27/h4-9,15,21H,10-14H2,1-3H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394817

(CHEMBL2163832)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-30-8-10-31(11-9-30)22(32)21(12-15-2-3-16(25)13-19(15)27)29-23(33)24(6-7-24)18-5-4-17(26)14-20(18)28/h2-5,13-14,21H,6-12H2,1H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394815

(CHEMBL2163834)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26BrCl2N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394804

(CHEMBL2163828)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H29BrF3N3O3/c28-18-5-1-16(2-6-18)13-23(24(35)34-20-7-8-21(34)15-19(32)14-20)33-25(36)26(11-12-26)17-3-9-22(10-4-17)37-27(29,30)31/h1-6,9-10,19-21,23H,7-8,11-15,32H2,(H,33,36)/t19?,20-,21+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394808
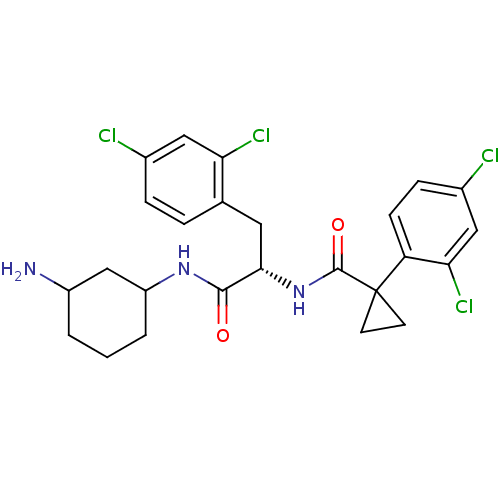
(CHEMBL2163824)Show SMILES NC1CCCC(C1)NC(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C25H27Cl4N3O2/c26-15-5-4-14(20(28)11-15)10-22(23(33)31-18-3-1-2-17(30)13-18)32-24(34)25(8-9-25)19-7-6-16(27)12-21(19)29/h4-7,11-12,17-18,22H,1-3,8-10,13,30H2,(H,31,33)(H,32,34)/t17?,18?,22-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091

(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394814

(CHEMBL2163836)Show SMILES C[C@@H]1CN(CCN1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-14-13-31(9-8-29-14)22(32)21(10-15-2-3-16(25)11-19(15)27)30-23(33)24(6-7-24)18-5-4-17(26)12-20(18)28/h2-5,11-12,14,21,29H,6-10,13H2,1H3,(H,30,33)/t14-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394806

(CHEMBL2163826)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H28BrCl2N3O2/c27-16-3-1-15(2-4-16)11-23(24(33)32-19-6-7-20(32)14-18(30)13-19)31-25(34)26(9-10-26)21-8-5-17(28)12-22(21)29/h1-5,8,12,18-20,23H,6-7,9-11,13-14,30H2,(H,31,34)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394819
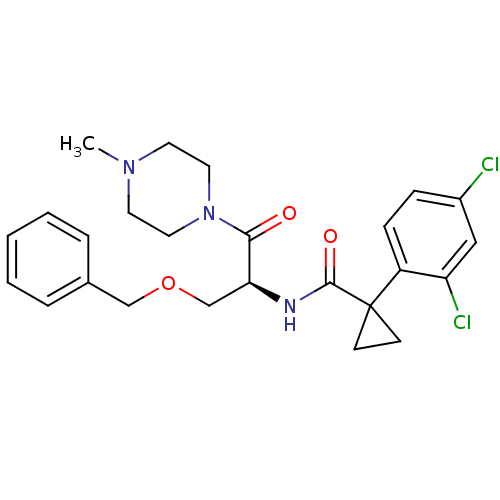
(CHEMBL2163830)Show SMILES CN1CCN(CC1)C(=O)[C@H](COCc1ccccc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C25H29Cl2N3O3/c1-29-11-13-30(14-12-29)23(31)22(17-33-16-18-5-3-2-4-6-18)28-24(32)25(9-10-25)20-8-7-19(26)15-21(20)27/h2-8,15,22H,9-14,16-17H2,1H3,(H,28,32)/t22-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394812

(CHEMBL2163838)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl4N3O2/c1-30(2)10-11-31(3)22(32)21(12-15-4-5-16(25)13-19(15)27)29-23(33)24(8-9-24)18-7-6-17(26)14-20(18)28/h4-7,13-14,21H,8-12H2,1-3H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394821
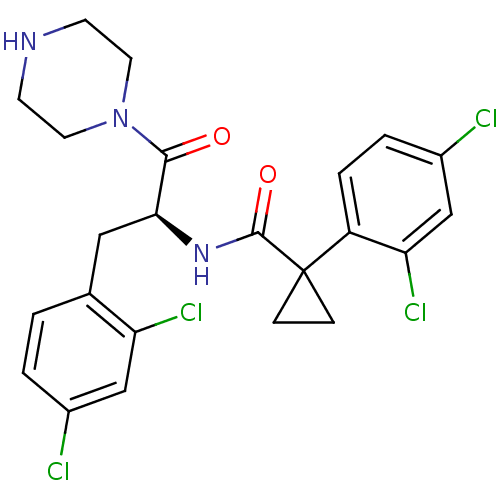
(CHEMBL2163835)Show SMILES Clc1ccc(C[C@H](NC(=O)C2(CC2)c2ccc(Cl)cc2Cl)C(=O)N2CCNCC2)c(Cl)c1 |r| Show InChI InChI=1S/C23H23Cl4N3O2/c24-15-2-1-14(18(26)12-15)11-20(21(31)30-9-7-28-8-10-30)29-22(32)23(5-6-23)17-4-3-16(25)13-19(17)27/h1-4,12-13,20,28H,5-11H2,(H,29,32)/t20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394818
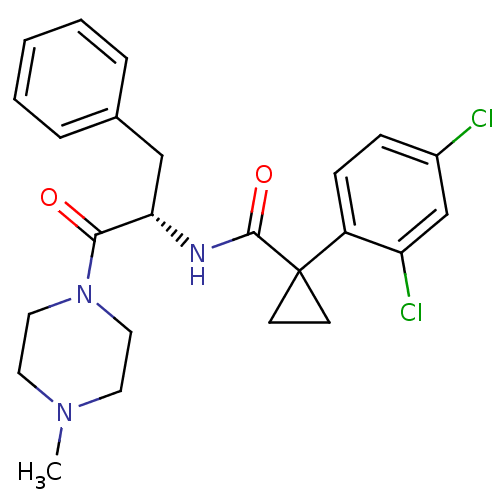
(CHEMBL2163831)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccccc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl2N3O2/c1-28-11-13-29(14-12-28)22(30)21(15-17-5-3-2-4-6-17)27-23(31)24(9-10-24)19-8-7-18(25)16-20(19)26/h2-8,16,21H,9-15H2,1H3,(H,27,31)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394813
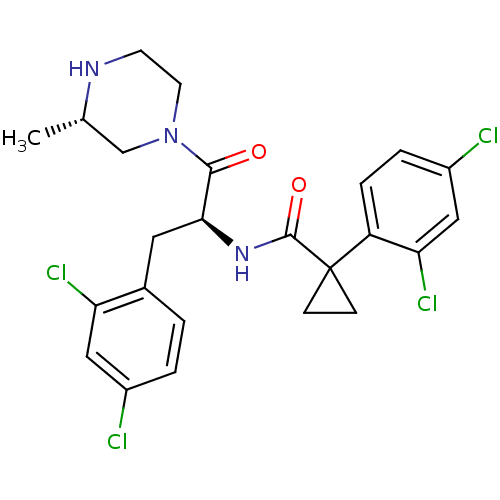
(CHEMBL2163837)Show SMILES C[C@H]1CN(CCN1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-14-13-31(9-8-29-14)22(32)21(10-15-2-3-16(25)11-19(15)27)30-23(33)24(6-7-24)18-5-4-17(26)12-20(18)28/h2-5,11-12,14,21,29H,6-10,13H2,1H3,(H,30,33)/t14-,21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394810
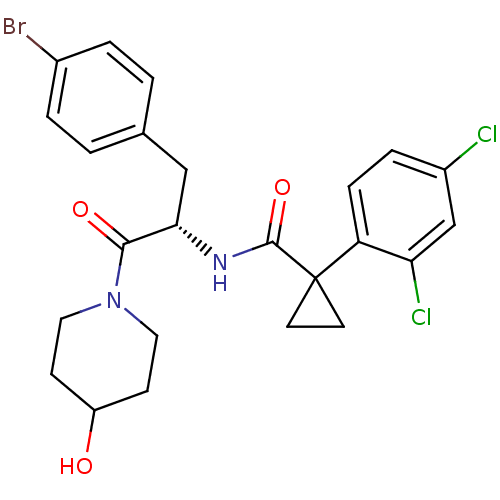
(CHEMBL2163822)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25BrCl2N2O3/c25-16-3-1-15(2-4-16)13-21(22(31)29-11-7-18(30)8-12-29)28-23(32)24(9-10-24)19-6-5-17(26)14-20(19)27/h1-6,14,18,21,30H,7-13H2,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394819

(CHEMBL2163830)Show SMILES CN1CCN(CC1)C(=O)[C@H](COCc1ccccc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C25H29Cl2N3O3/c1-29-11-13-30(14-12-29)23(31)22(17-33-16-18-5-3-2-4-6-18)28-24(32)25(9-10-25)20-8-7-19(26)15-21(20)27/h2-8,15,22H,9-14,16-17H2,1H3,(H,28,32)/t22-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394813

(CHEMBL2163837)Show SMILES C[C@H]1CN(CCN1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-14-13-31(9-8-29-14)22(32)21(10-15-2-3-16(25)11-19(15)27)30-23(33)24(6-7-24)18-5-4-17(26)12-20(18)28/h2-5,11-12,14,21,29H,6-10,13H2,1H3,(H,30,33)/t14-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394808

(CHEMBL2163824)Show SMILES NC1CCCC(C1)NC(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C25H27Cl4N3O2/c26-15-5-4-14(20(28)11-15)10-22(23(33)31-18-3-1-2-17(30)13-18)32-24(34)25(8-9-25)19-7-6-16(27)12-21(19)29/h4-7,11-12,17-18,22H,1-3,8-10,13,30H2,(H,31,33)(H,32,34)/t17?,18?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394807

(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394814

(CHEMBL2163836)Show SMILES C[C@@H]1CN(CCN1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-14-13-31(9-8-29-14)22(32)21(10-15-2-3-16(25)11-19(15)27)30-23(33)24(6-7-24)18-5-4-17(26)12-20(18)28/h2-5,11-12,14,21,29H,6-10,13H2,1H3,(H,30,33)/t14-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394815

(CHEMBL2163834)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26BrCl2N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394806

(CHEMBL2163826)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H28BrCl2N3O2/c27-16-3-1-15(2-4-16)11-23(24(33)32-19-6-7-20(32)14-18(30)13-19)31-25(34)26(9-10-26)21-8-5-17(28)12-22(21)29/h1-5,8,12,18-20,23H,6-7,9-11,13-14,30H2,(H,31,34)/t18?,19-,20+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394816

(CHEMBL2163833)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26Cl3N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394817

(CHEMBL2163832)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-30-8-10-31(11-9-30)22(32)21(12-15-2-3-16(25)13-19(15)27)29-23(33)24(6-7-24)18-5-4-17(26)14-20(18)28/h2-5,13-14,21H,6-12H2,1H3,(H,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394810

(CHEMBL2163822)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25BrCl2N2O3/c25-16-3-1-15(2-4-16)13-21(22(31)29-11-7-18(30)8-12-29)28-23(32)24(9-10-24)19-6-5-17(26)14-20(19)27/h1-6,14,18,21,30H,7-13H2,(H,28,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394818

(CHEMBL2163831)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccccc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl2N3O2/c1-28-11-13-29(14-12-28)22(30)21(15-17-5-3-2-4-6-17)27-23(31)24(9-10-24)19-8-7-18(25)16-20(19)26/h2-8,16,21H,9-15H2,1H3,(H,27,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394812

(CHEMBL2163838)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl4N3O2/c1-30(2)10-11-31(3)22(32)21(12-15-4-5-16(25)13-19(15)27)29-23(33)24(8-9-24)18-7-6-17(26)14-20(18)28/h4-7,13-14,21H,8-12H2,1-3H3,(H,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394819

(CHEMBL2163830)Show SMILES CN1CCN(CC1)C(=O)[C@H](COCc1ccccc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C25H29Cl2N3O3/c1-29-11-13-30(14-12-29)23(31)22(17-33-16-18-5-3-2-4-6-18)28-24(32)25(9-10-25)20-8-7-19(26)15-21(20)27/h2-8,15,22H,9-14,16-17H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394805

(CHEMBL2163827)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H28Cl2F3N3O3/c28-17-4-1-15(22(29)12-17)11-23(24(36)35-19-5-6-20(35)14-18(33)13-19)34-25(37)26(9-10-26)16-2-7-21(8-3-16)38-27(30,31)32/h1-4,7-8,12,18-20,23H,5-6,9-11,13-14,33H2,(H,34,37)/t18?,19-,20+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394804

(CHEMBL2163828)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H29BrF3N3O3/c28-18-5-1-16(2-6-18)13-23(24(35)34-20-7-8-21(34)15-19(32)14-20)33-25(36)26(11-12-26)17-3-9-22(10-4-17)37-27(29,30)31/h1-6,9-10,19-21,23H,7-8,11-15,32H2,(H,33,36)/t19?,20-,21+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate after 45 mins |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394822
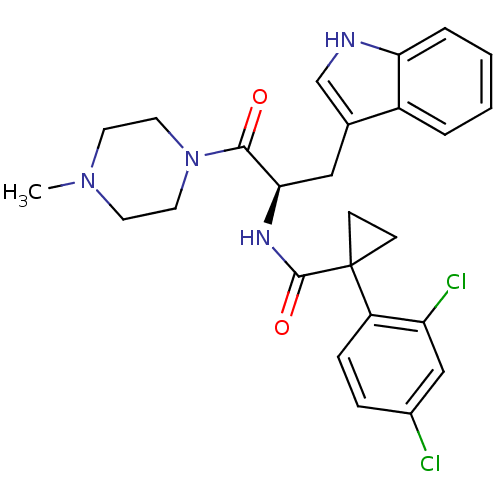
(CHEMBL2163819)Show SMILES CN1CCN(CC1)C(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O2/c1-31-10-12-32(13-11-31)24(33)23(14-17-16-29-22-5-3-2-4-19(17)22)30-25(34)26(8-9-26)20-7-6-18(27)15-21(20)28/h2-7,15-16,23,29H,8-14H2,1H3,(H,30,34)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data