

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300767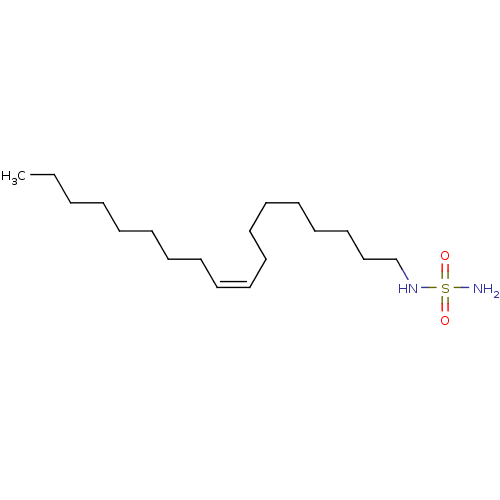 (CHEMBL570566 | {[(9Z)-octadec-9-en-1-yl]sulfamoyl}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300765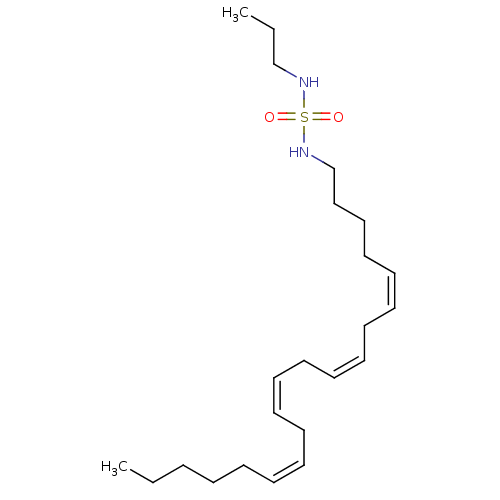 (CHEMBL583108 | N-(cis-5-cis-8-cis-11-cis-14-eicosa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300770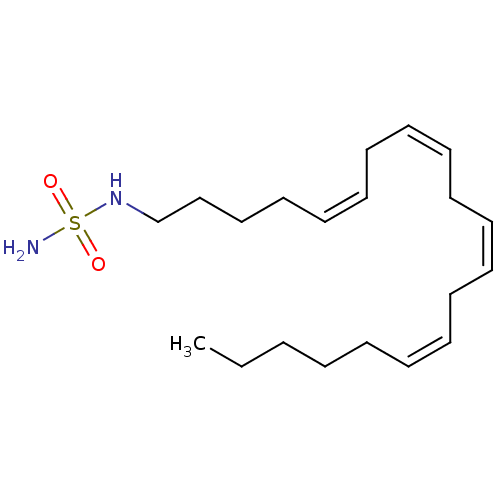 (CHEMBL568489 | N-(cis-5-cis-8-cis-11-cis-14-eicosa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300769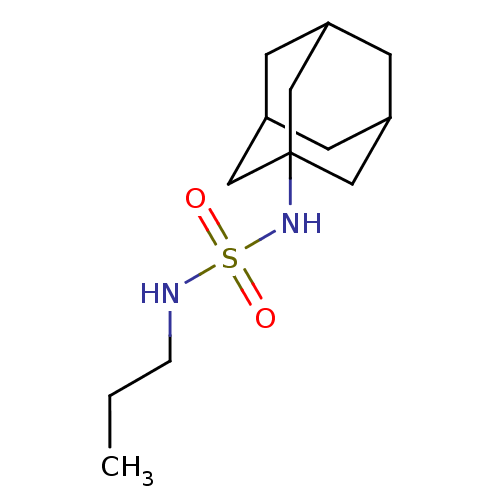 (CHEMBL374746 | N-(1-adamantyl)-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]WIN55212-2 from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM12915 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Competitive inhibition of DNA-PK (unknown origin) in the presence of ATP | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300771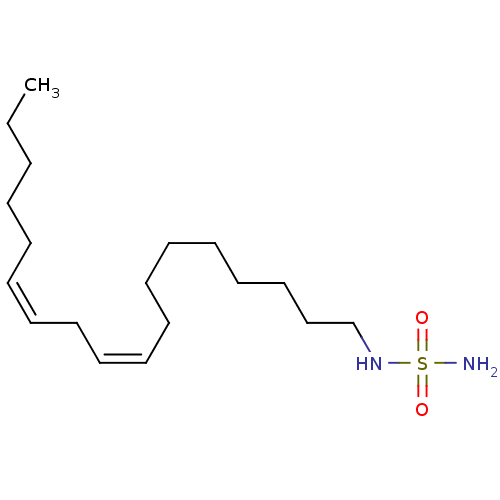 (CHEMBL570988 | N-(cis-9-cis-12-octadecadienyl)sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202584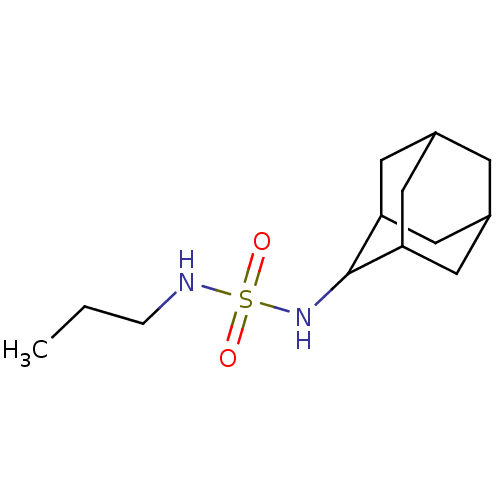 (CHEMBL218643 | N-(2-adamantyl)-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300766 (CHEMBL569658 | N-(cis-9-cis-12-octadecadienyl)-N'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300769 (CHEMBL374746 | N-(1-adamantyl)-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202583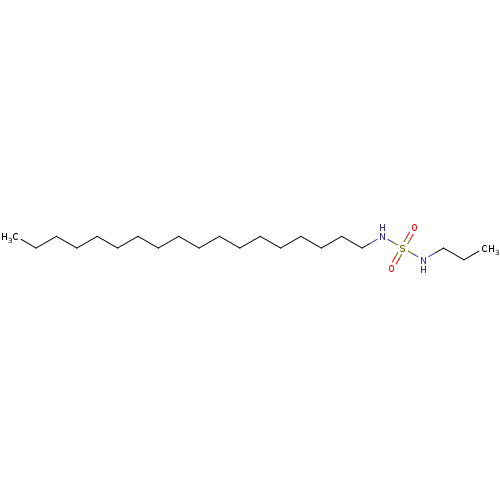 (CHEMBL219156 | N-octadecyl-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202583 (CHEMBL219156 | N-octadecyl-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]WIN55212-2 from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300768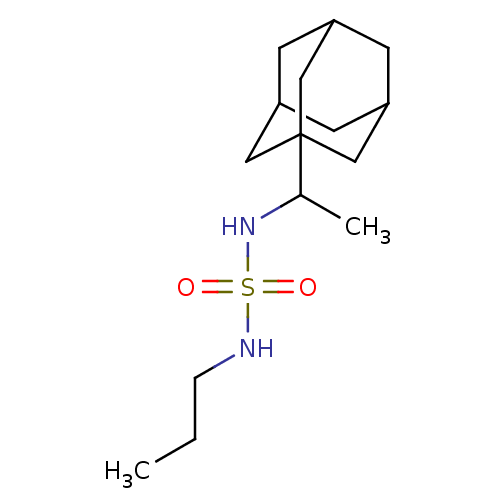 (CHEMBL437157 | N-(1-(1-adamantyl)ethyl)-N'-propyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202579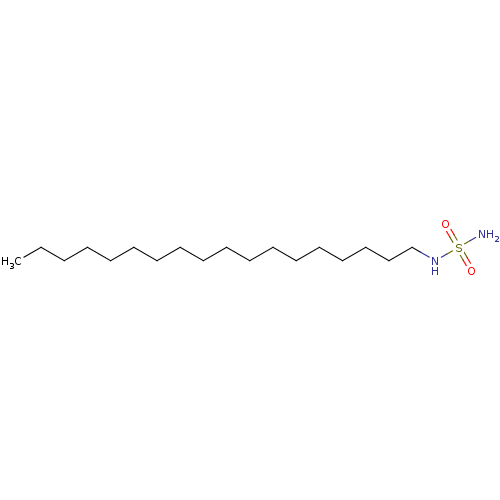 (CHEMBL218794 | N-octadecylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50202584 (CHEMBL218643 | N-(2-adamantyl)-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]WIN55212-2 from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300764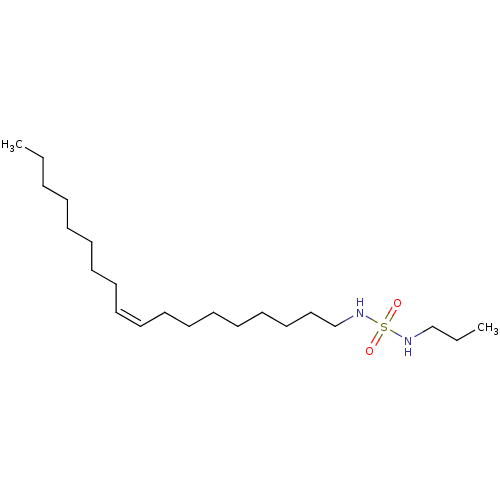 (CHEMBL571044 | N-oleyl-N'-propylsulfamide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50300768 (CHEMBL437157 | N-(1-(1-adamantyl)ethyl)-N'-propyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]WIN55212-2 from CB1 receptor in rat cerebellar membrane after 90 mins by scintillation counting | Eur J Med Chem 44: 4889-95 (2009) Article DOI: 10.1016/j.ejmech.2009.08.003 BindingDB Entry DOI: 10.7270/Q2PR7W2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50319926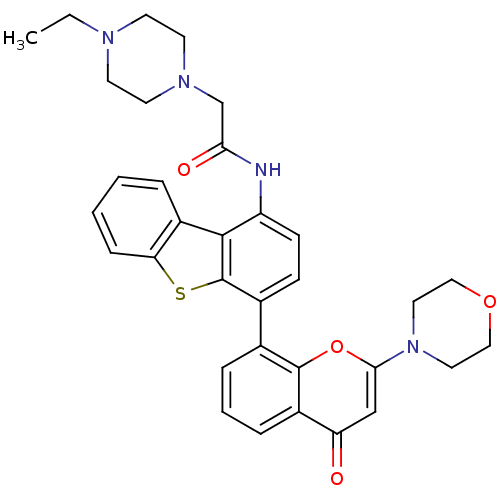 (2-(4-ethylpiperazin-1-yl)-N-(4-(2-morpholino-4-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of PI-3K delta (unknown origin) | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427143 (1-({[(1R)-2-[(4-chloro-2-methanesulfonylphenyl)met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427143 (1-({[(1R)-2-[(4-chloro-2-methanesulfonylphenyl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427127 (1-({[(1R)-2-[(4-chloro-2-methanesulfonylphenyl)met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50319926 (2-(4-ethylpiperazin-1-yl)-N-(4-(2-morpholino-4-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of PI-3K beta (unknown origin) | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427127 (1-({[(1R)-2-[(4-chloro-2-methanesulfonylphenyl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250082 (US9447106, 27b (peak 2) | US9556188, Compound 27a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00216g BindingDB Entry DOI: 10.7270/Q2DF6W53 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00216g BindingDB Entry DOI: 10.7270/Q2DF6W53 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427195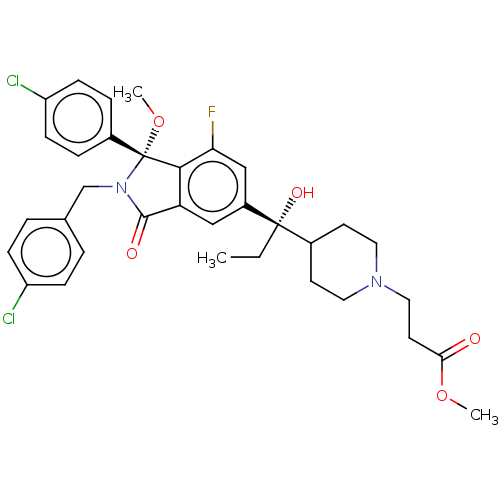 (US10544132, Example 136 | US10981898, Example 137 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427196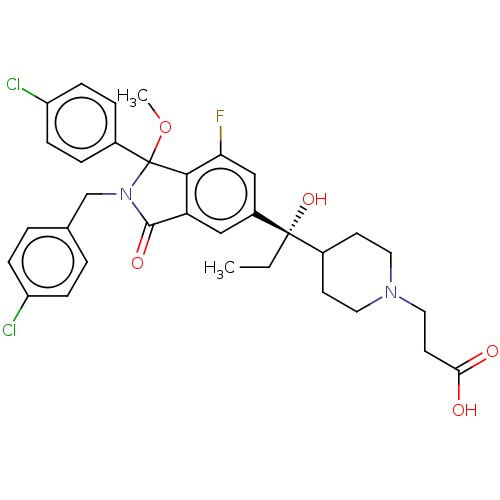 (US10544132, Example 137) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427156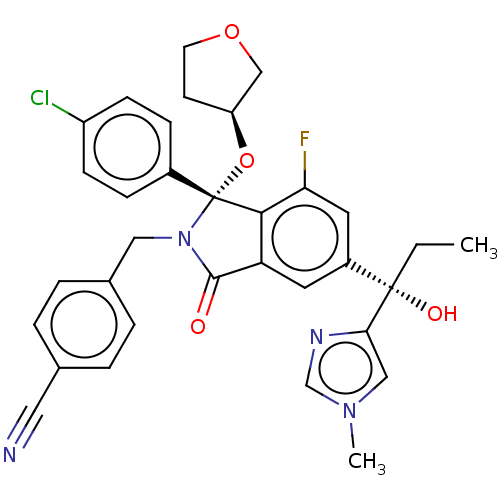 (4-{[(1R)-1-(4-chlorophenyl)-7-fluoro-5-[I-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427156 (4-{[(1R)-1-(4-chlorophenyl)-7-fluoro-5-[I-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427123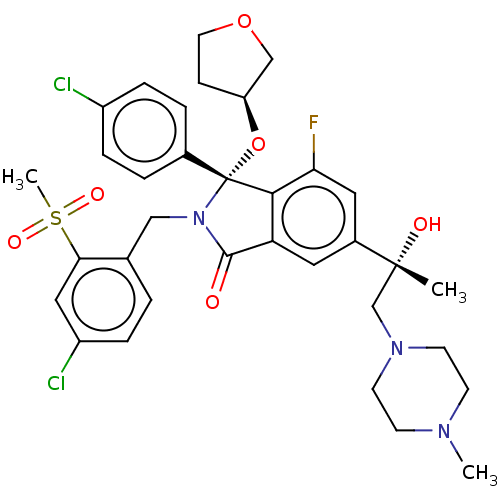 (US10544132, Example 70 | US10981898, Example 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427122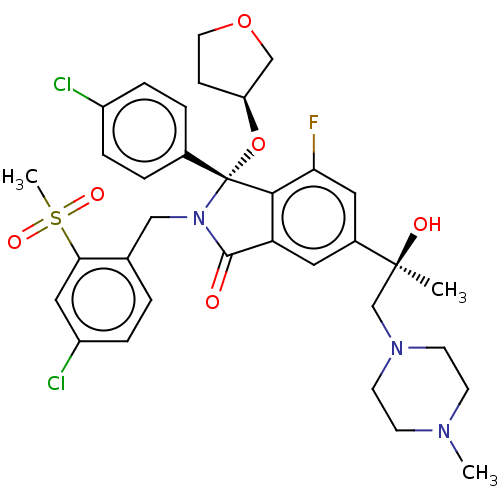 (US10544132, Example 69 | US10981898, Example 70) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427147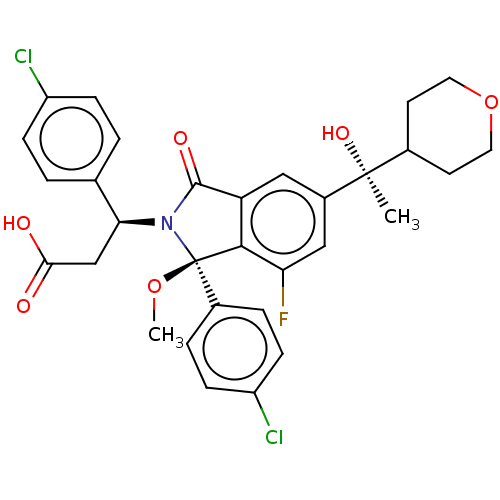 ((3S)-3-(4-chlorophenyl)-3-[(1R)-1-(4-chlorophenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427147 ((3S)-3-(4-chlorophenyl)-3-[(1R)-1-(4-chlorophenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50235359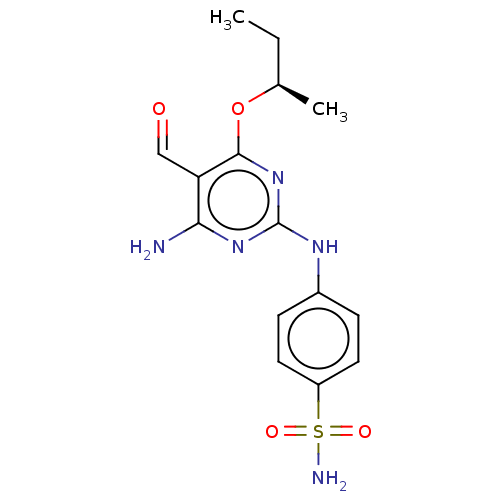 (CHEMBL4088242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description In vitro inhibitory concentration against angiotensin-converting enzyme | J Med Chem 60: 1746-1767 (2017) Article DOI: 10.1021/acs.jmedchem.6b01254 BindingDB Entry DOI: 10.7270/Q2833V86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427098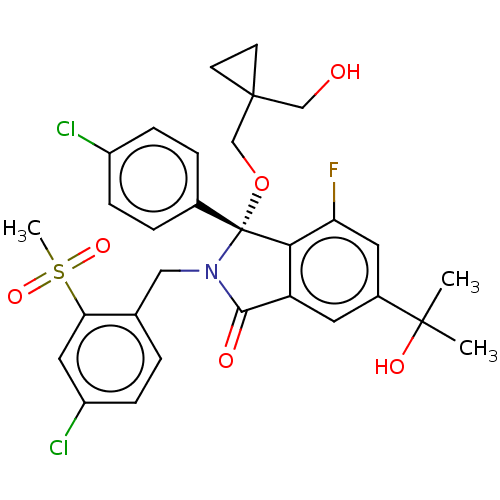 (US10544132, Example 50 | US10981898, Example 62 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427158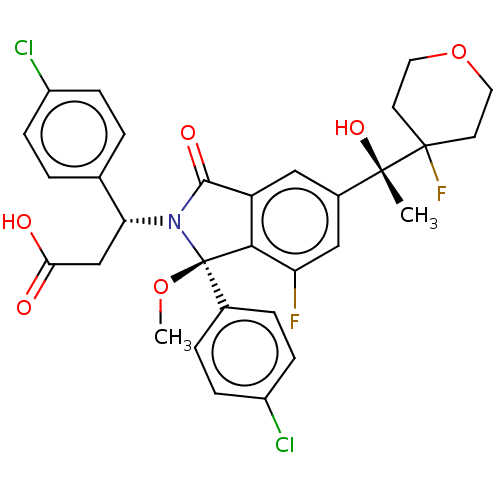 ((3S)-3-(4-chlorophenyl)-3-[(1R)-1-(4-chlorophenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427098 (US10544132, Example 50 | US10981898, Example 62 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM492987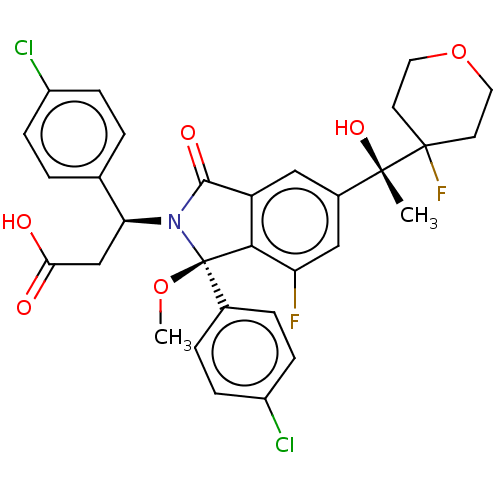 (US10981898, Example 98) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427136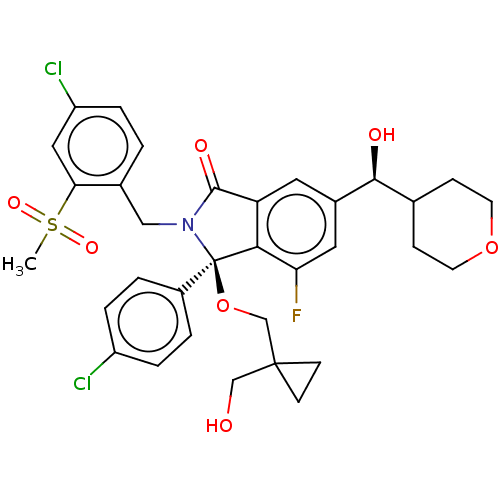 ((3R)-2-[(4-chloro-2-methanesulfonylphenyl)methyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427139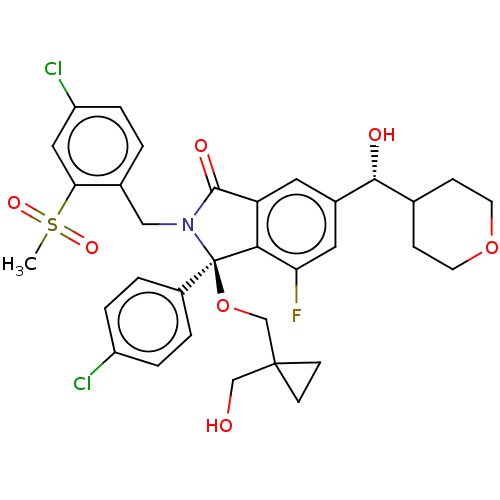 ((3R)-2-[(4-chloro-2-methanesulfonylphenyl)methyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2 (Bos taurus) | BDBM50235343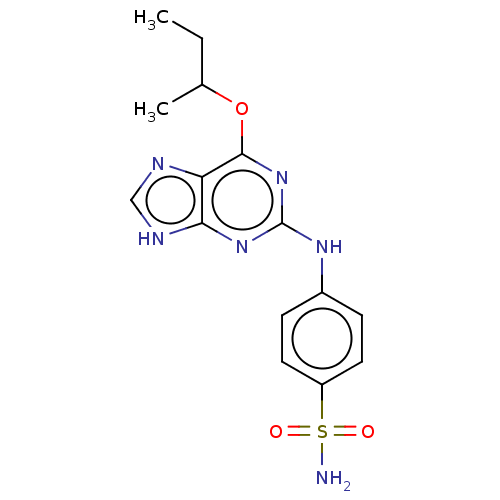 (CHEMBL4103249) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of human GST-tagged CDK2/bovine His-tagged Cyclin A | J Med Chem 60: 1746-1767 (2017) Article DOI: 10.1021/acs.jmedchem.6b01254 BindingDB Entry DOI: 10.7270/Q2833V86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427194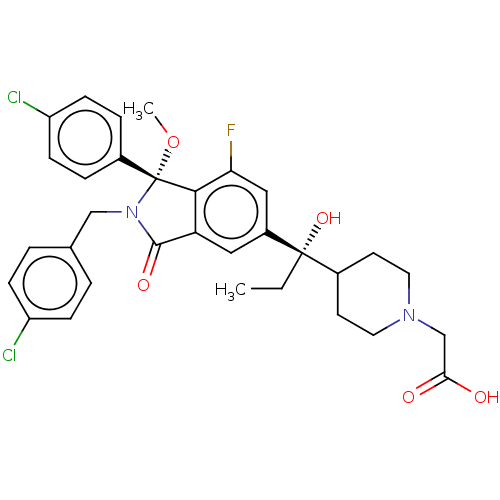 (US10544132, Example 135 | US10981898, Example 134 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427193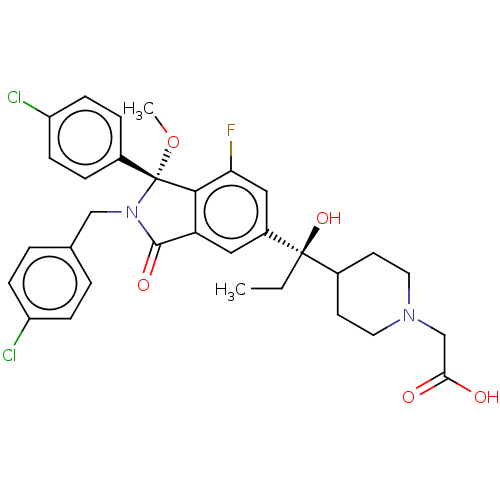 (US10544132, Example 134 | US11236047, Example 135) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50539763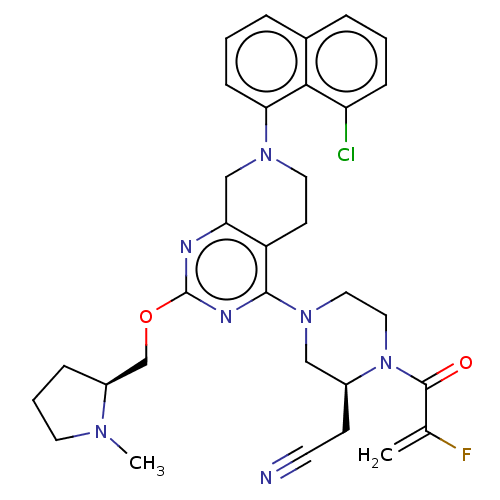 (Adagrasib | Mrtx-849 | Mrtx849) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d2md00216g BindingDB Entry DOI: 10.7270/Q2DF6W53 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427195 (US10544132, Example 136 | US10981898, Example 137 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427195 (US10544132, Example 136 | US10981898, Example 137 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM427125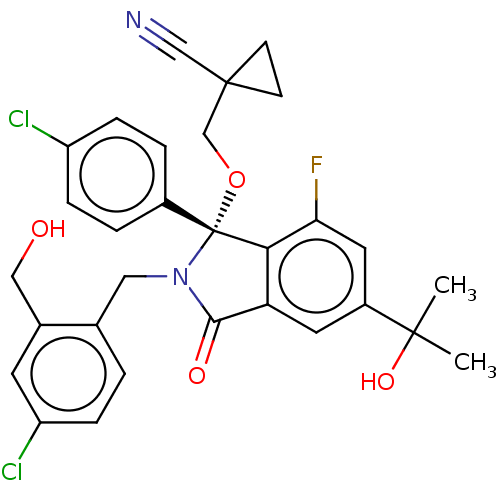 (US10544132, Example 72 | US10981898, Example 72 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10544132 (2020) BindingDB Entry DOI: 10.7270/Q27H1N06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 5 (Homo sapiens (Human)) | BDBM50434072 (CHEMBL2381342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of GST-tagged MEK5 (unknown origin) expressed in baculovirus expression system incubated for 90 mins by HTS assay | Eur J Med Chem 178: 530-543 (2019) Article DOI: 10.1016/j.ejmech.2019.05.057 BindingDB Entry DOI: 10.7270/Q23F4T0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM427125 (US10544132, Example 72 | US10981898, Example 72 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... | US Patent US10981898 (2021) BindingDB Entry DOI: 10.7270/Q2154M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 5 (Homo sapiens (Human)) | BDBM50434072 (CHEMBL2381342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01756 BindingDB Entry DOI: 10.7270/Q20R9TH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 963 total ) | Next | Last >> |