

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50443232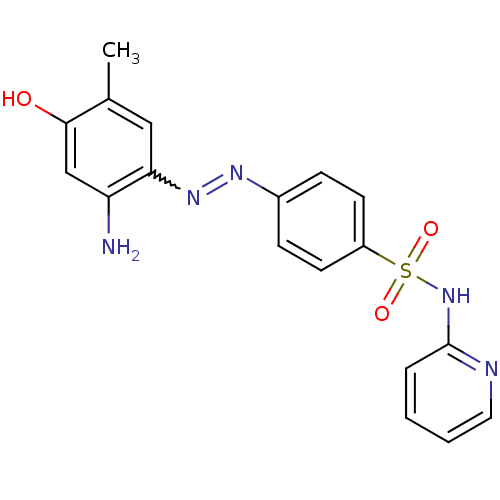 (CHEMBL3086883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human 6x-His-tagged BRD4 bromodomain 1 expressed in Escherichia coli | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 BindingDB Entry DOI: 10.7270/Q25T3PRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 protease using fluorogenic peptide substrate incubated for 30 mins prior to substrate addition measured after 10 mins b... | J Med Chem 57: 6468-78 (2014) Article DOI: 10.1021/jm5008352 BindingDB Entry DOI: 10.7270/Q2V126G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50443232 (CHEMBL3086883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human 6x-His-tagged BRD3 bromodomain 1 expressed in Escherichia coli | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 BindingDB Entry DOI: 10.7270/Q25T3PRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50443232 (CHEMBL3086883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human 6x-His-tagged BRD3 bromodomain 2 expressed in Escherichia coli | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 BindingDB Entry DOI: 10.7270/Q25T3PRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of multidrug resistant HIV-1 protease L10I/L63P/A71V/G73S/I84V/L90M mutant using fluorogenic peptide substrate incubated for 30 mins prior... | J Med Chem 57: 6468-78 (2014) Article DOI: 10.1021/jm5008352 BindingDB Entry DOI: 10.7270/Q2V126G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50443232 (CHEMBL3086883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human 6x-His-tagged BRD4 bromodomain 2 expressed in Escherichia coli | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 BindingDB Entry DOI: 10.7270/Q25T3PRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50058936 (CHEMBL3393116) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of multidrug resistant HIV-1 protease L10I/L63P/A71V/G73S/I84V/L90M mutant using fluorogenic peptide substrate incubated for 30 mins prior... | J Med Chem 57: 6468-78 (2014) Article DOI: 10.1021/jm5008352 BindingDB Entry DOI: 10.7270/Q2V126G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50058936 (CHEMBL3393116) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 protease using fluorogenic peptide substrate incubated for 30 mins prior to substrate addition measured after 10 mins b... | J Med Chem 57: 6468-78 (2014) Article DOI: 10.1021/jm5008352 BindingDB Entry DOI: 10.7270/Q2V126G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50277189 ((R)-6-methyl-N-(2-(thiazol-2-ylmethylamino)-2,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MTP in human HepG2 cells assessed as apolipoprotein B production by ELISA | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50107789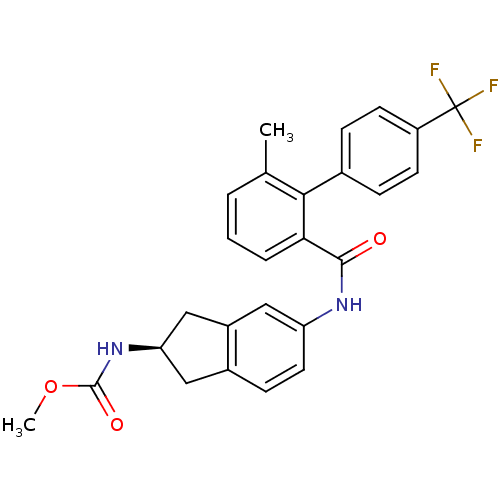 ((R)-methyl 5-(6-methyl-4'-(trifluoromethyl)bipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MTP in human HepG2 cells assessed as apolipoprotein B production by ELISA | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50107786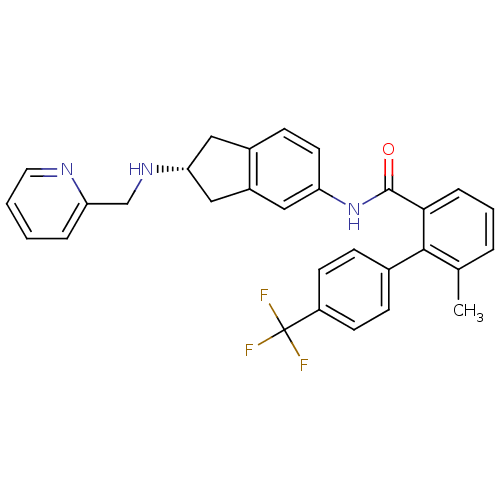 ((R)-6-methyl-N-(2-(pyridin-2-ylmethylamino)-2,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MTP in human HepG2 cells assessed as apolipoprotein B production by ELISA | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277330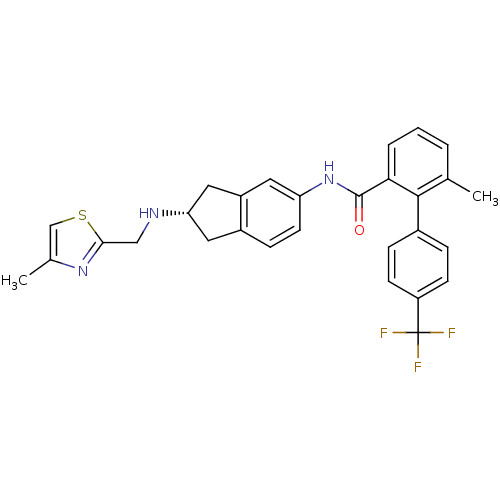 ((S)-6-methyl-N-(2-((4-methylthiazol-2-yl)methylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50107813 ((S)-methyl 5-(6-methyl-4'-(trifluoromethyl)bipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MTP in human HepG2 cells assessed as apolipoprotein B production by ELISA | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277332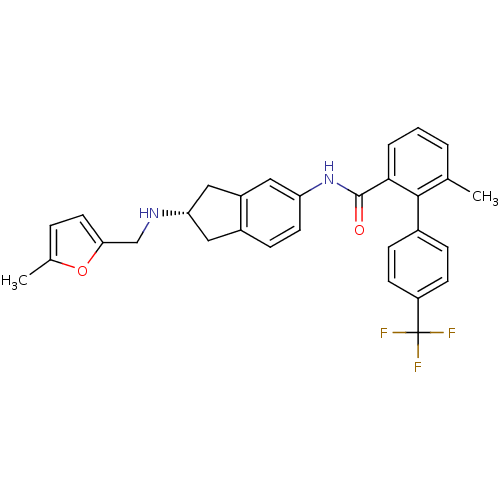 ((S)-6-methyl-N-(2-((5-methylfuran-2-yl)methylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277330 ((S)-6-methyl-N-(2-((4-methylthiazol-2-yl)methylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277297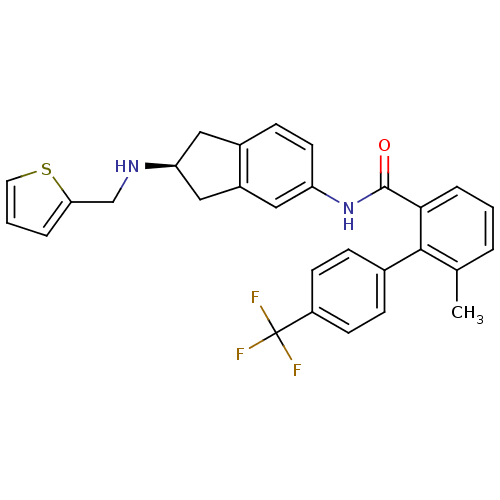 ((S)-6-methyl-N-(2-(thiophen-2-ylmethylamino)-2,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277258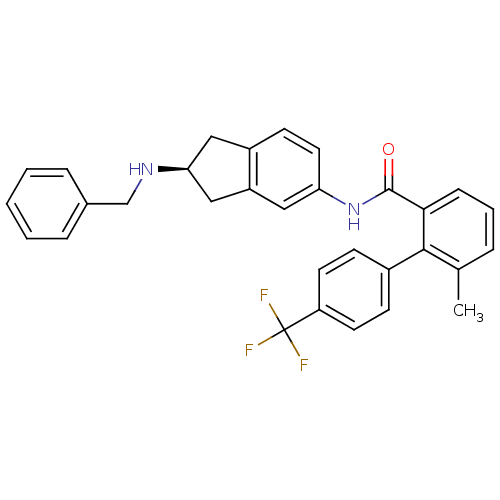 ((S)-N-(2-(benzylamino)-2,3-dihydro-1H-inden-5-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277297 ((S)-6-methyl-N-(2-(thiophen-2-ylmethylamino)-2,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277190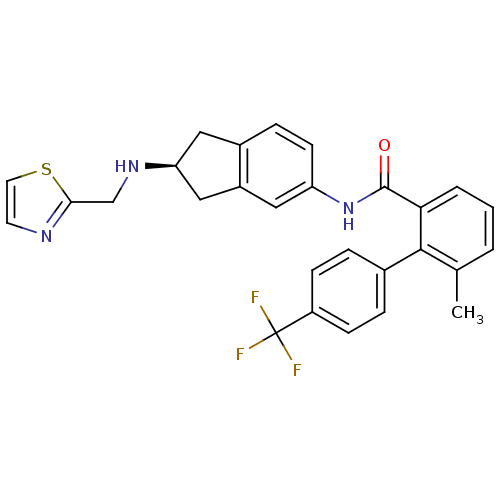 ((S)-6-methyl-N-(2-(thiazol-2-ylmethylamino)-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277332 ((S)-6-methyl-N-(2-((5-methylfuran-2-yl)methylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277298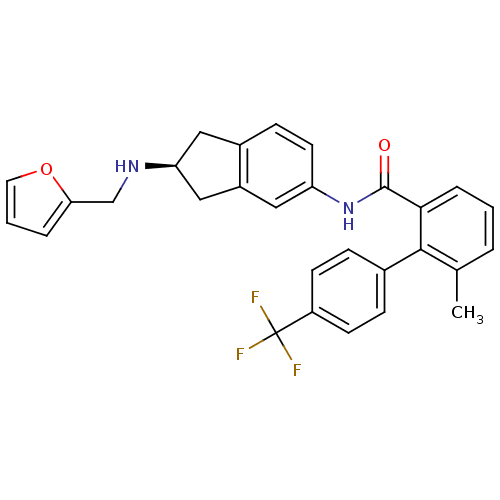 ((S)-N-(2-(furan-2-ylmethylamino)-2,3-dihydro-1H-in...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277258 ((S)-N-(2-(benzylamino)-2,3-dihydro-1H-inden-5-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277293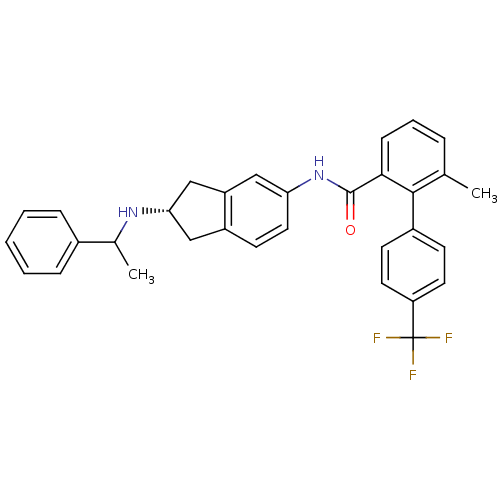 (6-methyl-N-((2S)-2-(1-phenylethylamino)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277190 ((S)-6-methyl-N-(2-(thiazol-2-ylmethylamino)-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277293 (6-methyl-N-((2S)-2-(1-phenylethylamino)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277294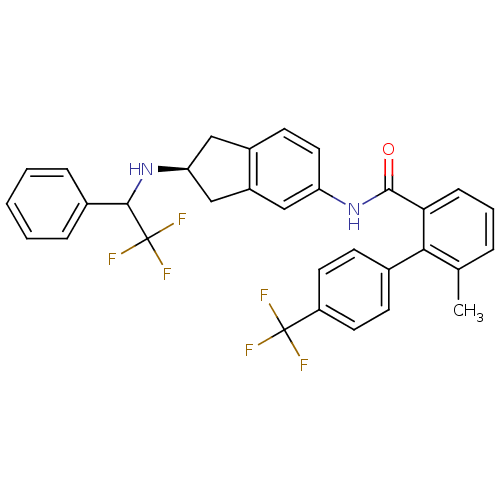 (6-methyl-N-((2S)-2-(2,2,2-trifluoro-1-phenylethyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50107787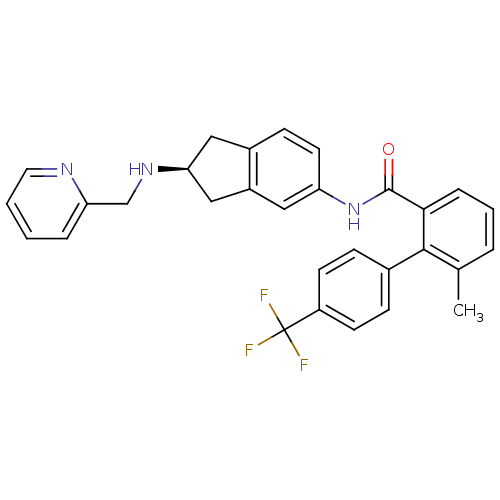 ((S)-6-methyl-N-(2-(pyridin-2-ylmethylamino)-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277294 (6-methyl-N-((2S)-2-(2,2,2-trifluoro-1-phenylethyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to BRD4 BD2 (unknown origin) by fluorescence anisotropy method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00933 BindingDB Entry DOI: 10.7270/Q2PR80VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to BRD4 BD1 (unknown origin) by fluorescence anisotropy method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00933 BindingDB Entry DOI: 10.7270/Q2PR80VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277296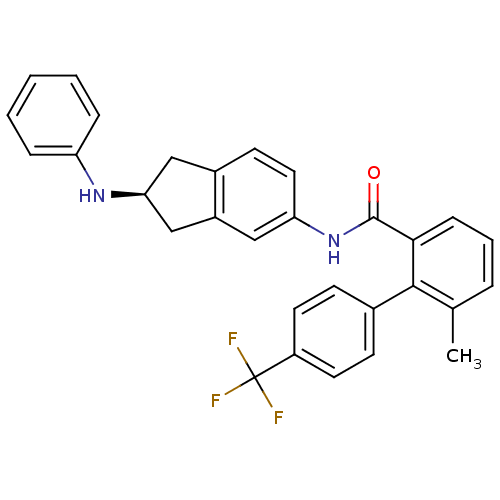 ((S)-6-methyl-N-(2-(phenylamino)-2,3-dihydro-1H-ind...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277260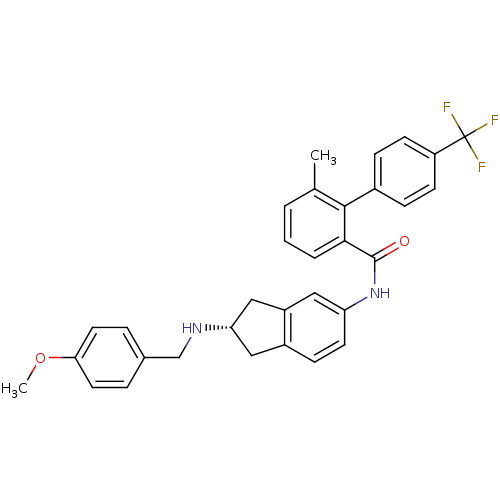 ((S)-N-(2-(4-methoxybenzylamino)-2,3-dihydro-1H-ind...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277151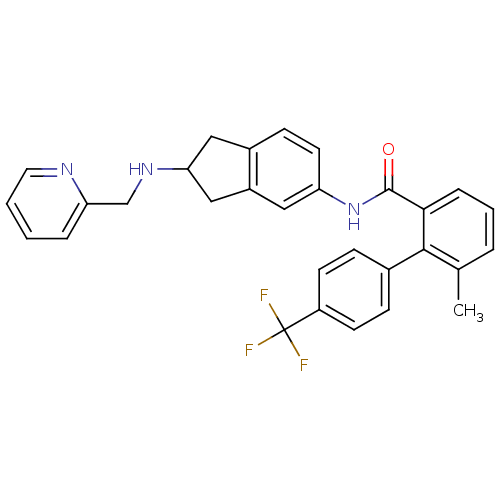 (CHEMBL474281 | rac-6-methyl-N-(2-(pyridin-2-ylmeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277331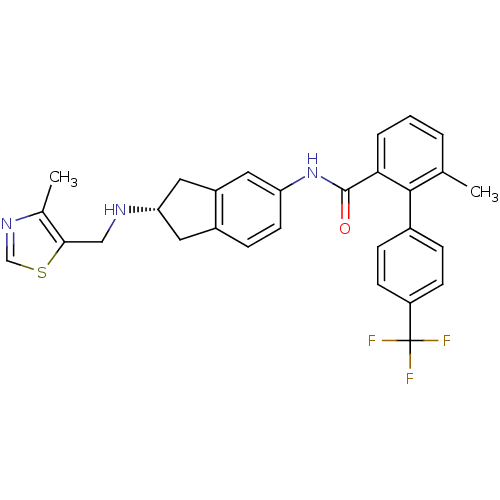 ((S)-6-methyl-N-(2-((4-methylthiazol-5-yl)methylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277260 ((S)-N-(2-(4-methoxybenzylamino)-2,3-dihydro-1H-ind...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50107787 ((S)-6-methyl-N-(2-(pyridin-2-ylmethylamino)-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073630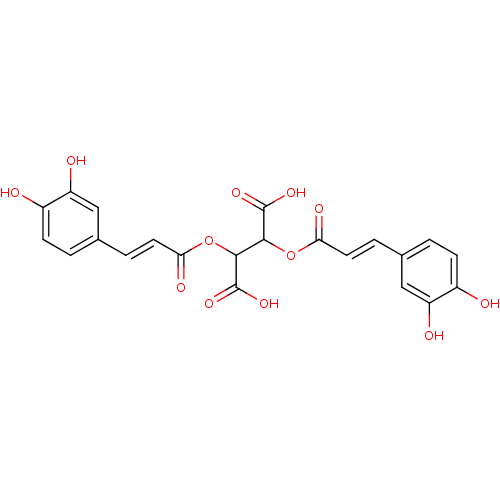 (2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acryloyloxy]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Tested for inhibition of HIV-1 integrase, under 1 uM for the strand transfer | J Med Chem 43: 2100-14 (2000) BindingDB Entry DOI: 10.7270/Q27D2VTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277296 ((S)-6-methyl-N-(2-(phenylamino)-2,3-dihydro-1H-ind...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50582040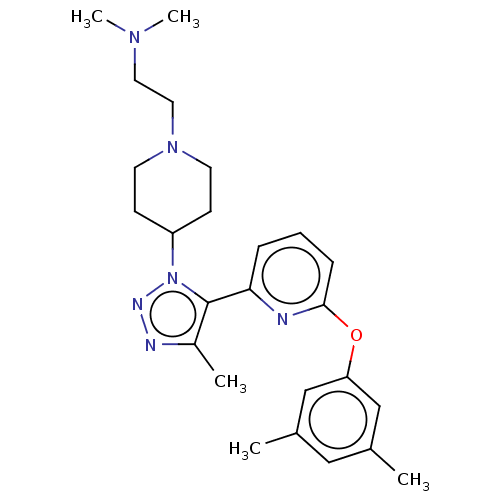 (CHEMBL5083660) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to BRD4 BD1 (unknown origin) by fluorescence anisotropy method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00933 BindingDB Entry DOI: 10.7270/Q2PR80VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50277190 ((S)-6-methyl-N-(2-(thiazol-2-ylmethylamino)-2,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MTP in human HepG2 cells assessed as apolipoprotein B production by ELISA | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50277297 ((S)-6-methyl-N-(2-(thiophen-2-ylmethylamino)-2,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MTP in human HepG2 cells assessed as apolipoprotein B production by ELISA | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50277298 ((S)-N-(2-(furan-2-ylmethylamino)-2,3-dihydro-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MTP in human HepG2 cells assessed as apolipoprotein B production by ELISA | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073630 (2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acryloyloxy]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase, under 1 uM for the 3''-preprocessing | J Med Chem 43: 2100-14 (2000) BindingDB Entry DOI: 10.7270/Q27D2VTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277331 ((S)-6-methyl-N-(2-((4-methylthiazol-5-yl)methylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50277293 (6-methyl-N-((2S)-2-(1-phenylethylamino)-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MTP in human HepG2 cells assessed as apolipoprotein B production by ELISA | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50277259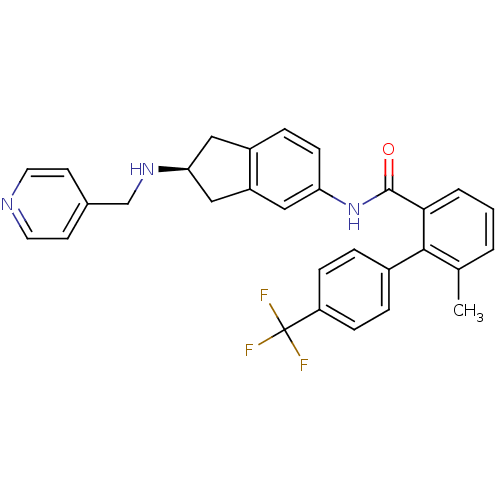 ((S)-6-methyl-N-(2-(pyridin-4-ylmethylamino)-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 1 (Homo sapiens (Human)) | BDBM104065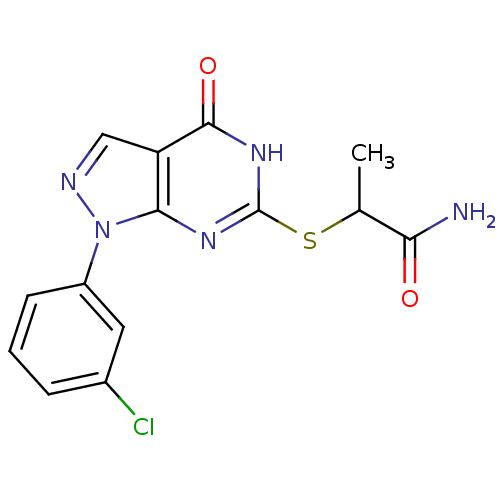 (HS38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center | Assay Description HS38 was evaluated using a P-33 ATP filter-binding assay by the International Centre for Kinase Profiling (University of Dundee) against 124 purified... | ACS Chem Biol 8: 2715-23 (2013) Article DOI: 10.1021/cb400407c BindingDB Entry DOI: 10.7270/Q24B2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM104065 (HS38) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center | Assay Description HS38 was evaluated using a P-33 ATP filter-binding assay by the International Centre for Kinase Profiling (University of Dundee) against 124 purified... | ACS Chem Biol 8: 2715-23 (2013) Article DOI: 10.1021/cb400407c BindingDB Entry DOI: 10.7270/Q24B2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277295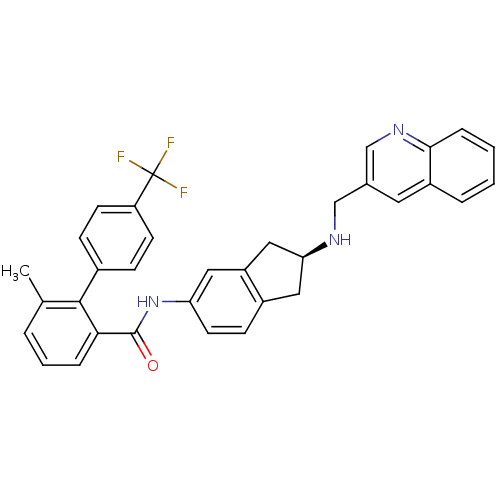 ((S)-6-methyl-N-(2-(quinolin-3-ylmethylamino)-2,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50277259 ((S)-6-methyl-N-(2-(pyridin-4-ylmethylamino)-2,3-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human Smo receptor expressed in CHO cells by [3H]Hh-Ag binding assay | Bioorg Med Chem Lett 19: 328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.11.096 BindingDB Entry DOI: 10.7270/Q29887ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 395 total ) | Next | Last >> |