 Found 162 hits with Last Name = 'chang' and Initial = 'js'
Found 162 hits with Last Name = 'chang' and Initial = 'js' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50481948
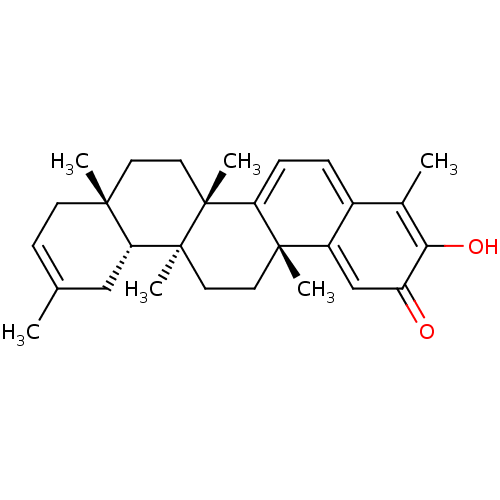
(Iguesterin | acs.jmedchem.1c00409_ST.224)Show SMILES [H][C@@]12CC(C)=CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C |r,c:4,24,t:14,16,19| Show InChI InChI=1S/C28H36O2/c1-17-9-10-25(3)11-13-27(5)22-8-7-19-18(2)24(30)21(29)16-20(19)26(22,4)12-14-28(27,6)23(25)15-17/h7-9,16,23,30H,10-15H2,1-6H3/t23-,25-,26+,27-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot |
Bioorg Med Chem Lett 20: 1873-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.152
BindingDB Entry DOI: 10.7270/Q28P63CF |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50481947
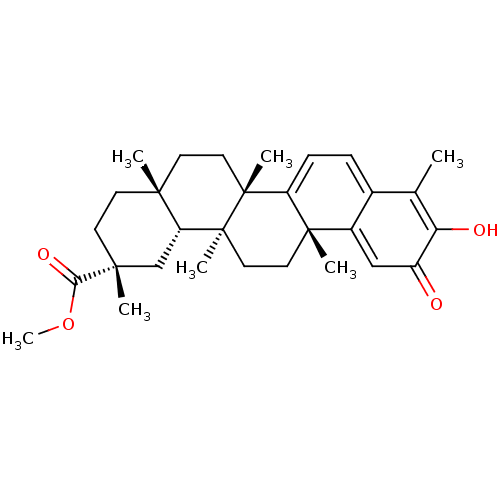
(CHEBI:8416 | GNF-Pf-476 | PRISTIMERIN | Pristimeri...)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OC |r,c:24,t:14,16,19| Show InChI InChI=1S/C30H40O4/c1-18-19-8-9-22-28(4,20(19)16-21(31)24(18)32)13-15-30(6)23-17-27(3,25(33)34-7)11-10-26(23,2)12-14-29(22,30)5/h8-9,16,23,32H,10-15,17H2,1-7H3/t23-,26-,27-,28+,29-,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot |
Bioorg Med Chem Lett 20: 1873-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.152
BindingDB Entry DOI: 10.7270/Q28P63CF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565931
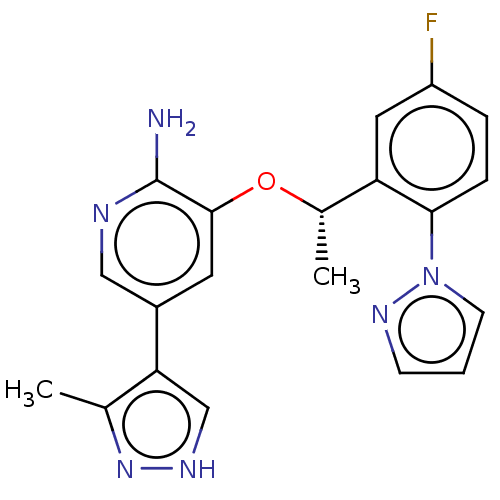
(CHEMBL4787096)Show SMILES C[C@H](Oc1cc(cnc1N)-c1c[nH]nc1C)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50071055
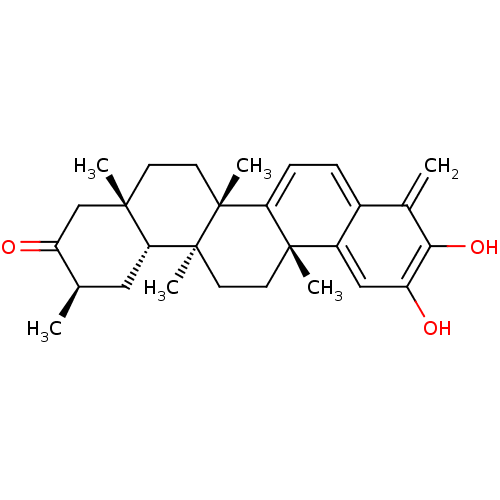
((6bS,8aS,11R,12aR,12bS,14aR)-3-Hydroxy-4,6b,8a,11,...)Show SMILES C[C@@H]1C[C@@H]2[C@@](C)(CC[C@]3(C)C4=CC=c5c(cc(O)c(O)c5=C)[C@]4(C)CC[C@@]23C)CC1=O |r,c:12,t:10| Show InChI InChI=1S/C28H36O3/c1-16-13-23-25(3,15-21(16)30)9-11-27(5)22-8-7-18-17(2)24(31)20(29)14-19(18)26(22,4)10-12-28(23,27)6/h7-8,14,16,23,29,31H,2,9-13,15H2,1,3-6H3/t16-,23-,25+,26+,27-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot |
Bioorg Med Chem Lett 20: 1873-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.152
BindingDB Entry DOI: 10.7270/Q28P63CF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565919
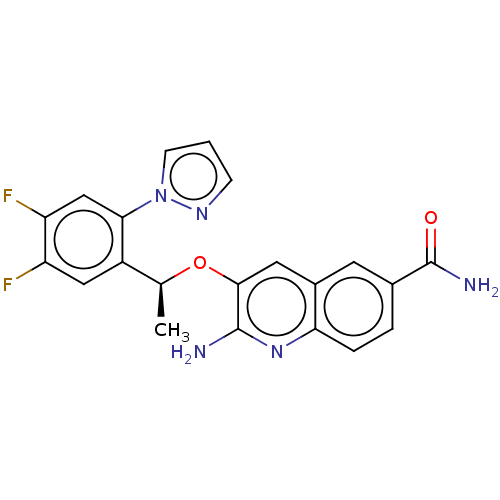
(CHEMBL4794362)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50071058
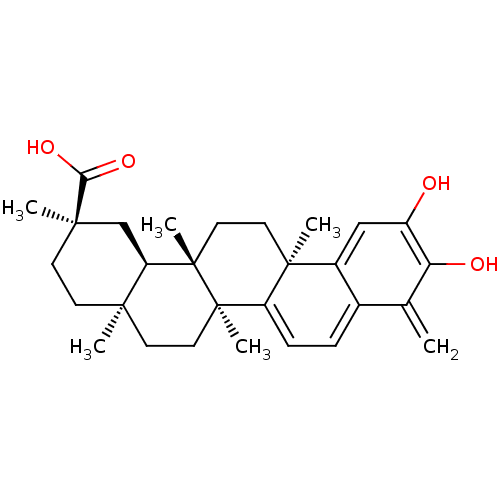
((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...)Show SMILES C[C@]12CC[C@](C)(C[C@H]1[C@]1(C)CC[C@]3(C)C(=CC=c4c3cc(O)c(O)c4=C)[C@@]1(C)CC2)C(O)=O |r,c:15,17| Show InChI InChI=1S/C29H38O4/c1-17-18-7-8-21-27(4,19(18)15-20(30)23(17)31)12-14-29(6)22-16-26(3,24(32)33)10-9-25(22,2)11-13-28(21,29)5/h7-8,15,22,30-31H,1,9-14,16H2,2-6H3,(H,32,33)/t22-,25-,26-,27+,28-,29+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot |
Bioorg Med Chem Lett 20: 1873-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.152
BindingDB Entry DOI: 10.7270/Q28P63CF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565920
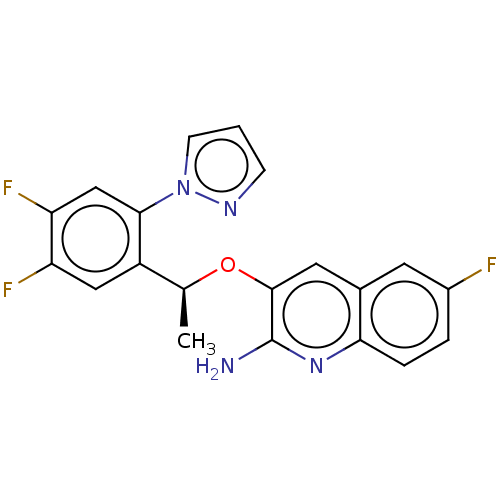
(CHEMBL4784517)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565925
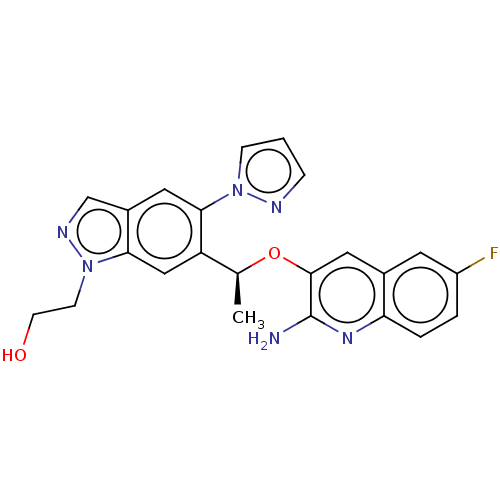
(CHEMBL4778780)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc2n(CCO)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565921
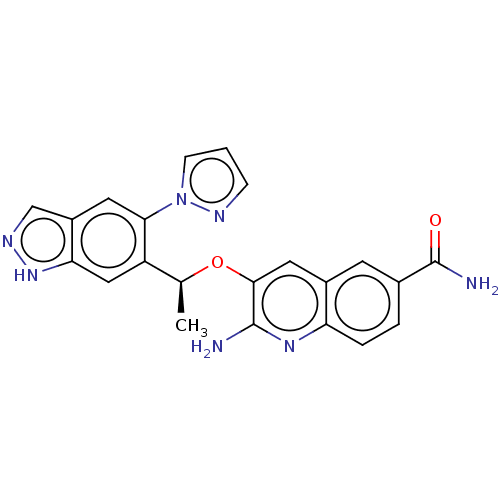
(CHEMBL4781765)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc2[nH]ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis |
Bioorg Med Chem 18: 7940-7 (2010)
Article DOI: 10.1016/j.bmc.2010.09.035
BindingDB Entry DOI: 10.7270/Q2MG7SBP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565918

(CHEMBL4778108)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C#N)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565917
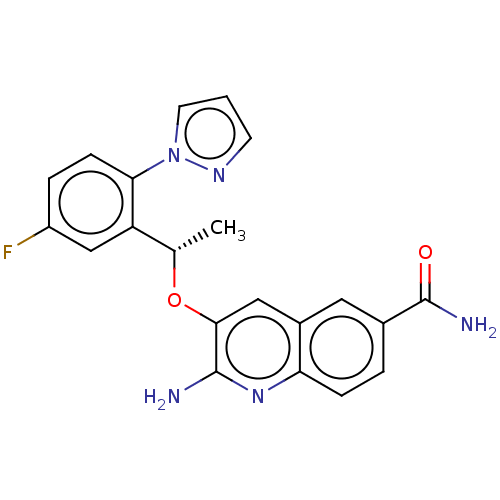
(CHEMBL4783261)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323199
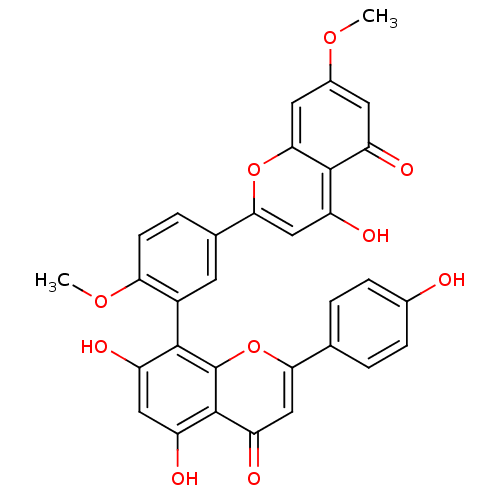
(5,7-dihydroxy-8-(5-(5-hydroxy-7-methoxy-4-oxo-4H-c...)Show SMILES COc1cc2oc(cc(O)c2c(=O)c1)-c1ccc(OC)c(c1)-c1c(O)cc(O)c2c1oc(cc2=O)-c1ccc(O)cc1 |(-8.64,-2.46,;-8.63,-4,;-7.29,-4.77,;-5.96,-3.99,;-4.63,-4.75,;-3.3,-3.96,;-1.95,-4.73,;-1.94,-6.28,;-3.28,-7.06,;-3.27,-8.6,;-4.61,-6.3,;-5.94,-7.06,;-5.93,-8.6,;-7.27,-6.31,;-.63,-3.95,;-.64,-2.41,;.68,-1.63,;2.03,-2.39,;3.35,-1.62,;3.34,-.08,;2.04,-3.94,;.71,-4.71,;3.37,-4.69,;3.38,-6.24,;2.05,-7.01,;4.72,-7,;6.05,-6.22,;7.39,-6.98,;6.04,-4.68,;4.7,-3.92,;4.68,-2.38,;6.02,-1.6,;7.36,-2.36,;7.36,-3.9,;8.7,-4.67,;6.02,-.06,;7.35,.72,;7.34,2.26,;6,3.02,;5.99,4.56,;4.67,2.23,;4.68,.7,)| Show InChI InChI=1S/C32H22O10/c1-39-18-10-20(34)30-23(37)13-27(41-28(30)11-18)16-5-8-25(40-2)19(9-16)29-21(35)12-22(36)31-24(38)14-26(42-32(29)31)15-3-6-17(33)7-4-15/h3-14,33,35-37H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis |
Bioorg Med Chem 18: 7940-7 (2010)
Article DOI: 10.1016/j.bmc.2010.09.035
BindingDB Entry DOI: 10.7270/Q2MG7SBP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565922

(CHEMBL4797664)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1ncc(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323206
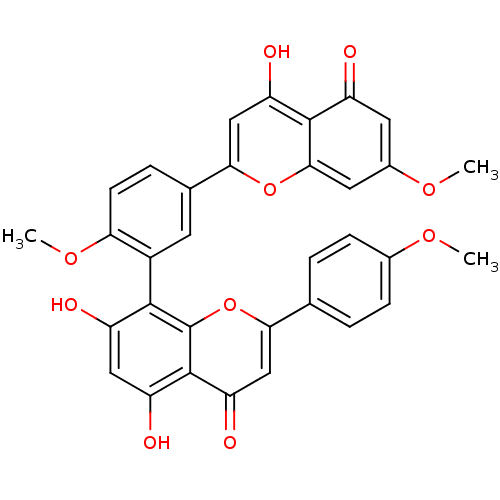
(CHEMBL208908 | sciadopitisin | sciadopitysin)Show SMILES COc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3OC)-c3cc(O)c4c(cc(OC)cc4=O)o3)c2o1 |(10.61,-33.01,;9.27,-32.25,;7.94,-33.02,;7.94,-34.56,;6.61,-35.34,;5.28,-34.57,;5.27,-33.03,;6.6,-32.26,;3.95,-35.34,;3.95,-36.87,;2.63,-37.63,;2.63,-39.17,;1.31,-36.87,;-.03,-37.66,;-.03,-39.2,;-1.36,-36.89,;-1.36,-35.35,;-2.69,-34.58,;-.02,-34.58,;-.02,-33.04,;-1.36,-32.27,;-1.35,-30.73,;-.03,-29.96,;1.31,-30.73,;1.31,-32.27,;2.65,-33.04,;3.98,-32.27,;-2.68,-29.97,;-2.68,-28.42,;-4.03,-27.64,;-4.03,-26.1,;-5.36,-28.42,;-5.36,-29.97,;-6.7,-30.74,;-8.03,-29.97,;-9.37,-30.74,;-10.7,-29.97,;-8.03,-28.42,;-6.7,-27.64,;-6.7,-26.1,;-4.02,-30.74,;1.3,-35.35,;2.62,-34.57,)| Show InChI InChI=1S/C33H24O10/c1-39-18-7-4-16(5-8-18)27-15-25(38)32-23(36)13-22(35)30(33(32)43-27)20-10-17(6-9-26(20)41-3)28-14-24(37)31-21(34)11-19(40-2)12-29(31)42-28/h4-15,35-37H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis |
Bioorg Med Chem 18: 7940-7 (2010)
Article DOI: 10.1016/j.bmc.2010.09.035
BindingDB Entry DOI: 10.7270/Q2MG7SBP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565929

(Pf-07059013)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1[nH]c(=O)ccc1-n1cccn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565927

(CHEMBL4779453)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc(ccc1-n1cccn1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565928

(CHEMBL4785484)Show SMILES C[C@H](Oc1cc2cc(F)cc(F)c2nc1N)c1cc(ccc1-n1cccn1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565916

(CHEMBL4777878)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C#N)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565924

(CHEMBL4795396)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc2n(CCO)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565923

(CHEMBL4762748)Show SMILES C[C@H](Oc1cc2cc(cc(F)c2nc1N)C(N)=O)c1ncc(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565926

(CHEMBL4778770)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc2n(CC(O)=O)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565930

(CHEMBL4796436)Show SMILES C[C@H](Oc1cc2cc(F)cc(F)c2nc1N)c1[nH]c(=O)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323196
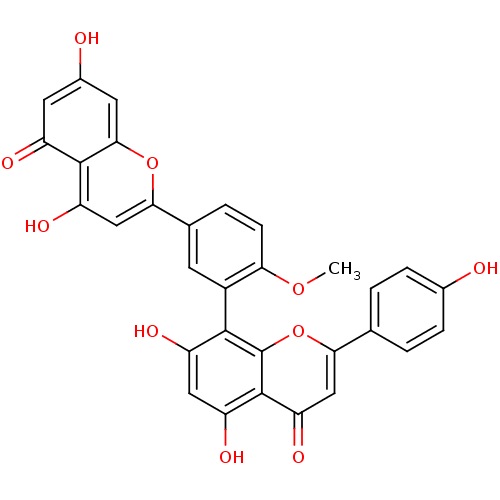
(4'-methylamentoflavone | CHEMBL378188 | bilobetin)Show SMILES COc1ccc(cc1-c1c(O)cc(O)c2c1oc(cc2=O)-c1ccc(O)cc1)-c1cc(O)c2c(cc(O)cc2=O)o1 |(3.27,-31.58,;3.27,-33.11,;1.94,-33.89,;.61,-33.11,;-.73,-33.89,;-.73,-35.42,;.61,-36.2,;1.94,-35.42,;3.27,-36.2,;4.6,-35.42,;4.6,-33.89,;5.93,-36.2,;5.93,-37.73,;7.27,-38.51,;4.6,-38.51,;3.27,-37.73,;1.94,-38.51,;1.94,-40.04,;3.27,-40.81,;4.6,-40.04,;5.93,-40.81,;.61,-40.81,;.61,-42.35,;-.73,-43.12,;-2.06,-42.35,;-3.39,-43.12,;-2.06,-40.81,;-.73,-40.04,;-2.06,-36.2,;-2.06,-37.73,;-3.39,-38.51,;-3.39,-40.04,;-4.73,-37.73,;-4.73,-36.2,;-6.06,-35.42,;-7.39,-36.2,;-8.72,-35.42,;-7.39,-37.73,;-6.06,-38.51,;-6.06,-40.04,;-3.39,-35.42,)| Show InChI InChI=1S/C31H20O10/c1-39-24-7-4-15(26-12-22(37)29-19(34)9-17(33)10-27(29)40-26)8-18(24)28-20(35)11-21(36)30-23(38)13-25(41-31(28)30)14-2-5-16(32)6-3-14/h2-13,32-33,35-37H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis |
Bioorg Med Chem 18: 7940-7 (2010)
Article DOI: 10.1016/j.bmc.2010.09.035
BindingDB Entry DOI: 10.7270/Q2MG7SBP |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500
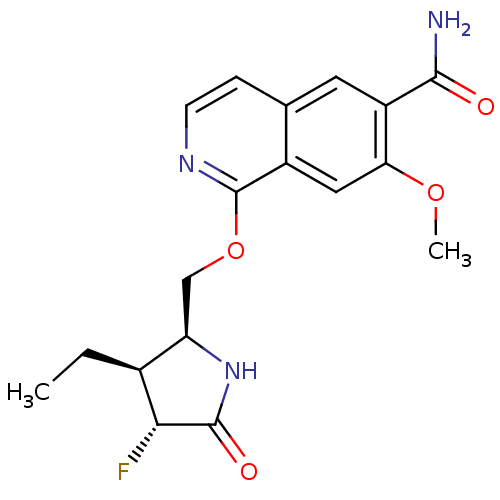
(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239498
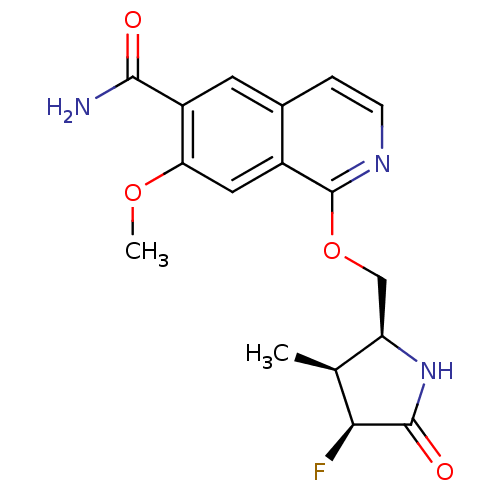
(CHEMBL4093120 | US10329302, Example 189 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239507
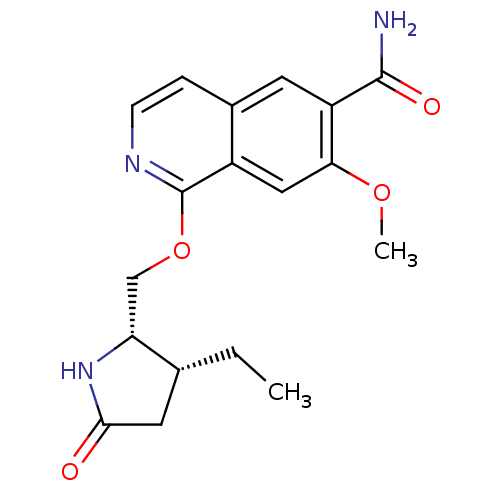
(CHEMBL4091434 | US10329302, Example 246 | US107935...)Show SMILES CC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H21N3O4/c1-3-10-7-16(22)21-14(10)9-25-18-12-8-15(24-2)13(17(19)23)6-11(12)4-5-20-18/h4-6,8,10,14H,3,7,9H2,1-2H3,(H2,19,23)(H,21,22)/t10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239508
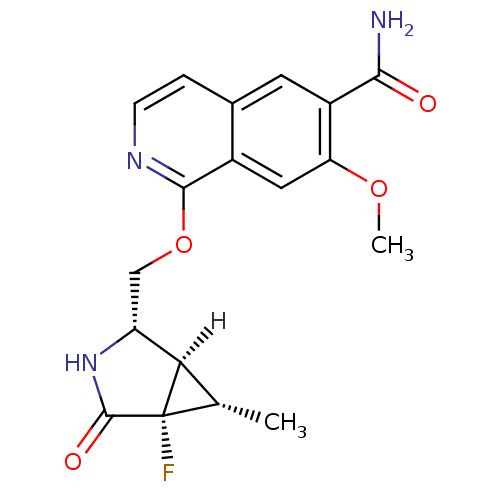
(CHEMBL4085199 | US10329302, Example 309 | US107935...)Show SMILES [H][C@]12[C@H](C)[C@@]1(F)C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H18FN3O4/c1-8-14-12(22-17(24)18(8,14)19)7-26-16-10-6-13(25-2)11(15(20)23)5-9(10)3-4-21-16/h3-6,8,12,14H,7H2,1-2H3,(H2,20,23)(H,22,24)/t8-,12+,14+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11390
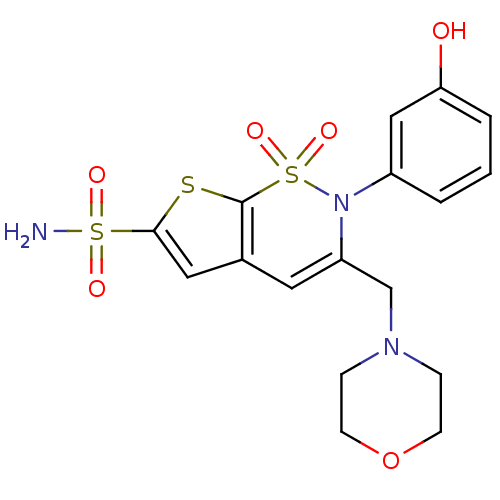
(2-(3-Hydroxyphenyl)-3-(4-morpholinylmethyl)-2H-thi...)Show SMILES NS(=O)(=O)c1cc2C=C(CN3CCOCC3)N(c3cccc(O)c3)S(=O)(=O)c2s1 |t:7| Show InChI InChI=1S/C17H19N3O6S3/c18-28(22,23)16-9-12-8-14(11-19-4-6-26-7-5-19)20(29(24,25)17(12)27-16)13-2-1-3-15(21)10-13/h1-3,8-10,21H,4-7,11H2,(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Concentration which produces 50% inhibition of human Carbonic anhydrase II |
J Med Chem 45: 888-93 (2002)
Article DOI: 10.1021/jm010163d
BindingDB Entry DOI: 10.7270/Q2P84FN0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11391
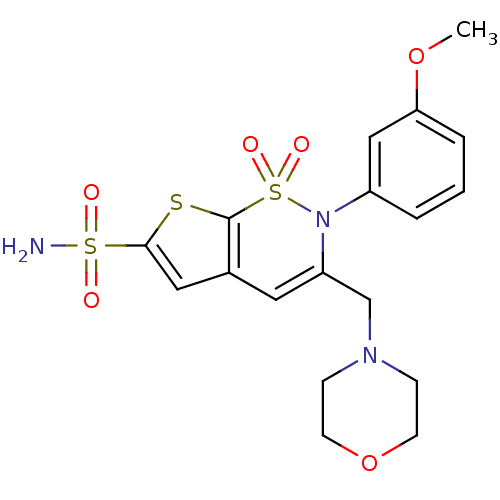
(2-(3-Methoxyphenyl)-3-[(4-morpholinyl)methyl]-2H-t...)Show SMILES COc1cccc(c1)N1C(CN2CCOCC2)=Cc2cc(sc2S1(=O)=O)S(N)(=O)=O |c:18| Show InChI InChI=1S/C18H21N3O6S3/c1-26-16-4-2-3-14(11-16)21-15(12-20-5-7-27-8-6-20)9-13-10-17(29(19,22)23)28-18(13)30(21,24)25/h2-4,9-11H,5-8,12H2,1H3,(H2,19,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Concentration which produces 50% inhibition of human Carbonic anhydrase II |
J Med Chem 45: 888-93 (2002)
Article DOI: 10.1021/jm010163d
BindingDB Entry DOI: 10.7270/Q2P84FN0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239493
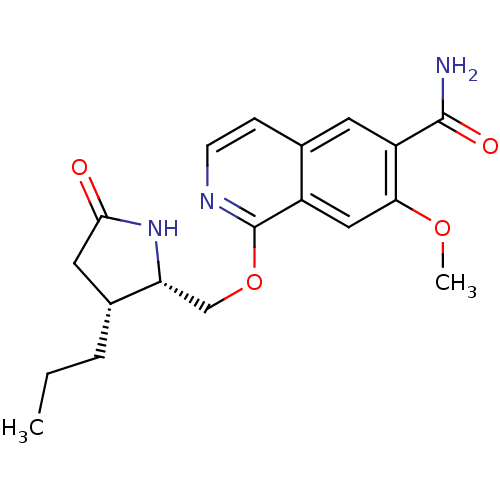
(CHEMBL4103497 | US10329302, Example 312 | US107935...)Show SMILES CCC[C@@H]1CC(=O)N[C@@H]1COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C19H23N3O4/c1-3-4-12-8-17(23)22-15(12)10-26-19-13-9-16(25-2)14(18(20)24)7-11(13)5-6-21-19/h5-7,9,12,15H,3-4,8,10H2,1-2H3,(H2,20,24)(H,22,23)/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239491
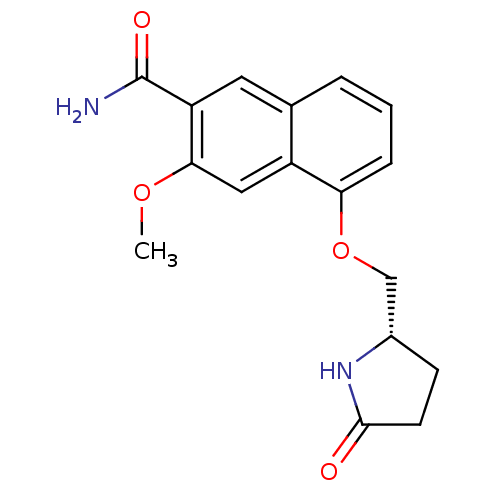
(CHEMBL4083655 | US10329302, Example 173 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)cccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18N2O4/c1-22-15-8-12-10(7-13(15)17(18)21)3-2-4-14(12)23-9-11-5-6-16(20)19-11/h2-4,7-8,11H,5-6,9H2,1H3,(H2,18,21)(H,19,20)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11392

((4R)-4-amino-2-(3-methoxypropyl)-1,1-dioxo-2H,3H,4...)Show SMILES COCCCN1C[C@H](N)c2cc(sc2S1(=O)=O)S(N)(=O)=O |r| Show InChI InChI=1S/C10H17N3O5S3/c1-18-4-2-3-13-6-8(11)7-5-9(20(12,14)15)19-10(7)21(13,16)17/h5,8H,2-4,6,11H2,1H3,(H2,12,14,15)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Concentration which produces 50% inhibition of human Carbonic anhydrase II |
J Med Chem 45: 888-93 (2002)
Article DOI: 10.1021/jm010163d
BindingDB Entry DOI: 10.7270/Q2P84FN0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239506

(CHEMBL4071526 | US10329302, Example 188 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@H](F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H18FN3O4/c1-8-12(21-16(23)14(8)18)7-25-17-10-6-13(24-2)11(15(19)22)5-9(10)3-4-20-17/h3-6,8,12,14H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as reduction R848-induced IL-6 secretion by measuring plasma protein binding corrected IC50 preincu... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production by measuring plasma protein binding corrected IC50 af... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM92626
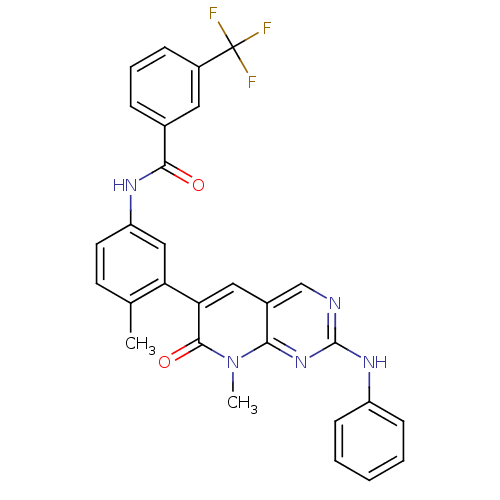
(Pyrido analog, 3)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cc2cnc(Nc3ccccc3)nc2n(C)c1=O Show InChI InChI=1S/C29H22F3N5O2/c1-17-11-12-22(34-26(38)18-7-6-8-20(13-18)29(30,31)32)15-23(17)24-14-19-16-33-28(35-21-9-4-3-5-10-21)36-25(19)37(2)27(24)39/h3-16H,1-2H3,(H,34,38)(H,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer
| Assay Description
A Lance TR-FRET assay (Perkin Elmer) was used to measure and compare the potency of compounds against Syk kinase domain. |
Chem Biol Drug Des 80: 657-64 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01443.x
BindingDB Entry DOI: 10.7270/Q2BG2MKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239497
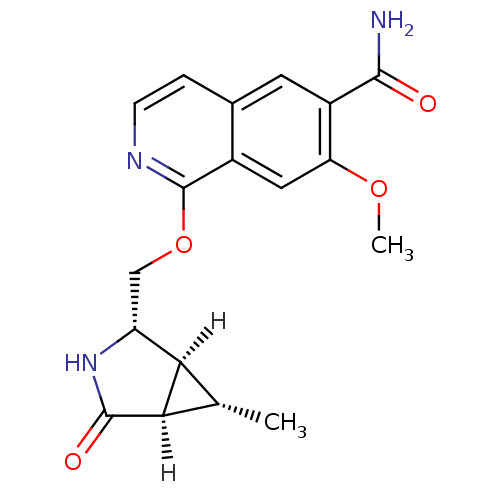
(CHEMBL4084228 | US10329302, Example 271 | US107935...)Show SMILES [H][C@]12[C@H](C)[C@@]1([H])C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C18H19N3O4/c1-8-14-12(21-17(23)15(8)14)7-25-18-10-6-13(24-2)11(16(19)22)5-9(10)3-4-20-18/h3-6,8,12,14-15H,7H2,1-2H3,(H2,19,22)(H,21,23)/t8-,12+,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239492

(CHEMBL4070515 | US10329302, Example 211 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)C[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C17H19N3O4/c1-9-5-15(21)20-13(9)8-24-17-11-7-14(23-2)12(16(18)22)6-10(11)3-4-19-17/h3-4,6-7,9,13H,5,8H2,1-2H3,(H2,18,22)(H,20,21)/t9-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Concentration which produces 50% inhibition of human Carbonic anhydrase II |
J Med Chem 45: 888-93 (2002)
Article DOI: 10.1021/jm010163d
BindingDB Entry DOI: 10.7270/Q2P84FN0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239505
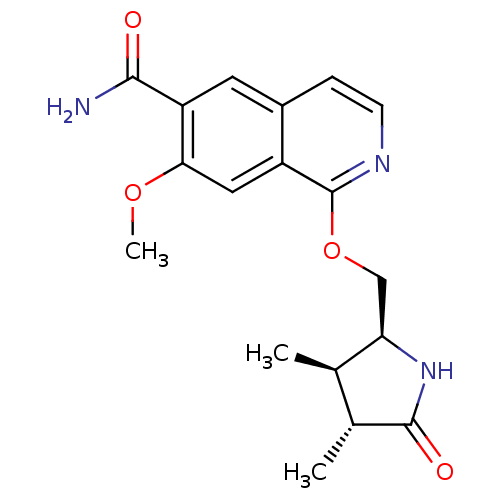
(CHEMBL4061801 | US10329302, Example 248 | US107935...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@H](C)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H21N3O4/c1-9-10(2)17(23)21-14(9)8-25-18-12-7-15(24-3)13(16(19)22)6-11(12)4-5-20-18/h4-7,9-10,14H,8H2,1-3H3,(H2,19,22)(H,21,23)/t9-,10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM11390

(2-(3-Hydroxyphenyl)-3-(4-morpholinylmethyl)-2H-thi...)Show SMILES NS(=O)(=O)c1cc2C=C(CN3CCOCC3)N(c3cccc(O)c3)S(=O)(=O)c2s1 |t:7| Show InChI InChI=1S/C17H19N3O6S3/c18-28(22,23)16-9-12-8-14(11-19-4-6-26-7-5-19)20(29(24,25)17(12)27-16)13-2-1-3-15(21)10-13/h1-3,8-10,21H,4-7,11H2,(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Concentration which produces 50% Inhibition of human Carbonic anhydrase IV |
J Med Chem 45: 888-93 (2002)
Article DOI: 10.1021/jm010163d
BindingDB Entry DOI: 10.7270/Q2P84FN0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239488
(CHEMBL4092338 | US10329302, Example 26 | US1079357...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CCC(=O)N3)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H21N3O4/c1-10(2)25-15-8-13-11(7-14(15)17(19)23)5-6-20-18(13)24-9-12-3-4-16(22)21-12/h5-8,10,12H,3-4,9H2,1-2H3,(H2,19,23)(H,21,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239496

(CHEMBL4075552 | US10329302, Example 264 | US107935...)Show SMILES [H][C@]12C[C@@]1([H])C(=O)N[C@@H]2COc1nccc2cc(C(N)=O)c(OC)cc12 |r| Show InChI InChI=1S/C17H17N3O4/c1-23-14-6-9-8(4-12(14)15(18)21)2-3-19-17(9)24-7-13-10-5-11(10)16(22)20-13/h2-4,6,10-11,13H,5,7H2,1H3,(H2,18,21)(H,20,22)/t10-,11+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239504

(CHEMBL4061890 | US10329302, Example 262 | US107935...)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)ccnc2cc1C(N)=O |r| Show InChI InChI=1S/C16H17N3O4/c1-22-14-7-10-12(6-11(14)16(17)21)18-5-4-13(10)23-8-9-2-3-15(20)19-9/h4-7,9H,2-3,8H2,1H3,(H2,17,21)(H,19,20)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50402996

(CHEMBL2207440)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1cc(n[nH]1)-c1ccc2ncccc2c1)C(C)(C)C Show InChI InChI=1S/C27H27N7O/c1-17-7-10-20(11-8-17)34-25(16-23(33-34)27(2,3)4)30-26(35)29-24-15-22(31-32-24)19-9-12-21-18(14-19)6-5-13-28-21/h5-16H,1-4H3,(H3,29,30,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal His-tagged PYK2 using ATP as substrate incubated for 1 hr prior to substrate addition |
Bioorg Med Chem Lett 22: 7523-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.039
BindingDB Entry DOI: 10.7270/Q2319X2C |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500

(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM11391

(2-(3-Methoxyphenyl)-3-[(4-morpholinyl)methyl]-2H-t...)Show SMILES COc1cccc(c1)N1C(CN2CCOCC2)=Cc2cc(sc2S1(=O)=O)S(N)(=O)=O |c:18| Show InChI InChI=1S/C18H21N3O6S3/c1-26-16-4-2-3-14(11-16)21-15(12-20-5-7-27-8-6-20)9-13-10-17(29(19,22)23)28-18(13)30(21,24)25/h2-4,9-11H,5-8,12H2,1H3,(H2,19,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Concentration which produces 50% Inhibition of human Carbonic anhydrase IV |
J Med Chem 45: 888-93 (2002)
Article DOI: 10.1021/jm010163d
BindingDB Entry DOI: 10.7270/Q2P84FN0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data